Abstract
Ticks are important disease vectors affecting animal health and causing substantial economic loss, especially in the tropics and subtropics. To examine the tick burden of cattle and associated risk factors for tick infestation, ticks were collected from 388 cattle within five regions in Ghana. Most of the cattle were males (50.3%) and generally older than 3 years (65%). Of the animals sampled, 2187 ticks were collected with a mean tick burden of 5.6 ticks per cattle, and the average tick burden on the udder/scrotum being significantly higher than in the anal region (Generalized Linear Mix Model [GLMM], p = 0.01197). The tick species identified were predominantly Amblyomma variegatum (42.6%) and Hyalomma rufipes (26.2%). High proportions of cattle examined were found to have A. variegatum infesting the udder/scrotum. Furthermore, H. rufipes infested mostly the anal region compared to other examined body parts (OR 14.8, 95% CI 8.6–25.4, p < 0.001). Using the GLMM, tick abundance was found to be significantly higher in cattle older than 3 years. The tick burden in the udder/scrotum was higher than that from the chest and leg/thigh of the cattle (GLMM, p < 0.05). The tick burden at the anal region was also significantly higher than the leg/thigh and chest. This study indicates that the preferred attachment sites of ticks on cattle are species-dependent and effective treatment with acaricides should take into consideration the udder/scrotum and anal regions as well as prioritizing older cattle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the tropical and subtropical regions, ticks are vectors for multiple pathogens that cause diseases in animals and humans (Balinandi et al. 2020; Estrada-Peña and De La Fuente 2014). With the increasing trade of livestock among African countries (Chand 2020) and the extensive transboundary migrations either in search of pasture or due to conflicts (Zannou et al. 2021), ticks can more readily infiltrate new regions and further spread tick-borne pathogens. Tick-borne disease negatively affects animal health and associated economic activity resulting in significant financial loss (Rajput et al. 2006; Vesco et al. 2011), with nearly 80% of livestock mortality caused by tick-borne diseases (Cleaveland et al. 2001; Cumming 2002). Multiple tick-borne pathogens can cause acute and chronic morbidity in humans, which are threats to public health (Rajput et al. 2006; Sarani et al. 2014). Despite this impact on human and animal health, control efforts have proven ineffective due to a lack of knowledge on the ecology of ticks and their resting preferences as well as human, biological and environmental factors that affect their biology (Cleaveland et al. 2001; Cumming 2002). To better understand the ecology of ticks, there is a need to investigate how demographic factors such as age, sex and the ecological zones they live in affect tick infestation on cattle (Cumming 1999; Dantas-Torres 2008).
Ticks prefer areas on an animal's body where the skin is thin and blood flow plentiful, like the inguinal region and external genital area (Hurtado and Giraldo-Ríos 2018). This can be observed in the case of A. variegatum which has a preference for the udder of livestock leading to drastically reduced milk production, serious wounds (Stachurski 2000) and impaired growth (Pegram and Oosterwijk 1990).
Within Africa, about 50 tick species have been documented to infest domestic animals (Walker et al. 2003) with the genera Rhipicephalus, Hyalomma and Amblyomma having the greatest impact on livestock and human health (Reye et al. 2012; Balinandi et al. 2020). In Ghana, tick species of the genera Amblyomma, Hyalomma and Rhipicephalus have been identified to infest livestock (Ntiamoa-Baidu et al. 2004). Additionally, tick-borne pathogens including Crimean-Congo Haemorrhagic Fever Virus (CCHFV), Dugbe Virus and Rickettsia africae have been reported in tick species (Akuffo et al. 2016; Kobayashi et al. 2017; Addo et al. 2023a). However, there is limited data on the ecology of ticks and how this affects tick infestation in the country. Given the veterinary and economic importance of ticks, there is a need to understand their biology, especially concerning their feeding and resting site preferences on cattle. This will provide essential information to develop effective control methods, given the apparent threat of zoonotic pathogen transmission in Ghana due to the dependence on livestock production. In this study, the burden of tick infestation and their feeding and resting preferences on cattle from different ecological zones in Ghana were investigated.
Methods
Study sites
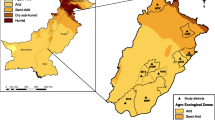
Ticks were collected from five different sampling locations in Ghana namely, Upper East (Abattoir, Cattle Market, Nakong), Northern (Airforce Base, Airborne Force, Kamina, Daboya), Bono East (Sunuase, Dawadawa, Cattle Market, Abattoir), Ashanti (Kumasi-abattoir) and Greater Accra (3MTD-Burma Camp, 1BN-Michel Camp, Asutsuare) (Fig. 1). The sampling took place from January to August 2020.
Tick collection and Identification
Using Epi Info version 6, a required sample size of 388 livestock (rounded up from the calculated number of 374) was calculated. Sample size calculations were made with the following assumptions: population size (estimated number of livestock in the study areas based upon local veterinarian estimate) of 16,633 livestock; anticipated prevalence rate of 45%; and a 5% confidence level. Eighty livestock were selected from each of the five regions and with the help of the herdsmen, livestock infested with ticks were identified and sampled. All body parts of the livestock were examined and ticks identified were collected using blunt forceps and placed in labelled vials containing RNA later (Qiagen, Germany). Ticks obtained from the same region of the animal were put into the same vial. In the laboratory, ticks were morphologically identified with the aid of a dissecting microscope using taxonomic keys (Walker et al. 2003) and individually stored at − 80 °C in 2 ml Eppendorf vials containing RNA Later. The taxonomic keys used in the morphological identification is an illustrated guide that outlines a straightforward three-step method for recognizing domestic animal ticks in Africa.
Generally, the mouthparts of the hard ticks identified in this study project forward, they have a scutum and often have eyes that are visible dorsally. Ticks of the genera Amblyomma and Hyalomma are bigger with large eyes, long mouthparts projecting to the anterior part of the body and pale rings on segments of their legs. With the genus Rhipicephalus, the ticks are medium in size with short mouthparts, eyes present and coxae 1 having large and equal paired spurs. Boophilus ticks are smaller in size, have short mouthparts, the eyes are small or absent and the coxae 1 has small paired spurs or single spur. To further identify ticks at the species level, the taxonomic keys provide pictorial unique features. For example, A. variegatum has small to medium punctuations, the posterior lips of the female genital aperture is U shaped and the males have enamel patterns often without lateral spots and no enamel on the festoons.
Animal data such as sex and age were obtained from the animal handler and recorded. Animals less than or equal to 3 years old were considered young while those 4 years or more were considered old.
Molecular identification of tick species
Ticks that were engorged and difficult to morphologically identify to the species level were subject to molecular identification. Total nucleic acid was extracted from each tick using the QIAamp Mini Kit (Qiagen, Valencia, CA, USA) (Crowder et al. 2010). To determine the tick species, primers TickCO1-F (TACTCTACTAATCATAAAGACATTGG) and TickCO1-R (CCTCCTCCTGAAGGGTCAAAAAATGA) that amplify the 660-bp segment of the mitochondrial COI gene were used (Barrett and Hebert 2005).
A total of 50 μl was used for each PCR reaction, which included 25 μl of GoTaq® Green Master Mix (2x), 1 μM of both forward and reverse primers, 18 μl of nuclease-free water and 5 μl of DNA as template. Each PCR reaction contained both positive (tick isolate) and negative (nuclease-free water) controls. Mastercycler X50-PCR Thermocycler (Eppendorf, Germany) was used to run the PCR. The cycling conditions were as follows: a first hold at 95 °C for 5 min; a second hold at 95 °C for 30 s, 48 °C for 30 s and 72 °C for 1 min at 34 cycles; and a third hold at 72 °C for 5 min. At 4 °C, the reaction was maintained. Using 2 g of agarose in 100 ml of 1 × Tris Acetate EDTA, a 2% agarose gel was made. It was then stained with 5 μl of SYBR® Safe DNA gel stain. Each PCR product (5 μl) was placed onto the gel along with a 100 bp ladder from New England BioLabs and run for 30 min at 100 V. Afterwards, the gel was examined using a Molecular Imager® Gel Doc (Bio-Rad).
For Sanger sequencing, the PCR products were sent to Macrogen Europe B.V. in Amsterdam, Netherlands. Using Chromas (version 2.6.6), each sequence obtained in this study was viewed after which MEGA (version 10.0.5) was used to edit, clean and generate a consensus sequence. The consensus sequences were subsequently compared to various sequences that had been submitted to the NCBI database (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Data analysis
Statistical analysis was done using R version 4.1.0. Descriptive statistics were used to compare animal and tick characteristics. Categorical variables were described using percentages and frequencies. Count variables were described using mean and standard deviations. Kruskal Wallis or Mann Whitney testing was used to determine the association between animal demographic characteristics and the count of ticks from different body parts of livestock. To determine the association between tick species and the animal’s age, sex and region from where it was sampled, each tick species was treated as a dichotomous variable (dependent) and a univariate logistic regression was fitted with age, sex and the region sampled as an independent variable. To identify the risk factors of tick burden on the livestock, a negative binomial generalized linear mixed model (GLMM) was used. Statistical significance was set at p < 0.05.
Results
Demographic characteristics of cattle sampled
A total of 388 cattle were examined from the study sites (10 farms, 3 abattoirs and 2 cattle markets). Out of the total number of cattle examined, approximately 50% were females while 65.2% were greater than 3 years old (Table 1, S1Table). The total number of ticks obtained from the cattle were 2187 with a majority (35.1%) obtained from the Ashanti region of Ghana. Approximately 54% of the ticks were obtained from the udder/scrotum of the cattle with the average number of ticks collected being 3.0 (SE = 0.27) (Table 2, S2 Table).
Tick species composition and area where they were collected
Of the total number of ticks collected, 42.9% were A. variegatum and 26.3% were H. rufipes. It was observed that more male cattle were infested with A. variegatum than females (49.7% compared to 34.2%; OR 1.9, 95% CI 1.3–2.9, p = 0.002) (Table 3). Amblyomma variegatum infestation did not significantly differ among the younger and older cattle (OR 1.0, 95% CI 0.7–1.6, p = 0.977). Also, high proportions of cattle examined were found to have A. variegatum attached to the udder/scrotum (Fig. 2). Cattle from the Deciduous Forest had a higher likelihood of A. variegatum infestation as compared to those from the other ecological zones (OR 3.8, 95% CI 2.3–6.5, p < 0.001). The male cattle were less likely to have H. rufipes infestation as compared to the females (OR 0.5, 95% CI 04–0.8, p = 0.007). Regarding the preferred site of attachment, anal regions were more likely to be infested with H. rufipes as compared to the other body parts. Cattle in the Deciduous Forest ecological zone were also more likely to be infested with H. rufipes than those in the other ecological zones (OR 0.3, 95% CI 0.2–0.6, p = 0.001). Cattle from the Transition zone were more likely to be infested with H. truncatum (OR 0.2, 95% CI 0.0–0.9, p = 0.032) compared to cattle from the Coastal savannah which are more likely to be infested with Rhipicephalus microplus (OR 2.3, 95% CI 1.1–5.0, p = 0.031) (S3Table; Table 4).
Risk factors for tick infestation
In the GLMM, tick abundance was significantly higher in cattle > 3 years old compared to ≤ 3 years old (p < 0.001). Tick burden in male cattle was not significantly higher than the females (GLMM, p = 0.0744). Ticks were identified on the udder/scrotum of 43% of the cattle. The tick burden in the udder/scrotum was higher than that from the anal, chest, head/neck, abdomen and leg/thigh of the cattle (GLMM, p < 0.05). However, tick burden at the anal region was significantly higher than leg/thigh, head/neck, abdomen and chest. Tick burden from the abdomen was also significantly higher than the leg/thigh and head/neck (Table 5).
The tick sequences generated in this study have been submitted to GenBank: R. microplus (acc. nrs. OR960937–OR960941), R. turanicus (OR960942), R. annulatus (acc. nrs. OR960943–OR960945), R. decoloratus (acc. nrs. OR960946 and OR960947), R. geigyi (acc. nrs. OR960948- OR960950), R. evertsi evertsi (acc. nrs. OR960951 and OR960952), H. dromedarii (acc. nrs. OR960953–OR960955), A. variegatum (acc. nrs. OR960956 and OR960957), H. marginatum (OR960958) and H. rufipes (OR960959).
Discussion
Effect of cattle characteristics on tick burden
Although nearly the same number of male and female animals were examined for tick infestation, the tick burden in male cattle was significantly higher than in females. This finding could be explained by reports that males are more often used for farming activities and hence stay longer in the field and move over longer distances to graze exposing them over a greater period to ticks ( Opara and Ezeh 2011; Musa et al. 2014).
Tick burden was significantly higher in cattle older than 3 years. Low tick burden in younger livestock has been observed in other studies (Rehman et al. 2017), and may be due to the small surface area of younger animals, the more frequent grooming (Mooring et al. 2000), or the suggested innate and cell-mediated immunity that confers protection from ticks (Okello-Onen et al. 1999). This impact of innate immunity warrants further study and may have implications for future control strategies.
Tick species
Similar to previous studies in Ghana, A. variegatum was the predominant species found infesting cattle in this study (Bell-Sakyi et al. 1996; Walker and Koney 1999). Amblyomma variegatum impairs livestock growth (Stachurski et al. 1993) and has been reported as the main vector of Ehrlichia ruminantium which causes heartwater disease in animals ( Stachurski 2000; Esemu et al. 2013). It also transmits Rickettsia africae which causes African tick bite fever in humans (Kelly et al. 1996; Tomassone et al. 2018). Hyalomma ticks were also identified from the study sites. These ticks transmit pathogens that negatively affect cattle production (Jongejan and Uilenberg 2004). It is important to note that all the Hyalomma species identified in this study have been implicated as reservoirs or potential reservoirs of CCHFV (Bakheit et al. 2012; Gargili et al. 2017), suggesting a risk to cattle owners and abattoir workers. This study reports the first molecular identification of Hyalomma dromedarii in Ghana. Hyalomma dromedarii is primarily a camel tick but has been reported to infest domestic animals such as cattle, goats and sheep at a reduced prevalence rate (Abdullah et al. 2018). Hyalomma dromedarii is reported to transmit Theileria annulata, the causative agent of cattle theileriosis (Mamman et al. 2021; Omer et al. 2021).
In this study, Rhipicephalus evertsi evertsi was collected from cattle which can be compared to a previous study in Ghana (Addo et al. 2023b). Furthermore, this study again reports the first molecular identification of Rhipicephalus turanicus. Rhipicephalus turanicus is closely related to Rhipicephalus sanguineus and can be found in the Palaearctic and Afrotropical regions, infesting domestic animals such as dogs, cattle and sheep (Walker et al. 2003). It has been reported in Nigeria (Lorusso et al. 2013) and Angola (Sili et al. 2021). Rhipicephalus (Boophilus) microplus were also identified in this study. This invasive species has been previously reported in countries such as Burkina Faso, Cameroon, Ghana, Mali, Nigeria and Togo (Adakal et al. 2013; Madder et al. 2012; Biguezoton et al. 2016; Muhanguzi et al. 2020; Addo et al. 2023b). It exhibits high resistance to acaricides and aids in the transmission of Babesia species which affects cattle production (Jonsson 2006; Rodrigues and Leite 2013) and zoonotic pathogens R. africae (Ehounoud et al. 2016; Mediannikov et al. 2012; Reye et al. 2012) and Coxiella burnetii (Diarra et al. 2017). Apart from its resistance to the majority of acaricides, R. microplus has a higher rate of reproduction and a shorter generation duration (Baffi et al. 2008; Madder et al. 2011). This allows it to quickly populate an area and displace other closely related Boophilus species (Adakal et al. 2013; Tønnesen et al. 2004). Rhipicephalus decoloratus, R. annulatus and R. geigyi were other Boophilus species identified in this study. These species have been reported in Ghana (Addo et al. 2023b; Ntiamoa-Baidu et al. 2004), Cameroon (Awa et al. 2015; Silatsa et al. 2019) and Nigeria (Lorusso et al. 2013). There is an increased risk of diverse tick infestation in Ghana due to the transboundary movement of herders and their livestock. This calls for regular surveillance efforts to provide useful information needed to formulate tick control measures.
It was observed in this study that tick species were significantly associated with cattle across the ecological zones. For instance, H. rufipes and H. truncatum were more likely to infest cattle from the Deciduous Forest and Transition zone, respectively. The same was seen for A. variegatum and R. microplus which were more likely to infest cattle in the Deciduous Forest and Coastal savannah ecological zones, respectively. This finding is consistent with studies that suggest that tick abundance is influenced by habitats and ecological zones (Okello-Onen et al. 1999). The results of this study can also be compared to a recent report in Ghana that indicated tick species distribution varies across three ecological zones (Coastal, Guinea and Sudan Savanna zones) (Nimo-Paintsil et al. 2022). For control measures to be effective, they have to take into account the unique tick species distribution in each ecological zone. This information will aid in the choice of a suitable acaricide to control the tick populations and reduce infestation in cattle.
Tick infestation and preferred attachment site on cattle
Amblyomma variegatum was found mostly attached to the udder/scrotum of the cattle which is similar to studies in Burkina Faso and Cameroon (Stachurski 2000, 2006). Furthermore, A. variegatum often attaches to the hairless regions of a host where it is safe to feed (Huruma et al. 2015). The second most predominant species was H. rufipes which had a high preference for the anal region of the cattle sampled. This preference for the anal region could be due to the moist nature of the anal region which could prevent desiccation during feeding. The tail of the animal, which often covers the anal region, prevents direct sunlight from reaching the ticks during the feeding process and further conceals them from the sight of animal handlers who sometimes remove them. Thus, acquiring a blood meal from the anal region would prove safer and conducive for H. rufipes. It was further observed that H. rufipes infestation was significantly different among the sexes of the cattle examined.
The seasonal distribution of tick species was not recorded in this study due to the different collection periods across the study areas. Furthermore, the breed of cattle was not recorded to give a clear indication of which breeds are more susceptible to tick infestation. Future investigations should include seasonal tick collections as well as specific cattle breeds to provide more information for creating effective control measures.
Conclusion
In this study, cattle sampled within the different ecological regions of Ghana were infested with ticks of medical and veterinary importance. Host animal factors including age and sex significantly influenced the tick burden. The predominant tick species A. variegatum and H. rufipes were found mostly attached to the udder/scrotum and anal region of cattle, respectively. These tick species are known to cause significant damage to cattle production suggesting the need for the continuous monitoring of tick populations as well as tick-borne diseases within the country. Finally, risk factor identification can help formulate effective control measures to protect both animals as well as humans. Effective chemical control strategies should take into consideration the resting and feeding preferences of ticks on cattle.
Data availability
The article and its additional files contain supporting information for the conclusions drawn in this work. Upon justifiable request, the raw datasets utilized and examined in this study can be made accessible.
References
Abdullah HHAM, El-Shanawany EE, Abdel-Shafy S, Abou-Zeina HAA, Abdel-Rahman EH (2018) Molecular and immunological characterization of Hyalomma dromedarii and Hyalomma excavatum (Acari: Ixodidae) vectors of Q fever in camels. Vet World 11(8):1109–1119. https://doi.org/10.14202/vetworld.2018.1109-1119
Adakal H, Biguezoton A, Zoungrana S, Courtin F, de Clercq EM, Madder M (2013) Alarming spread of the Asian cattle tick Rhipicephalus microplus in West Africa-another three countries are affected: Burkina Faso, Mali and Togo. Exp Appl Acarol 61(3):383–386. https://doi.org/10.1007/S10493-013-9706-6
Addo SO, Bentil RE, Baako BOA, Yartey KN, Behene E, Asiamah B, Nyarko AA, Asoala V, Sallam M, Mate S, Dunford JC, Larbi JA, Baidoo PK, Wilson MD, Diclaro JW II, Dadzie SK (2023a) Occurrence of Rickettsia spp. and Coxiella burnetii in ixodid ticks in Kassena-Nankana. Ghana Exp Appl Acarol. https://doi.org/10.1007/S10493-023-00808-0
Addo SO, Bentil ER, Baako BAO, Addae CA, Larbi JA, Baidoo PK, Wilson MD, Asoala V, Oduro D, Mate S, Diclaro JW II, Dadzie SK (2023b) First record of Rhipicephalus (Boophilus) microplus in Ghana, a potential risk to livestock production. Exp Appl Acarol. https://doi.org/10.1007/s10493-023-00793-4
Akuffo R, Brandful JAM, Zayed A, Adjei A, Watany N, Fahmy NT, Hughes R, Doman B, Voegborlo SV, Aziati D, Pratt D, Awuni JA, Adams N, Dueger E (2016) Crimean-Congo hemorrhagic fever virus in livestock ticks and animal handler seroprevalence at an abattoir in Ghana. BMC Infect Dis 16(1):1–5. https://doi.org/10.1186/s12879-016-1660-6
Awa DN, Adakal H, Luogbou NDD, Wachong KH, Leinyuy I, Achukwi MD (2015) Cattle ticks in Cameroon: Is Rhipicephalus (Boophilus) microplus absent in Cameroon and the Central African region? Ticks and Tick-Borne Diseases 6(2):117–122. https://doi.org/10.1016/j.ttbdis.2014.10.005
Baffi MA, de Souza GRL, de Sousa CS, Ceron CR, Bonetti AM (2008) Esterase enzymes involved in pyrethroid and organophosphate resistance in a Brazilian population of Riphicephallus (Boophilus) microplus (Acari, Ixodidae). Mol Biochem Parasitol 160(1):70–73. https://doi.org/10.1016/j.molbiopara.2008.03.009
Bakheit MA, Latif AA, Vatansever Z, Seitzer U, Ahmed J (2012) The huge risks due to hyalomma ticks. Arthropods as vectors of emerging diseases. Springer, Berlin, Heidelberg, pp 167–194
Balinandi S, Chitimia-Dobler L, Grandi G, Nakayiki T, Kabasa W, Bbira J, Lutwama JJ, Bakkes DK, Malmberg M, Mugisha L (2020) Morphological and molecular identification of ixodid tick species (Acari: Ixodidae) infesting cattle in Uganda. Parasitol Res 119(8):2411–2420. https://doi.org/10.1007/s00436-020-06742-z
Barrett RD, Hebert PD (2005) Identifying spiders through DNA barcodes. Can J Zool 83(3):481–491. https://doi.org/10.1139/z05-024
Bell-Sakyi L, Koney EB, Dogbey O, Sumption KJ (1996) Heartwater in Ghana: implications for control of ticks. Trop Anim Health Prod 28(2 Suppl):59–64. https://doi.org/10.1007/bf02310701
Biguezoton A, Adehan S, Adakal H, Zoungrana S, Farougou S, Chevillon C (2016) Community structure, seasonal variations and interactions between native and invasive cattle tick species in Benin and Burkina Faso. Parasit Vectors 9(1):1–16. https://doi.org/10.1186/s13071-016-1305-z
Chand A (2020) Livestock trade maps in West Africa. Nat Food 1(6):326–326. https://doi.org/10.1038/s43016-020-0105-y
Cleaveland S, Laurenson MK, Taylor LH (2001) Diseases of humans and their domestic mammals: pathogen characteristics, host range and the risk of emergence. Phil Trans R Soc B 356(1411):991–999. https://doi.org/10.1098/rstb.2001.0889
Crowder CD, Matthews HE, Schutzer S, Rounds MA, Luft BJ, Nolte O, Campbell SR, Phillipson CA, Li F, Sampath R, Ecker DJ, Eshoo MW (2010) Genotypic variation and mixtures of lyme borrelia in ixodes ticks from North America and Europe. PLoS ONE 5(5):e10650. https://doi.org/10.1371/journal.pone.0010650
Cumming GS (1999) Host distributions do not limit the species ranges of most African ticks (Acari: Ixodida). Bull Entomol Res 89(4):303–327. https://doi.org/10.1017/s0007485399000450
Cumming GS (2002) Comparing climate and vegetation as limiting factors for species ranges of African ticks. Ecology 83(1):255–268. https://doi.org/10.1890/0012-9658(2002)083[0255:CCAVAL]2.0.CO;2
Dantas-Torres F (2008) The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol 152(3–4):173–185. https://doi.org/10.1016/j.vetpar.2007.12.030
Diarra AZ, Almeras L, Laroche M, Berenger JM, Koné AK, Bocoum Z, Dabo A, Doumbo O, Raoult D, Parola P (2017) Molecular and MALDI-TOF identification of ticks and tick-associated bacteria in Mali. PLoS Negl Trop Dis 11(7):1–25. https://doi.org/10.1371/journal.pntd.0005762
Ehounoud CB, Yao KP, Dahmani M, Achi YL, Amanzougaghene N, Kacou N’Douba A, N’Guessan JD, Raoult D, Fenollar F, Mediannikov O (2016) Multiple Pathogens Including Potential New Species in Tick Vectors in Côte d’Ivoire. PLoS Negl Trop Dis 10(1):1–18. https://doi.org/10.1371/journal.pntd.0004367
Esemu SN, Besong WO, Ndip RN, Ndip LM (2013) Prevalence of Ehrlichia ruminantium in adult Amblyomma variegatum collected from cattle in Cameroon. Exp Appl Acarol 59(3):377–387. https://doi.org/10.1007/s10493-012-9599-9
Estrada-Peña A, De La Fuente J (2014) The ecology of ticks and epidemiology of tick-borne viral diseases. Antiviral Res 108(1):104–128. https://doi.org/10.1016/j.antiviral.2014.05.016
Gargili A, Estrada-Peña A, Spengler JR, Lukashev A, Nuttall PA, Bente DA (2017) The role of ticks in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus: a review of published field and laboratory studies. Antiviral Res 144:93–119. https://doi.org/10.1016/j.antiviral.2017.05.010
Hurtado OJB, Giraldo-Ríos C (2018) Economic and health impact of the ticks in production animals. In: Abubakar M, Perera PK (eds) Ticks and tick-borne pathogens. IntechOpen
Huruma G, Abdurhaman M, Gebre S, Deresa B (2015) Identification of bovine tick species and their prevalence in and around Sebeta Town, Ethiopia. J Parasitol Vector Biol 7:1–8. https://doi.org/10.5897/JPVB2014.0172
Jongejan F, Uilenberg G (2004) The global importance of ticks. Parasitology 129:S3-14
Jonsson NN (2006) The productivity effects of cattle tick (Boophilus microplus) infestation on cattle, with particular reference to Bos indicus cattle and their crosses. Vet Parasitol 137(1–2):1–10. https://doi.org/10.1016/j.vetpar.2006.01.010
Kelly PJ, Beati L, Mason PR, Matthewman LA, Roux V, Raoult D (1996) Rickettsia africae sp. nov., the etiological agent of African tick bite fever. Int J System Bacteriol 46(2):611–614. https://doi.org/10.1099/00207713-46-2-611
Kobayashi D, Ohashi M, Osei JHN, Agbosu E, Opoku M, Agbekudzi A, Joannides J, Fujita R, Sasaki T, Bonney JHK, Dadzie S, Isawa H, Sawabe K, Ohta N (2017) Detection of a novel putative phlebovirus and first isolation of Dugbe virus from ticks in Accra, Ghana. Ticks Tick-Borne Dis 8(4):640–645. https://doi.org/10.1016/j.ttbdis.2017.04.010
Lorusso V, Picozzi K, De Bronsvoort BMC, Majekodunmi A, Dongkum C, Balak G, Igweh A, Welburn SC, De Bronsvoort BMC, Majekodunmi A, Dongkum C (2013) Ixodid ticks of traditionally managed cattle in central Nigeria: Where Rhipicephalus (Boophilus) microplus does not dare (yet?). Parasit Vectors 6(1):1–10. https://doi.org/10.1186/1756-3305-6-171
Madder M, Thys E, Achi L, Touré A, De Deken R (2011) Rhipicephalus (Boophilus) microplus: a most successful invasive tick species in West Africa. Exp Appl Acarol 53(2):139–145. https://doi.org/10.1007/s10493-010-9390-8
Madder M, Adehan S, De Deken R, Adehan R, Lokossou R (2012) New foci of Rhipicephalus microplus in West Africa. Exp Appl Acarol 56(4):385–390. https://doi.org/10.1007/S10493-012-9522-4
Mamman AH, Lorusso V, Adam BM, Dogo GA, Bown KJ, Birtles RJ (2021) First report of Theileria annulata in Nigeria: findings from cattle ticks in Zamfara and Sokoto States. Parasit Vectors 14(1):1–9. https://doi.org/10.1186/s13071-021-04731-4
Mediannikov O, Diatta G, Zolia Y, Balde MC, Kohar H, Trape JF, Raoult D (2012) Tick-borne rickettsiae in Guinea and Liberia. Ticks Tick-Borne Dis 3(1):43–48. https://doi.org/10.1016/j.ttbdis.2011.08.002
Mooring M, Benjamin J, Harte C, Herzog N (2000) Testing the interspecific body size principle in ungulates: the smaller they come, the harder they groom. Anim Behav 60(1):35–45. https://doi.org/10.1006/ANBE.2000.1461
Muhanguzi D, Byaruhanga J, Amanyire W, Ndekezi C, Ochwo S, Nkamwesiga J, Mwiine FN, Tweyongyere R, Fourie J, Madder M, Schetters T, Horak I, Juleff N, Jongejan F (2020) Invasive cattle ticks in East Africa: Morphological and molecular confirmation of the presence of Rhipicephalus microplus in south-eastern Uganda. Parasit Vectors 13(1):1–9. https://doi.org/10.1186/s13071-020-04043-z
Musa HI, Jajere SM, Adamu NB, Atsanda NN, Lawal R, Adamu SG, Lawal EK (2014) Prevalence of tick infestation in different breeds of cattle in Maiduguri, Northeastern Nigeria. Bangladesh Journal of Veterinary Medicine 12(2):161–166
Nimo-Paintsil SC, Mosore M, Addo SO, Lura T, Tagoe J, Ladzekpo D, Addae C, Bentil RE, Behene E, Dafeamekpor C, Asoala V, Fox A, Watters CM, Koehler JW, Schoepp RJ, Arimoto H, Dadzie S, Letizia A, Ii JWD (2022) Ticks and prevalence of tick-borne pathogens from domestic animals in Ghana. Parasit Vectors. https://doi.org/10.1186/s13071-022-05208-8
Ntiamoa-Baidu Y, Carr-Saunders C, Matthews BE, Preston PM, Walker AR (2004) An updated list of the ticks of Ghana and an assessment of the distribution of the ticks of Ghanaian wild mammals in different vegetation zones. Bull Entomol Res 94(3):245–260. https://doi.org/10.1079/ber2004302
Okello-Onen J, Tukahirwa EM, Perry BD, Rowlands GJ, Nagda SM, Musisi G, Bode E, Heinonen R, Mwayi W, Opuda-Asibo J (1999) Population dynamics of ticks on indigenous cattle in a pastoral dry to semi-arid rangeland zone of Uganda. Exp Appl Acarol 23(1):79–88. https://doi.org/10.1023/A:1006058317111
Omer SA, Alsuwaid DF, Mohammed OB (2021) Molecular characterization of ticks and tick-borne piroplasms from cattle and camel in Hofuf, eastern Saudi Arabia. Saudi J Biol Sci 28(3):2023–2028. https://doi.org/10.1016/j.sjbs.2021.01.005
Opara NM, Ezeh ON (2011) Ixodid ticks of cattle in Borno and Yours truly, Obe states of northeastern Nigeria: Breed and coat colour preference. Animal Research International 8(1):1359–1365
Pegram RG, Oosterwijk GPM (1990) The effect of Amblyomma variegatum on liveweight gain of cattle in Zambia. Med Vet Entomol 4(3):327–330. https://doi.org/10.1111/j.1365-2915.1990.tb00448.x
Rajput ZI, Hu S, Chen W, Arijo AG, Xiao C (2006) Importance of ticks and their chemical and immunological control in livestock. J Zhejiang Univ Sci B 7(11):912–921. https://doi.org/10.1631/jzus.2006.B0912
Rehman A, Nijhof AM, Sauter-Louis C, Schauer B, Staubach C, Conraths FJ (2017) Distribution of ticks infesting ruminants and risk factors associated with high tick prevalence in livestock farms in the semi-arid and arid agro-ecological zones of Pakistan. Parasit Vectors 10(1):1–15. https://doi.org/10.1186/s13071-017-2138-0
Reye AL, Arinola OG, Hübschen JM, Muller CP (2012) Pathogen prevalence in ticks collected from the vegetation and livestock in Nigeria. Appl Environ Microbiol 78(8):2562–2568. https://doi.org/10.1128/AEM.06686-11
Rodrigues DS, Leite RC (2013) Economic impact of Rhipicephalus (Boophilus) microplus: estimate of decreased milk production on a dairy farm. Arquivo Brasileiro De Medicina Veterinaria e Zootecnia 65(5):1570–1572. https://doi.org/10.1590/S0102-09352013000500039
Sarani M, Telmadarraiy Z, Moghaddam AS, Azam K, Sedaghat MM (2014) Distribution of ticks (Acari: Ixodidae) infesting domestic ruminants in mountainous areas of Golestan province, Iran. Asian Pac J Trop Biomed 4(Suppl 1):S246–S251. https://doi.org/10.12980/APJTB.4.2014C746
Silatsa BA, Simo G, Githaka N, Mwaura S, Kamga RM, Oumarou F, Keambou C, Bishop RP, Djikeng A, Kuiate JR, Njiokou F, Pelle R (2019) A comprehensive survey of the prevalence and spatial distribution of ticks infesting cattle in different agro-ecological zones of Cameroon. Parasit Vectors 12(1):1–14. https://doi.org/10.1186/s13071-019-3738-7
Sili G, Byaruhanga C, Horak I, Steyn H, Chaisi M, Oosthuizen MC, Neves L (2021) Ticks and tick-borne pathogens infecting livestock and dogs in Tchicala-Tcholoanga, Huambo Province, Angola. Parasitol Res 120(3):1097–1102. https://doi.org/10.1007/S00436-020-07009-3/METRICS
Stachurski F (2000) Invasion of West African cattle by the tick Amblyomma variegatum. Med Vet Entomol 14(4):391–399. https://doi.org/10.1046/j.1365-2915.2000.00246.x
Stachurski F (2006) Attachment kinetics of the adult tick Amblyomma variegatum to cattle. Med Vet Entomol 20(3):317–324. https://doi.org/10.1111/j.1365-2915.2006.00633.x
Stachurski F, Musonge EN, Achu-kwi MD, Saliki JT (1993) Impact of natural infestation of Amblyomma variegatum on the liveweight gain of male Gudali cattle in Adamawa (Cameroon). Vet Parasitol 49(2–4):299–311. https://doi.org/10.1016/0304-4017(93)90128-A
Tomassone L, Portillo A, Nováková M, De Sousa R, Oteo JA (2018) Neglected aspects of tick-borne rickettsioses. Parasit Vectors 11(1):1–11. https://doi.org/10.1186/s13071-018-2856-y
Tønnesen MH, Penzhorn BL, Bryson NR, Stoltsz WH, Masibigiri T (2004) Displacement of Boophilus decoloratus by Boophilus microplus in the Soutpansberg region, Limpopo Province. SA Exp Appl Acarol 32(3):199–208. https://doi.org/10.1023/B:APPA.0000021789.44411.b5
Vesco U, Knap N, Labruna MB, Avšič-Županc T, Estrada-Peña A, Guglielmone AA, Bechara GH, Gueye A, Lakos A, Grindatto A, Conte V, De Meneghi D (2011) An integrated database on ticks and tick-borne zoonoses in the tropics and subtropics with special reference to developing and emerging countries. Exp Appl Acarol 54(1):65–83. https://doi.org/10.1007/S10493-010-9414-4
Walker A, Bouattour A, Camicas J, Estrada-Peña A, Horak I, Latif A, Pegram R, Preston P (2003) Ticks of domestic animals in Africa: a guide to identification of species. Bioscience reports University of Edinburgh
Walker AR, Koney EBM (1999) Distribution of ticks (Acari: Ixodida) infesting domestic ruminants in Ghana. Bull Entomol Res 89(5):473–479. https://doi.org/10.1017/s0007485399000619
Zannou OM, Ouedraogo AS, Biguezoton AS, Yao KP, Abatih E, Farougou S, Lenaert M, Lempereur L, Saegerman C (2021) First tick and tick damage perception survey among sedentary and transhumant pastoralists in Burkina Faso and Benin. Vet Med Sci 7(4):1216–1229. https://doi.org/10.1002/vms3.414
Acknowledgements
The authors appreciate the support of Major Ampadu of the Veterinary Department of Ghana Armed Forces and his team and the Public Health Team at the 37 Military Hospital. We are grateful to Patrick Obuam and Yaw Akuamoah-Boateng of the School of Public Health (KNUST) as well as Dominic Kambonga and Edmond Tampugre of the Navrongo Health Research Centre (NHRC) for their contribution towards this study. Many thanks to Madam Rebecca Awe (Veterinarian in Navrongo) and Mr. Joseph Otchere (Phlebotomist) for their assistance in sample collections.
Funding
This work was supported by the Armed Forces Health Surveillance Division, Global Emerging Infections Surveillance (GEIS) Branch; PROMIS number P0129_20_RD_01.
Author information
Authors and Affiliations
Contributions
SN, JWD and SKD conceived and designed the study; SOA, REB, MM, JA, JT, CY, BOAB, DA, SAK, VA and DLM realized the fieldwork; SOA and REB performed morphological identification; EB did the data analysis; SOA and EB wrote the first draft of the manuscript; SN, KP, EO, EON, ATF, AGL, JWD, JFH and SKD revised the manuscript; SN and SKD supervised the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This study was evaluated by the Naval Medical Research Command (NMRC) veterinarian and deemed to be a “field study” as defined in 1) DoD Instruction 3216.01 “Use of Animals in DOD Programs,” and 2) SECNAVINST 3900.38C “The Care and Use of Laboratory Animals in DOD Programs.” Therefore, it is not regulated by these references and the project did not require further NMRC Veterinary review.
Authors’ disclaimer statement
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the U.S. Navy.
Copyright assignment statement
CAPT Letizia, LCDR Diclaro II, LCDR Harwood and Dr. Nimo-Paintsil are military service members or employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. §105 provides that ‘Copyright protection under this title is not available for any work of the United States Government.’ Title 17 U.S.C. §101 defines a U.S. Government work as a work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Addo, S.O., Bentil, R.E., Mosore, Mt. et al. Risk factors affecting the feeding site predilection of ticks on cattle in Ghana. Exp Appl Acarol 92, 835–850 (2024). https://doi.org/10.1007/s10493-024-00906-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-024-00906-7