Abstract
Hyalomma dromedarii is the predominant tick species parasitizing camels in Egypt which leads to mortalities in young animals that result in economic losses. It can transmit a lot of pathogens to animals and humans, such as the Crimean-Congo hemorrhagic fever virus, the Dhori virus, Kadam virus, Theileria annulata and spotted fever rickettsia. The continuous use of chemical acaricides has negative impact on the environment and almost led to acaricidal resistance, and hence the plant extracts represent alternative methods for controlling ticks. The present study was carried out to assess the histopathological effects on the ovary of fed female Hyalomma dromedarii following immersion in the ethanolic extract of fruits of Citrullus colocynthis (100 mg/mL). Light, scanning and transmission electron microscopy observations provided evidence that Citrullus colocynthis caused extensive damage to oocytes. Destruction of the internal organelles of oocytes, along with delay and/or inhibition of vitellogenesis were demonstrated. This is the first histological study that points to damage in H. dromedarii ovaries following treatment with the ethanolic extract of fruits of C. colocynthis. The data presented suggest that the plant extract affects the ovary either directly by entering the oocytes and/or indirectly by damaging the gut cells and digestion of blood that interfere with the development of oocytes, so it can be used as a promising agent for tick control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ticks are blood-sucking ectoparasites with worldwide distribution, infesting animals and transmitting severe infectious diseases to animals and humans (Abdel Gawad et al. 2023). Ticks and tick-borne diseases (TBDs) are widespread in Egyptian localities (Abdelbaset et al. 2022). Hyalomma dromedarii (Ixodoidea: Ixodidae) is the predominant tick species parasitizing camels in Egypt (Abdel-Ghany et al. 2023). It causes mild to severe anemia; annoying bites, anorexia, skin spoilage, intense pruritus, loss of appetite, reduction in growth rate, decreased productivity, and mortalities in young animals that result in economic losses (Guglielmone et al. 2014).
Hyalomma dromedarii can transmit many diseases such as Q fever (Coxiella burnetii) (Bazlikova et al. 1984), theileriosis (Theileria camelensis and T. annulata) (Hoogstraal et al. 1981; Mohamed et al. 2021), Crimean-Congo hemorrhagic fever (Bendary et al. 2022), spotted fever (Rickettsia rickettsii) (Al-Deeb et al. 2015), and African tick-borne fever (R. africae) (Abdel-Shafy et al. 2012; Abdullah et al. 2019). So, its control is very important for the prevention of tick-borne diseases.
The usage of chemical acaricides resulted in contamination of the environment, residues in milk and meat products and the development of acaricide-resistant ticks (Iqbal et al. 2021; Mohammed et al. 2023). Alternative control strategies to overcome the drawbacks associated with acaricides are an urgent need (Mohamed et al. 2022; Eltaly et al. 2023). The use of herbal medications is one of the most promising alternative approaches for the treatment of various infectious agents because of their biodegradability, target efficiency and cost effectiveness (Nugraha et al. 2020; Zheoat et al. 2021; Abdel-Meguid et al. 2022).
The fruits of C. colocynthis contain a number of bioactive constituents, including glycosides, flavonoids and alkaloids (Hussain et al. 2022) with anti-inflammatory, anti-diabetic, anti-microbial, anti-helminthic, antibacterial, anti-carcinogenic, anti-ulcerogenic, hypolipidemic, hypoglycemic, and anti-oxidant properties (Ullah et al. 2013; Bhasin et al. 2020). The different extracts of it showed insecticidal activity against different insect species such as Culex quinquefasciatus and Aedes aegypti (Rahuman et al. 2008; Sakthivadivel and Daniel 2008), Aphis craccivora (Dimetry et al. 2015), Anopheles arabiensis (Hamid et al. 2016) and Brevicoryne brassicae (Ahmed et al. 2020). Its acaricidal efficacy was studied at biological levels only against mites such as Tetranychus urticae (Dahroug et al. 2000) and Rhizoglyph ustritici (Bashir et al. 2013), and against different stages of ticks; H. analoticum (Godara et al. 2015), Rhipicephalus microplus (Ullah et al. 2015), Rhipicephalus sp. (Hussian and Jasim 2018) and H. dromedarii (Mahran et al. 2020; Mohamed et al. 2022).
Many studies were carried out to detect the histopathological effects of plant extracts on the female reproductive system of hard ticks such as Amblyomma cajennense (Anholeto et al. 2018), R. sanguieus (Souza et al. 2019)d microplus (Reis et al. 2021). Although a few authors tested plant extracts against different developmental stages of H. dromedarii (Abdel-Shafy et al. 2007; Habeeb et al. 2007; Mahran et al. 2020; Mohamed et al. 2022; Abdel-Ghany et al. 2021a, b, 2023; Eltaly et al. 2023), none of them studied the histology of internal organs. Therefore, this work is aimed at studying the histopathological effect of an ethanolic ectract of the fruits of C. colothynthis on the ovary of the tick, H. dromedarii.
Materials and methods
Tick collection
Hyalomma dromedarii ticks were collected from the naturally infested camels at Birqash camel market (30°9′58.4″N, 31°2′13.2″E), Giza Governorate, Egypt. They were identified according to Hoogstraal and Kaiser (1958), and grouped into non-engorged, semi-engorged and engorged males and females according to Stark et al. (2018). The engorged females were kept in glass vials, covered by pieces of gauze securely held by rubber bands, in the incubator at 28 ± 2 °C and 75–80% relative humidity until the experiments were conducted (within 24 h of collection).
Preparation of the extract
Ripen dried fruits of Citrullus colocynthis were bought from the market, cleaned to remove dust, and ground using a stainless steel knife mill. Ethanolic extract was prepared according to the method of Twaij et al. (1988). Plant powder (50 g) was added to 80% ethyl alcohol (250 mL), covered with aluminum foil and kept in dark condition for 72 h, then it was filtrated using Whatman filter paper (110 mm diameter opening). The filtrate was poured in glass petri dishes and put in the incubator at 50 °C for alcohol evaporation. Finally, the dried extract was collected, weighed, transferred to glass vials, and kept at 4 °C until use. A concentration of 100 mg/mL was prepared by dissolving 1 g of the extract in 10 mL of distilled water.
Treatment
Adult immersion test (AIT) was performed as described by Drummond et al. (1973). Each engorged female tick was immersed in 10 mL of 100 mg/mL concentration for 5 min and transferred to sterile glass vials securely covered by gauze. Treated and untreated ticks were kept in the incubator at 28 ± 2 °C and 75–80% relative humidity.
Morphological and histological studies
Both untreated and treated ticks were dissected 4 and 7 days after engorgement and treatment, respectively. Thirty-six tick females were dissected throughout the experiments. Three replicates each with three females were kept for the untreated and treated specimens at each period.
Light microscopy
Female ticks were dissected in a Petri dish containing a mixture of paraffin wax and charcoal and covered by 0.85% NaCl solution under a dissecting binocular microscope. The dorsal integument was removed, and the tick was washed several times with saline solution for blood removal then fixed in Bouin̕̕s fixative for 24 h (Bancroft et al. 2008). Specimens were dehydrated in an ascending series of ethyl alcohol, then transferred to methyl benzoate for 24 h to soften the integument. Samples were then transferred to a solution of 2% celloidin in methyl benzoate for 24 h (Gatenby and Beams 1950), cleared in benzol, infiltrated in three changes of paraplast at 56 °C and then embedded in paraplast (Fisher Scientific Inc. USA).
Serial transverse sections were cut at 3 μm thick using a YD-335 computer microtome (Huran Kaida Scientific Instrument Comp., China), and stained with Mallory triple stain (MT) (Pantin 1946) or hematoxylin-eosin stain (HE) (Pears 1960), then photographed using a Samsung ES95 HD digital camera fixed on an Olympus microscope (Japan made).
Electron microscopy
Female ticks were dissected in cold phosphate buffer adjusted to pH 7.2, and the ovaries were removed and fixed for 2 h in 3% cold fresh glutaraldehyde. They were washed for 30 min in phosphate buffer.
Scanning electron microscopy (SEM)
After washing in phosphate buffer, ovaries were dehydrated in ascending series of ethanol. Samples were subjected to critical point drying, attached to aluminum stubs, coated with gold in a sputter-coating apparatus, and then examined and photographed under a Quanta FEG 250 scanning electron microscope (FEI Company, Hillsboro, Oregon, USA) at the Electron Microscope Unit, Desert Research Center, Cairo, Egypt.
Transmission electron microscopy (TEM)
After washing in phosphate buffer, ovaries were postfixed in cold 1% osmic acid for 2 h and washed again in fresh buffer. They were then dehydrated in ascending series of ethanol and embedded in an epoxy resin (Gatenby and Beams 1950).
Semithin Sections. (500 to 1000 nm) from blocks were prepared using Leica Ultra-cut (UCT ultra-microtome) and stained with toluidine blue stain (TB) (Dawes 1971) for light microscope examination before ultrasectioning. With a sharp diamond knife and the same ultratome, ultrathin Sections. (75–90 nm) were cut, mounted on copper grids (grid size 300 mesh × 83 μm pitch) and stained with uranyl acetate and lead citrate (Reynold, 1963). Oocytes were examined by transmission electron microscope JEOL (JEM-1400 TEM) at the Electron Microscope Unit, Faculty of Agriculture, Cairo University, Egypt.
Results
The comparison between untreated and treated ovaries changes was summarized in Table 1. The ovary of untreated H. dromedarii is a tubular structure in the form of a horseshoe located in the postero-lateral region of the body cavity extending approximately in whole body length (Fig. 1).
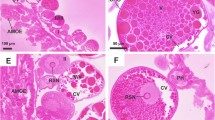
Scanning electron microscopical examination (SEM) revealed that the ovary appears as a grape-like structure (Fig. 2a and c). It contains numerous oocytes at different developmental stages, from early (I and II) to late stages (III, IV, and V), (Fig. 2b and c). Oocytes are more or less oval in shape and develop asynchronously (Fig. 2a and c).
Scanning electron micrographs of Hyalomma dromedarii ovary. a 7 days after engorgement showing its grape-like appearance containing early (EO) and late (LO) stages of oocytes. b 4 days after engorgement showing early (EO) and first stage (III) of late oocytes (LO) with tuberculated surfaces (Ts). c 7 days after engorgement showing early (EO) and late stages (III and IV) of developing oocytes with tuberculated surfaces (Ts). d 4 days after treatment showing deformities in the shape of oocytes (O). e 4 days after treatment showing oocytes (O) appeared as fused molten globules with indistinct boundaries. f 7 days after treatment showing higher magnification of damaged early (EO) and late oocytes (LO) with fused boundaries and wrinkled surfaces
Four days after engorgement, the late stages of oocytes begin to form, especially stage III, in the presence of a large number of early developing ones (Fig. 2b). The differentiation of late stages was clearly demonstrated as protruding from the ovarian surface, with a few early ones after seven days of engorgement (Fig. 2c). The outer surface of more developed oocytes (Stage V) is completely covered by a rough tuberculated surface called the eggshell (chorion) (Fig. 2c).
The ovary of the treated female H. dromedarii showed elongation with damaged and deformed oocytes (Fig. 2d). It was observed that after 4 days of treatment, the development of oocytes was markedly suppressed, and it was difficult to differentiate between early stages and stage III (Fig. 2e) when compared with the untreated ones. Oocytes appeared like fused molten globules with indistinct boundaries (Fig. 2e).
Seven days after the treatment, the ovary showed highly deformed oocytes with no late stages of oocytes (Fig. 2f). Some oocytes were fused together with no distinct boundaries between them, especially the early stages that found in large number (Fig. 2f). Scanning electron microscopical examination of late stages of oocytes revealed signs of damage represented by loss of their ovoid shape and wrinkling or eroding of their surfaces (Fig. 2f).
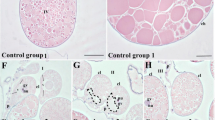
Examination of the H. dromedarii ovary under a light microscope revealed a grape-like hollow organ enclosing a lumen surrounded by a thin ovarian wall (Fig. 3a). This wall consisted of epithelial cells, oogonia and different oocyte stages protruding into hemolymph and connected to the ovarian wall by a thin stalk, the pedicle (Fig. 3a).
Light micrographs of transverse sections of Hyalomma dromedarii ovary. a Paraffin Section 4 days after engorgement showing protruding oocytes giving it the grape-like appearance, ovarian epithelium (Ep), pedicle cells (Pe) and ovarian lumen (L). I: Oocyte stage I. II: Oocyte stage II, III: Oocyte stage III, IV: Oocyte stage IV, Og: Oogonia, Ow: Ovarian wall. MT stain. b Paraffin Section 7 days after engorgement showing oocyte stage III (III) with almost rounded nucleus (N), beginning of yolk granules (YG) formation, eggshell (ES) in its early stage and the connection to pedicle cells (Pe). MT stain. c Paraffin Section 7 days after engorgement showing oocyte stage V with fully formed yolk granules (YG) and covered with eggshell (ES) (chorion). MT stain. d Paraffin Section 7 days after treatment showing marked alterations in the ovarian epithelium (Ep), oocytes (O) of various developmental stages and pedicle cells (Pe). L: Lumen, Og: Oogonia. MT stain. e Paraffin Section 4 days after treatment showing higher magnification of oocyte stages II (II) and III (III) with vacuolation of nucleus (N) and abnormal shape of nucleoulus (Nu) in addition to damage of nuclear membrane (Nm). V: Vacuole. HE stain. f Semithin Section 4 days after treatment showing deformed oocyte stage III (III) with wrinkled boundaries, coalesced cytoplasm and deformed pedicle cell (Pe) with vacuoles (V). TB stain. g Semithin Section 7 days after treatment showing oocyte stage IV (IV) with large squashed cytoplasmic areas at its periphery and extensively damaged pedicle cells (Pe) containing numerous vacuoles (V). TB stain. h Semithin Section 7 days after treatment showing oocyte stage IV (IV) with damaged yolk granules (YG), cytoplasmic vacuoles (V) and disrupted eggshell (ES). TB stain
Oogonia were very small in size, oval in shape, appeared in densely packed clusters (Fig. 3a). They were characterized by their high nucleocytoplasmic ratio (Fig. 3a). Stage I oocytes resulted from the oogonial division. They were oval to polygonal in shape and faced the ovarian lumen (Fig. 3a). Their cytoplasm showed large oval to round nuclei that occupied a wide area. Stage II oocytes were characterized by great cytoplasmic growth. It’s formation extends until the beginning of yolk granules formation then changes into the next stage. They appeared oval in shape with dense and granulated cytoplasm that was connected to the ovarian wall by pedicle cells (Fig. 3a).
Stage III oocytes were virtually visible four days after engorgement and characterized by the appearance of yolk granules in addition to the early formation of the egg shell (chorion) (Fig. 3a and b). The nucleus was not easily detected and if so, it appeared almost rounded with dispersed euchromatin and a relatively large, rounded nucleolus (Fig. 3a and b). Stage IV oocytes were oval in shape (Fig. 3a). It contained large yolk granules that fill most of the cytoplasm with increasing eggshell deposition indicated by high intensity of staining (Fig. 3a). Stage V oocytes became clearly visible seven days after engorgement, containing fully formed yolk granules that became very large and completely filled the oocyte cytoplasm (Fig. 3c). The eggshell is well developed and very thick.
Generally, marked alterations in epithelial cells, oocytes and pedicle cells were noticed after treatment. The number of oocytes decreased, and the remaining ones were damaged (Fig. 3d). Oogonia and stage I oocytes were completely damaged and appeared as misshapen and heterogeneous cell masses (Fig. 3d). Four days after treatment, stage II oocytes showed ruptured membranes with vacuolated karyolysed nuclei (Fig. 3e). On the other hand, stage III oocytes showed pycnotic nuclei with ruptured membrane (Fig. 3e). Some of these oocytes exhibited wrinkled boundaries with great deformities in their shapes (Fig. 3f). The cytoplasm coalesced, shrunk, and condensed in some areas, with vacuoles (Fig. 3f).
Seven days after treatment, oocyte IV appeared irregular in shape and highly damaged with coalesced cytoplasm (Fig. 3g). A large squashed cytoplasmic area at the periphery of the oocytes was observed (Fig. 3g). Fluid-filled spaces were detected at the attachment site of pedicle cells. Cytoplasmic vacuolation was observed (Fig. 3g and h). Yolk granules were highly deformed with chorion disruption in some areas (Fig. 3h).
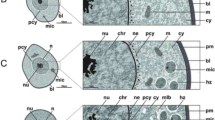
Using TEM, cytoplasm of stage I oocytes of untreated ticks appeared contained mitochondria with free ribosomes and large oval to round nuclei that occupy a wide area of the cytoplasm (Fig. 4a). The nucleoplasm density is low exhibiting mainly euchromatin with few dense masses of heterochromatin beneath the nuclear membrane (Fig. 4a). After treatment with the extract, it was difficult to distinguish this stage as it was completely damaged.
Transmission electron micrographs of stages I, II and III oocytes from Hyalomma dromedarii ovary. a. stage I (I) oocyte 4 days after engorgement showing nucleus (N), mitochondria (M), ribosomes (R) and low density nucleoplasm with euchromatin (Ec) and few dense masses of heterochromatin (Hc) beneath the nuclear membrane (Nm). b Stage II (II) oocyte 4 days after engorgement showing nucleus (N), nucleolus (Nu), rough endoplasmic reticulum (RER), ribosomes (R), mitochondria (M), lipid droplets (LD) and the connection to pedicle cells (Pe) with microvilli (Mv). c Stage II (II) oocyte 4 days after treatment showing great deformation of its shape and presence of numerous vacuoles (V) in the cytoplasm. Nu: Nucleolus, N: Nucleus. d. Stage II (II) oocyte 7 days after treatment showing cytoplasm with numerous vacuoles (V), abnormal nucleus (N) and damaged nucleolus (Nu) with vacant space (astrisk). e Stage III (III) oocyte 4 days after engorgement showing the presence of tunica propria (T), microvilli (Mv) and cytoplasm with Golgi complex (G), rough endoplasmic reticulum (RER), lipid droplets (LD), multi-vesicular bodies (MVB) and small electron dense homogeneous yolk granules (YG). f Stage III (III) oocyte 7 days after engorgement showing the presence of thin eggshell (ES) below the tunica propria (T). Mv: Microvilli. Vc: Vesicle. g Stage III (III) oocyte 4 days after treatment showing irregular nucleus (N), nucleolus (Nu) with vacuoles (V) and many yolk granules (YG). h Stage III (III) oocyte 4 days after treatment showing damaged microvilli (Mv), aggregated areas of cytoplasm at the periphery of oocyte and vacuoles (V) in the cytoplasm. T: Tunica propria, YG: Yolk granules. i Stage III (III) oocyte 7 days after treatment showing lysed mitochondria (M), irregular and crescent shape yolk granules (YG) and vacuoles (V) at the periphery of the oocyte. ES: Eggshell, RER: Rough endoplasmic reticulum
Transmission electron microscopical examination of stage II oocyte reveals that the nucleoplasm is occupied with euchromatin and a prominently large nucleolus (Fig. 4b). Their cytoplasm contained numerous rough endoplasmic reticulum, ribosomes, lipid droplets and small mitochondria (Fig. 4b). Short microvilli appeared between this oocyte stage and pedicle cells (Fig. 4b).
Four days after treatment, numerous vacuoles in the cytoplasm of stage II oocytes were observed (Fig. 4c). Their nuclei were without heterochromatin and the nucleolus was relatively large (Fig. 4c). Vacuolation increased seven days after treatment with the presence of vacant space inside nucleolus (Fig. 4d).
In the stage III oocytes, the cytoplasmic organelles were well developed (Fig. 4e). A layer of tunica propria, that separates the ovarian tissue from the haemolymph, was clearly observed (Fig. 4e and f). Yolk originated from small vesicles derived from Golgi bodies and rough endoplasmic reticulum, joined together to form larger multi-vesicular bodies (Fig. 4e). Through repeated fusions between these bodies, electron dense homogeneous yolk spheres are formed. Lipid droplets were observed in few numbers (Fig. 4e). Deposition of eggshell began slightly later to the beginning of yolk formation. Eggshell (chorion) developed from vesicles, derived from Golgi bodies, which reached cell membrane and fuse with it and/or microvilli discharging their content (exocytosis) into the extracellular space between the plasma membrane and the tunica propria (Fig. 4f). The eggshell at this stage was very thin below the tunica propria and the surface of the oocyte membrane showed few microvilli (Fig. 4e and f).
Transmission electron microscopical examination of treated ticks after 4 days displayed folded nuclear membrane and squashing of nucleoplasm and chromatin granules of stage III oocyte (Fig. 4g). Nucleolus contained numerous vacuoles (Fig. 4g). A lysis of cell organelles such as mitochondria and rough endoplasmic reticulum was noticed (Fig. 4h). The presence of cytoplasmic vacuoles and yolk granules were rarely appeared (Fig. 4h). Seven days after treatment, vacuolation increased in the cytoplasm (Fig. 4i). Yolk granules appeared irregular or crescent-like shape and decreased in number (Fig. 4i). There was marked damage in the oocytes’ microvilli (Fig. 4h) that led to abnormal formation of eggshell (Fig. 4i) after seven days of treatment.
Stage IV oocytes were markedly observed seven days after engorgement in untreated tick. Each contained large electron dense homogeneous yolk granules which fill most of the oocyte cytoplasm (Fig. 5a and b). Some Golgi bodies and mitochondria were observed between yolk granules. Lipid droplets markedly increased in size and quantity (Fig. 5a and b). Obvious increase in eggshell deposition resulted in stretching of the tunica propria that appeared with fewer fibers (Fig. 5b).
Transmission electron micrographs of stages IV and V oocytes from Hyalomma dromedarii ovary. a stage IV (IV) oocyte 7 days after engorgement showing its cytoplasm with large electron dense yolk granules (YG), Golgi complex (G), mitochondria (M) and lipid droplets (LD). Ly: Lysosomes. b Stage IV (IV) oocyte 7 days after engorgement showing the increase in eggshell (ES) deposition that resulted in stretching of tunica propria (T). LD: Lipid droplets, M: Mitochondria, Mv: Microvilli, YG: Yolk granules. c Stage IV (IV) oocyte 7 days after treatment showing deformed and lysed yolk granules (YG) and abnormal eggshell (ES). V: Vacuoles. d Stage IV (IV) oocyte 7 days after treatment showing the separation between the eggshell (ES) and tunica propria (T) and damaged microvilli (Mv). LD: Lipid droplets, V: Vacuoles, YG: Yolk granules. e Stage V oocyte 7 days after engorgement showing the fully formed yolk granules (YG) in the cytoplasm and the thick eggshell (ES). Mv: Microvilli
Transmission electron microscopical examination of these oocytes showed great damage or disappearance of cytoplasmic organelles (Fig. 5c). There was deformation of yolk granules, and some were fragmented (Fig. 5c). The extract caused distinct separation between cell membrane and tunica propria in addition to damaged microvilli (Fig. 5d) leading to abnormal or defect in eggshell formation.
Stage V oocytes became clearly visible seven days after engorgement. It is characterized by containing fully formed large yolk granules completely filled the cytoplasm (Fig. 5e). The microvilli reached their maximum length and appeared as straight tubes with stretched tunica propria and the eggshell became very thick (Fig. 5e).
Stage V oocytes could not be observed after treatment in the ovaries of females during the examined period.
Discussion
The female genital system is the most complicated system in the ixodid tick body (Balashov 1972). In the present study based on microscopical examination of untreated female Hyalomma dromedarii, there was a single, tubular, U-shaped ovary lying in the posterior half of the body. Similar to that described in other ticks (Balashov 1972, 1983), ovary appeared as a hollow organ enclosing a lumen surrounded by a thin ovarian wall that was composed of epithelial cells, oogonia, and oocytes in various developmental stages. An outer tunica propria that separates the ovarian tissue from the hemolymph was also observed. After feeding and mating, oocytes protrude into the haemocoel, giving the ovary a grape-like appearance, with oocytes attached to the ovarian wall through pedicles. The ovary then increases in size considerably exhibiting eggs (Rey 1973) until they are ready for oviposition (Oliveira et al. 2006).
Oocytes in the present study might be classified into oogonia and stages I, II, III, IV and V based on Roshdy (1969) and Balashov (1983) classifications. Similarly, oocytes were classified into five stages in Haemaphysalis longicornis (Yano et al. 1989; Mihara et al. 2018), Amblyomma cajennense (Denardi et al. 2004), Rhipicephalus microplus (Saito et al. 2005), R. sanguineus (Oliveira et al. 2005; Sanches et al. 2012a), A. triste (Oliveira et al. 2006), A. brasiliense (Sanches et al. 2010), A. rotundatum (Sanches et al. 2012b), A. varium (Sanches et al. 2014)d annulatus (Kanapadinchareveetil et al. 2015, 2019).
In the present study, scanning electron microscopical examination of the ovary of fed H. dromedarii immersed in ethanolic extract of C. colocynthis (100 mg/mL) showed stretched ovary with deformed oocytes having indistinct boundaries, and wrinkled, eroded surfaces.
Microscopical examinations of the tick ovary revealed that the oocytes of the treated females exhibited changes that affected their growth and development when the plant extract was applied after engorgement. The overall damage observed included great deformity in the ovarian wall and a decrease in the number of oocytes. According to Oliveira et al. (2005), the ovarian epithelium is involved in the oocyte’s attachment and transportation of the yolk elements from the hemolymph to the oocytes. Thus, it was suggested that the damage to the ovarian wall affected the metabolism and development of oocytes.
There was lysis of cell organelles, especially mitochondria, and the appearance of large cytoplasmic vacuoles after treatment. It was suggested that these vacuoles are autophagic and are responsible for the degradation of organelles that have been damaged by the action of plant extract. Damaged mitochondria suggested a decrease in ATP production, causing an effect on respiratory metabolism (Iturbe-Requena et al. 2020) and all cell activities (Alberts et al. 2010) interfering in the development of these cells and even preventing the continuation of vitellogenesis (Oliveira et al. 2017). The energy loss was also reported by Denardi et al. (2012) and Remedio et al. (2015) in R. sanguineus treated with neem extract and neem oil, respectively. Carvalho and Recco-Pimentel (2007) explained the presence of vacuoles as structures found mainly in cells where degradation and recycling of damaged portions and cytoplasmic organelles take place. They may be a result of a detoxification mechanism to isolate interfering substances or organelles without functions (Arnosti et al. 2011a, b) or caused by increased permeability of the oocyte membrane (Vendramini et al. 2012; Konig et al. 2019).
In the present study, the nucleus suffered from an abnormal appearance with a vacuolated nucleolus reflecting the ability of the plant extract to reach it. The nucleolus plays a pivotal role in the synthesis of proteins (Scheer and Hock 1999) that are important for oocyte development (Konig et al. 2020). The presence of a vacuolated nucleolus led to degeneration of genetic material (Remedio et al. 2015), and thus cell death (Denardi et al. 2011; Vendramini et al. 2012; Remedio et al. 2014), preventing the completion of vitellogenesis (Oliveira et al. 2019).
Fragmentation and decrease in the number of yolk granules were observed after treatment. As the vacuolated areas increase, the areas occupied by the yolk granules decrease, which affect the vitellogenesis, the formation of embryos and also impair the offspring’s survival. Iturbe-Requena et al. (2020) reported that the yolk granules store the nutrients of embryos, their damage would affect the survival of the progeny.
Damaged microvilli were clearly demonstrated in the present study between cell membrane and tunica propria. This may be due to the result of extract penetration through the egg that ruptures cell membrane and damages the microvilli. This considered a defense mechanism resulted in decrease in the surface area adhering to the haemolymph and/or decrease in the exogenous uptake of yolk precursors explaining the delay or inhibition of vitellogenesis (Marzouk et al. 2020).
A separation between tunica propria and the cell membrane was observed that led to disruption in the formation of chorion and demonstrated the capability of the plant extract to damage the first protective cell barrier. Vitellogenesis was inhibited or delayed where stage V oocytes were not formed during the examined period. Vitellogenin is a protein whose concentration increases with female engorgement, and regulation occurs through its uptake by oocytes (Seixas et al. 2010). The C. colocynthis extract interfered with hormonal regulation, leading to a decrease in the production of vitellogenic elements and reducing the release of vitellogenin through the inhibition of ecdysteroid hormones.
The chorion allows the oxygenation of the embryo, protects oocytes, and regulates the entry and exit of elements (Oliveira et al. 2016). The disruption of this membrane would expose oocytes to the hemolymph and allow toxic compounds to penetrate the oocyte (Arnosti et al. 2011b). This caused an increase in vacuoles and yolk granules lost their round shape, became irregular and began to lyse which led to major changes in the cytoplasm of oocytes (Arnosti et al. 2011b). As the chorion deposition would start in oocyte stage III and be completed in stage V, oocytes I and II are more susceptible to the intake of toxic compounds (Oliveira et al. 2019; Souza et al. 2019), which explain the damage to oocytes observed in our results. In some cases, the chorion is not able to prevent the total absorption of the product due to the damage caused to its structure, which can be confirmed by the presence of folds and rupture in the membranes of these cells. Thus, the chorion loses its original protective function, allowing the endocytosis of the extract by the oocytes and consequent harmful action in the cells (Vendramini et al. 2012).
In studies evaluating the effect of botanical acaricides on ticks, morphophysiological alterations of the oocytes during their development were observed (Nwanade et al. 2020). The fully engorged tick females reached their critical weight and then detached from the host (Kanapadinchareveetil et al. 2019). After detachment, the nervous system of the tick had no influence on the reproductive system (Weiss and Kaufman 2001). Botanical acaricides can affect the morphophysiology of reproductive organs (Konig et al. 2020). In blood-feeding arthropods, blood provides a rich source of proteins for vitellogenesis and egg production (Horn et al. 2009). During digestion of the blood, a large amount of lipids is secreted into the hemolymph and will be taken up by the growing oocytes to produce vitellogenin (Atella et al. 2005). According to Balashov (1983) and Xavier et al. (2018), vitellogenin is also synthesized in the tick gut for yolk production.
Despite the presence of a defense mechanism exhibited by oocytes to overcome the effect of extract, these cells were not able to recover this damage leading to cell death and vitellogenesis stopped. It is suggested that plant extract could affect different stages of oocytes in the tick ovary in two ways, directly by absorption through the tick integument and transport through the hemolymph until reaching the oocytes, causing their damage, or indirectly entering the gut through mouthparts, then affecting either gut cells or the process of blood digestion, leading to disturbances in vitellogenin production which prevent vitellogenesis.
Conclusion
Histological observations of fed female Hyalomma dromedarii ovaries, after the treatment with ethanolic extract of Citrullus colocynthis, provided evidence that it suffered from extensive damage of different types of oocytes. Destruction of the internal organelles of oocytes and/or inhibition of vitellogenesis were demonstrated. This is the first histological study that points to the damage in H. dromedarii ovaries following treatment with ethanolic extract of C. colocynthis. The data presented suggest that this plant extract can be used as a promising agent for tick control generally including H. dromedarii.
Code availability
None.
References
Abdel Gawad S, Baz MM, Taie HAA, Selim A, Mustafa S, Khater HF (2023) Novel acaricidal efficacy of nine Egyptian plants against the camel tick, Hyalomma dromedarii (Ixodida: Ixodidae). Persian J Acarol 12(1):121–136
Abdel-Ghany HSM, Abdel-Shafy S, Abuowarda M, El-Khateeb RM, Hoballah EM, Fahmy MM (2021aa) Acaricidal activity of Artemisia herba-alba and Melia azedarach oil nanoemulsion against Hyalomma dromedarii and their toxicity on Swiss albino mice. Exp Appl Acarol 84(241):262
Abdel-Ghany HSM, Abdel-Shafy S, Abuowarda MM, El-Khateeb RM, Hoballah E, Hammam AMM, Fahmy MM (2021b) In vitro acaricidal activity of green synthesized nickel oxide nanoparticles against the camel tick, Hyalomma dromedarii (Ixodidae), and its toxicity on Swiss albino mice. Exp Appl Acarol 83:611–633
Abdel-Ghany HSM, Allam SA, Khater HF, Selim A, Abdel-Shafy S (2023) Effects of commercial oils on the camel tick, Hyalomma dromedarii (Acari: Ixodidae) and their enzyme activity. Persian J Acarol 12(1):137–149
Abdel-Shafy S, Soliman MMM, Habeeb SM (2007) In vitro acaricidal effect of some crude extracts and essential oils of wild plants against certain tick species. Acarologia 47(1–2):33–42
Abdel-Shafy S, Allam NA, Mediannikov O, Parola P, Raoult D (2012) Molecular detection of spotted fever group rickettsiae associated with ixodid ticks in Egypt. Vector Borne Zoonotic Dis 12(5):346–359
Abdelbaset AE, Nonaka N, Nakao R (2022) Tick-borne diseases in Egypt: a one health perspective. One Health. https://doi.org/10.1016/j.onehlt.2022.100443
AbdelMeguid AD, Ramadan MY, Khater HF, Radwan IT (2022) Louicidal efficacy of essential oils against the dog louse, trichodectes canis (Mallophaga: Trichodectidae). Egypt. Acad J Biol Sci 14(1):1–16
Abdullah HHAM, El-Molla A, Salib AF, Allam ATN, Ghazy AA, Sanad MY, Abdel-Shafy S (2019) Molecular characterization of Rickettsiae infecting camels and their ticks vectors in Egypt. Bact Emp 2(1):10–18
Ahmed M, Peiwen Q, Gu Z, Liu Y, Sikandar A, Hussain D, Javeed A, Shafi J, Iqbal MF, An R, Guo H, Du Y, Wang W, Zhang Y, Ji M (2020) Insecticidal activity and biochemical composition of Citrullus colocynthis, Cannabis indica and Artemisia argyi extracts against cabbage aphid (Brevicoryne brassicae L.). Sci Rep 10(1):522. https://doi.org/10.1038/s41598-019-57092-5
Al-Deeb MA, Bin Muzafar S, Abu-Zeid YA, Enan MR, Karim S (2015) First record of a spotted fever group Rickettsia sp. and Theileria annulata in Hyalomma dromedarii (Acari: Ixodidae) ticks in the United Arab Emirates, Florida. Entomology 98:135–139
Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2010) Biologia molecular Da célula 5th ed. Artmed, Porto Alegre, p 1396
Anholeto LA, Oliveira PR, Rodrigues RAF, Yamane LT, Castro KNC, Camargo-Mathias MI (2018) Morphological alterations in the ovaries Amblyomma cajennense semi-engorged ticks exposed to ethanolic extract of Acmella oleraceae. Microsc Res Tech 81(11):1347–1357
Arnosti A, Brienza PD, Furquim KCS, Chierice GO, Neto SC, Bechara GH, Sampieri BR, Camargo-Mathias MI (2011a) Effects of Ricinus communis oil esters on salivary glands of Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae). Exp Parasitol 127(2):569–574
Arnosti A, Brienza PD, Furquim KCS, Chierice GO, Bechara GH, Calligaris IB, Camargo-Mathias MI (2011b) Effects of ricinoleic acid esters from caster oil of Ricinus communis on the vitellogenesis of Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) ticks. Exp Parasitol 127(2):575–580
Atella GC, Gondim KC, Machado EA, Medeiros MN, Silva-Neto MAC, Masuda H (2005) Oogenesis and egg development in triatomines: a biochemical approach. An Acad Bras Cienc 77(3):405–430
Balashov YS (1972) Bloodsucking ticks (Ixodoidea) vectors of diseases of man and animals. Misc Publ Entomol Soc Am 8:161–376
Balashov YS, USSR AKAD Sci Nauka Publ Leningrad (1983) An atlas of ixodid tick ultrastructure. Entomological Society of America Headquarters, Annapolis
Bancroft JD, Marilyn (2008) Theory and Practice of histology techniques. Elsevier, Amsterdam
Bashir MH, Gogi MD, Ashfaq M, Afzal M, Khan MA, Ihsan M (2013) The efficacy of crude aqueous extracts of some plants as grain protectants against the stored grain mite, Rhizoglyphus Tritici. Turk J Agric for 37(5):585–594
Bazlikova M, Kazar J, Schramek S (1984) Phagocytosis of Coxiella burnetii by Hyalomma dromedarii tick haemocytes. Acta Virol 28(1):48–52
Bendary HA, Rasslan F, Wainwright M, Alfarraj S, Zaki AM, Abdulall AK (2022) Crimean-Congo hemorrhagic fever virus in ticks collected from imported camels in Egypt. Saudi J Biol Sci 29(4):2597–2603
Bhasin A, Singh S, Garg R (2020) Nutritional and medical importance of Citrullus colocynthis-a review. Plant Arch 20(2):3400–3406
Carvalho FH, Recco-Pimentel SM (eds) (2007) A célula. Manole, São Paulo, pp 200–210
Dahroug SM, Sobeiha AK, Farag AMJ, Bakr EM (2000) Toxicity of certain botanical extracts to red spider mite and black bean aphid. Ann Agric Sci 38(4):2551–2562
Dawes CJ (1971) Biological techniques in electron microscopy. (Barnes and Noble, Inc.), pp. 148–149
Denardi SE, Bechara GH, Oliveira PR, Nunes ET, Saito KC, Camargo-Mathias MI (2004) Morphological characterization of the ovary and vitellogenesis dynamics in the tick Amblyomma cajennense (Acari: Ixodidae). Vet Parasitol 125(3–4):379–395
Denardi SE, Bechara GH, Oliveira PR, Camargo-Mathias MI (2011) Inhibitory action of neem aqueous extract (Azadirachta indica A. Juss) on the vitellogenesis of Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) ticks. Microsc Res Tech 74(10):889–899
Denardi SE, Bechara GH, Oliveira PR, Camargo-Mathias MI (2012) Ultrastructural analysis of the oocytes of female Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) ticks subjected to the action of Azadirachta indica A. juss (neem). Ultrastruct Pathol 36(1):56–67
Dimetry NZ, El-Gengaihi S, Hafez M, Abbass MH (2015) Pesticidal activity of certain plant extracts and their isolates against the cowpea beetle Callosobruchus maculatus (F.) (Coleoptera: Chrysomelidae: Bruchinae). Herba Pol 61(3):77–92
Drummond RO, Ernst SE, Trevino JL, Gladney WJ, Graham OH (1973) Boophilus annulatus and Boophilus microplus: laboratory test of insecticides. J Econ Entomol 66:130–133
Eltaly RI, Baz MM, Radwan IT, Yousif M, Abosalem HS, Selim A, Taie HAA, Farag AAG, Khater HF (2023) Novel acaricidal activity of Vitex Castus and Zingiber officinale extracts against the camel tick, Hyalomma dromedarii. Int J Vet Sci 12(2):255–259
Gatenby JB, Beams HW (1950) The microtomist’s vade-mecum: a handbook of the methods of microscopic anatomy (Bolles Lee), (11th edn.) J. & A. Churcill LTD., London, pp 753–759
Godara R, Parveen S, Katoch R, Yadav A, Katoch M, Khajuria JK, Kaur D, Ganai A, Verma PK, Khajuria V, Singh NK (2015) Acaricidal activity of ethanolic extract of Artemisia absinthium against Hyalomma anatolicum ticks. Exp Appl Acarol 65(1):141–148
Guglielmone AA, Apanaskevich DA, Estrada-Peña A, Robbins RG, Petney TN, Horak IG (2014) The hard ticks of the world: (Acari: Ixodida: Ixodidae). Springer, Berlin
Habeeb SM, Abdel-Shafy S, Youssef AA (2007) Light, scanning electron microscopy and SDS-PAGE studies on the effect of the essential oil Citrus sinensis var. Balady on the embryonic development of camel tick Hyalomma dromedarii (Koch 1818) (Acari-Ixodidae). Pak J Biol Sci 10(8):1151–1160
Hamid NS, Kahil MA, Ibrahim NA (2016) Larvicidal activity of ethanol extract of Citrullus colocynthis seed and fruit pulp against Anopheles arabiensis and Culex quinquefasciatus. J Med Plants Stud 4(6):252–255
Hoogstraal H, Kaiser MN (1958) The ticks (Ixodoidea) of Iraq: keys, hosts, and distribution. J Iraqi Med Prof 6:58–84
Hoogstraal H, Wassef HY, Buttiker W (1981) Ticks (Acarina) of Saudi Arabia fam argasidae ixodidae. Fauna Saudi Arabia 3:25–110
Horn M, Nussbaumerová M, Šanda M, Kovářová Z, Srba J, Franta Z, Sojka D, Bogyo M, Caffrey CR, Kopa´cek P, Mareš M (2009) Hemoglobin digestion in blood-feeding ticks: mapping a multipeptidase pathway by functional proteomics. Chem Biol 16(10):1053–1063
Hussian HH, Jasim MA (2018) The lethal effects of Citrullus colocynthis against Rhipicephalus spp. Diyala J Agric Sci 10:399–406
Hussain AI, Rathore HA, Sattar MZ, Chatha SA, Sarker SD, Gilani AH (2022) Citrullus colocynthis (L.) Schrad (bitter apple fruit): a review of its phytochemistry, pharmacology, traditional uses and nutritional potential. J Ethnopharmacol 155(1):54–66
Iqbal T, Ahmed N, Shahjeer K, Ahmed S, Al-Mutairi KA, Khater HF, Ali RF (2021) Botanical insecticides and their potential as anti-insect/pests: are they successful against insects and pests? In: El-Shafie HAF (ed) Global decline of insects. IntechOpen, London
Iturbe-Requena SL, Prado-Ochoa MG, Velázquez-Sánchez AM, García-Hernández F, Cossío-Bayúgar R, Muñoz-Guzmán MA, Alba-Hurtado F (2020) Oogenesis and embryogenesis inhibition induced by two new ethyl-carbamates in the cattle tick Rhipicephalus microplus. Ticks Tick Borne Dis 11(2):101326. https://doi.org/10.1016/j.ttbdis.2019.101326
Kanapadinchareveetil S, Chandrasekhar L, Gopi J, Lenka DR, Vasa A, KGopalan AK, Nair SN, Ravindran R, Juliet S, Ghosh S (2015) Histoarchitecture of the ovary of Rhipicephalus (Boophilus) annulatus during pre- and postengorgement period. Sci World J. https://doi.org/10.1155/2015/126584
Kanapadinchareveetil S, Chandrasekhar L, Pious A, Kartha HS, Ravindran R, Juliet S, Nair SN, Ajithkumar KG, Ghosh S (2019) Molecular, histological and ultrastructural characterization of cytotoxic effects of amitraz on the ovaries of engorged females of Rhipicephalus (Boophilus) annulatus. Exp Parasitol 204:107732. https://doi.org/10.1016/j.exppara.2019.107732
Konig IFM, Gonçalves RRP, Oliveira MVS, Silva CM, Thomasi SS, Peconick AP, Remedio RN (2019) Sublethal concentrations of acetylcarvacrol strongly impact oocyte development of engorged female cattle ticks Rhipicephalus microplus (Canestrini, 1888) (Acari: Ixodidae). Ticks Tick Borne Dis 10(4):766–774
Konig IFM, Oliveira MVS, Gonçalves RRP, Peconick AP, Thomasi SS, Anholeto LA, Lima-de-Souza JR, Camargo-Mathias MI, Remedio RN (2020) Low concentrations of acetylcarvacrol induce drastic morphological damages in ovaries of surviving Rhipicephalus sanguineus Sensu Lato ticks (Acari: Ixodidae). Micron 129:102780. https://doi.org/10.1016/j.micron.2019.102780
Mahran MO, Wahba AA, Mansour KM (2020) In vitro acaricidal effect of neem leaves (Azadirachta Indica) and Citrullus colocynthis extracts against the camel ticks, Hyalomma dromedarii (Acari: Ixodidae). J Ecosys Ecograph 10(1):264–270
Marzouk AS, Swelim HH, Ali AAB, Oken (2020) Ultrastructural changes induced by the entomopathogenic fungus Beauveria bassiana in the ovary of the tick Argas (Persicargas) persicus (Oken). Ticks Tick Borne Dis. https://doi.org/10.1016/j.ttbdis.2020.101507
Mihara R, Umemiya-Shirafuji R, Abe Y, Matsuo T, Horiuch N, Kawano S, Fujisaki K, Suzuki H (2018) The development of oocytes in the ovary of a parthenogenetic tick, Haemaphysalis longicornis. Parasitol Int 67(4):465–471
Mohamed WMA, Ali AO, Mahmoud HY, Omar MA, Chatanga E, Salim B, Naguib D, Anders JL, Nonaka N, Moustafa MAM, Nakao R (2021) Exploring prokaryotic and eukaryotic microbiomes helps in detecting tick-borne infectious agents in the blood of camels. Pathogens 10(3):351. https://doi.org/10.3390/pathogens10030351
Mohamed SNA, Montasser AA, Ali AAB (2022) Acaricidal effect of Citrullus colocynthis fruit extract on the camel tick Hyalomma dromedarii. Ticks Tick Borne Dis 1844(5):101995. https://doi.org/10.1016/j.ttbdis.2022.101995. Koch
Mohammed SH, Baz MM, Ibrahim M, Radwan IT, Selim A, Dawood A-FD, Taie H, Abdalla S, Khater HF (2023) Acaricide resistance and novel photosensitizing approach as alternative acaricides against the camel tick, Hyalomma dromedarii. Photochem Photobiol Sci 22(1):87–101
Nugraha RV, Ridwansyah H, Ghozali M, Khairani AF, Atik N (2020) Traditional herbal medicine candidates as complementary treatments for COVID-19: a review of their mechanisms, pros and cons. Evid Based Complement Altern Med. https://doi.org/10.1155/2020/2560645
Nwanade CF, Yu Z, Liu J (2020) Botanical acaricides induced morphophysiological changes of reproductive and salivary glands in tick: a mini-review. Res Vet Sci 132:285–291
Oliveira PR, Bechara GH, Denardi SE, Nunes ET, Camargo-Mathias MI (2005) Morphological characterization of the ovary and oocytes vitellogenesis of the tick Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae). Exp Parasitol 110(2):146–156
Oliveira PR, Camargo-Mathias MI, Bechara GH (2006) Amblyomma triste (Koch, 1844) (Acari: Ixodidae): morphological description of the ovary and of vitellogenesis. Exp Parasitol 113(3):179–185
Oliveira PR, Castro KNC, Anholeto LA, Camargo-Mathias MI (2016) Cytotoxic effects of extract of Acmella oleraceae (Jambu) in Rhipicephalus microplus female ticks. Microsc Res Tech 79(8):744–753
Oliveira PR, Anholeto LA, Bechara GH, Camargo-Mathias MI (2017) Dinotefuran-induced morphophysiological changes in semi-engorged females Rhipicephalus sanguineus Latreille, 1806 (Acari: Ixodidae) ticks: ultra-structural evaluation. Acta Trop 166:139–154
Oliveira PR, Anholeto LA, Rodrigues RAF, Arnosti A, Bechara GH, Castro KNC, Camargo-Mathias MI (2019) Cytotoxic effects of extract of Acmella oleraceae in the ovaries and midgut of Rhipicephalus sanguineus Latreille, 1806 (Acari: Ixodidae) female ticks. J Microsc Ultrastruct 7(1):28–43
Pantin CFA (1946) Notes on microscopical technique for zoologists. Cambridge University Press, Cambridge
Pearse AGE (1960) Histochemistry, theoretical and applied, 2nd edn. Churchill Livingstone, Edinburgh
Rahuman AA, Venkatesan P, Gopalakrishnan G (2008) Mosquito larvicidal activity of oleic and linoleic acids isolated from Citrullus colocynthis (Linn.) Schrad. Parasitol Res 103(6):1383–1390
Reis AC, Konig IFM, Rezende DACS, Gonçalves RRP, Lunguinho AS, Ribeiro JCS, Cardoso MG, Remedio RN (2021) Cytotoxic effects of Satureja montana L. essential oil on oocytes of engorged Rhipicephalus microplus female ticks (Acari: Ixodidae). Microsc Res Tech 84(7):1375–1388
Remedio RN, Nunes PH, Anholeto LA, Camargo-Mathias MI (2014) Morphological alterations in the synganglion and integument of Rhipicephalus sanguineus ticks exposed to aqueous extracts of neem leaves (Azadirachta indica A. JUSS). Microsc Res Tech 77(12):989–998
Remedio RN, Nunes PH, Anholeto LA, Oliveira PR, Camargo-Mathias MI (2015) Morphological effects of neem (Azadirachta indica A. Juss) seed oil with known azadirachtin concentrations on the oocytes of semi-engorged Rhipicephalus sanguineus ticks (Acari: Ixodidae). Parasitol Res 114(2):431–444
Rey L (1973) Parasitologia. Guanabara Koogan, Rio de Janeiro, pp 633–641
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17(1):208–212
Roshdy MA (1969) Structure of the female reproductive system of Ixodes ricinus (L.) and its bearing on the affinity of Ixodes to other ixodid genera. J Parasitol 5:1078–1083
Saito KC, Bechara GH, Nunes ET, Oliveira PR, Denardi SE, Camargo-Mathias MI (2005) Morphological, histological, and ultrastructural studies of the ovary of the cattle-tick Boophilus microplus (Canestrini, 1887) (Acari: Ixodidae). Vet Parasitol 129(3–4):299–311
Sakthivadivel M, Daniel T (2008) Evaluation of certain insecticidal plants for the control of vector mosquitoes viz. Culex quinquefasciatus, Anopheles stephensi and Aedes aegypti. Appl Entomol Zool 43(1):57–63
Sanches GS, Bechara GH, Camargo-Mathias MI (2010) Ovary and oocyte maturation of the tick Amblyomma brasiliense Aragao, 1908 (Acari: Ixodidae). Micron 41(1):84–89
Sanches GS, de Oliveira PR, André MR, Machado RZ, Bechara GH, Camargo-Mathias MI (2012a) Copulation is necessary for the completion of a gonotrophic cycle in the tick Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae). J Insect Physiol 58(7):1020–1027
Sanches GS, Araujo AM, Martins TF, Bechara GH, Labruna MB, Camargo-Mathias MI (2012b) Morphological records of oocyte maturation in the parthenogenetic tick Amblyomma rotundatum Koch, 1844 (Acari: Ixodidae). Ticks Tick Dis 3(1):59–64
Sanches GS, André MR, do Prado AP, Allegretti SM, Remedio RN, Nunes PH, Machado RZ, Bechara GH, Camargo-Mathias MI (2014) Oocyte maturation in the sloth’s giant tick Amblyomma varium (Acari: Ixodidae) in an ecological context. Exp Appl Acarol 64(4):519–531
Scheer U, Hock R (1999) Structure and function of the nucleolus. Curr Opin Cell Biol 11(3):385–390
Seixas A, Oldiges DP, Vaz ISJR, Termignoni C (2010) Endocrinologia E controle Da vitelogˆenese em carrapatos. Acta Sci Vet 38(2):95–111
Souza JRL, Oliveira PR, Anholeto LA, Arnosti A, Daemon E, REmedio RN, Camargo-Mathias MI (2019) Effects of carvacrol on oocyte development in semi-engorged Rhipicephalus sanguineus sensu lato females ticks (Acari: Ixodidae). Micron 116:66–72
Starck JM, Mehnert L, Biging A, Bjarsch J, Franz-Guess S, Kleeberger D, Hörnig M (2018) Morphological responses to feeding in ticks (Ixodes ricinus). Zoological Lett 4:20. https://doi.org/10.1186/s40851-018-0104-0
Twaij HAA, Sayed-Ali HM, AlZohry AM (1988) Pharmacological phytochemical and antimicrobial studies on Myrtus communis Part I. Cardiovascular and Phytochemical studies. J Biol Sci Res 19(1):29–40
Ullah S, Khan MS, Sajid MS, Muhammud G (2013) Comparative anthelmintic efficacy of Curcuma longa, Citrullus colocynthis, Peganum harmala. Glob Vet 11(5):560–567
Ullah S, Khan MN, Sajid MS, Iqbal Z, Muhammad G (2015) Comparative efficacies of Curcuma longa, Citrullus colocynthis and Peganum harmala against Rhipicephalus microplus through modifie larval immersion test. Int J Agric Biol 17(1):216–220
Vendramini MCR, Camargo-Mathias MI, de Faria AU, Bechara GH, de Oliveira PR, Roma GC (2012) Cytotoxic effects of andiroba oil (Carapa guianensis) in reproductive system of Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae) semiengorged females. Parasitol Res 111(5):1885–1894
Weiss LB, Kaufman WR (2001) The relationship between ‘critical weight’ and 20- hydroxiecdysone in the female ixodid tick, Amblyomma hebraeum. J Insect Physiol 47(11):1261–1267
Xavier MA, Tirloni L, Pinto AFM, Diedrich JK, Yates JR, Mulenga A, Logullo C, Da Silva Vaz I, Seixas A, Termignoni C (2018) A proteomic insight into vitellogenesis during tick ovary maturation. Sci Rep 8(1):1–14
Yano Y, Mori T, Shiraishi S, Uchida T (1989) Ultrastructure of oogenesis in the adult cattle tick, Haemaphysalis longicornis. J Fac Agric Kyushu Univ 34:53–67
Zheoat AM, Alenezi S, Elmahallawy EK, Ungogo MA, Alghamdi AH, Watson DG, Igoli JO, Gray AI, Koning HP, Ferro VA (2021) Antitrypanosomal and antileishmanial activity of chalcones and flavanones from Polygonum salicifolium. Pathogens 10(2):175. https://doi.org/10.3390/pathogens10020175
Acknowledgements
Authors would like to thank Dr. Mostafa Hussein, a researcher in Egypt Desalination Research Center of Excellence, Desert Research Center, Cairo, Egypt and Dr. Ebtehal Hassan Mohamed, a researcher in Electron Microscope Unit, Faculty of Agriculture, Cairo University, Egypt for their helpful cooperation in photographing of electron microscopical samples.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
AABA and AAM contributed to the study conception and design. Material preparation, data collection and analysis were performed by AABA and SNAM. The first draft of this manuscript was written by AABA and all authors commented on the previous versions before this final one. All authors read and approved this final manuscript. AABA: Conceptualization, Validation, Investigation, Resources, Data curation, Writing—original draft, Writing—review & editing, Visualization, Supervision. AAM: Conceptualization, Writing—review & editing. SNAM: Conceptualization, Investigation, Resources, Data curation, Writing—review & editing.
Corresponding author
Ethics declarations
Competing interest
We the authors of this manuscript disclose and declare that there is no competing interest with any person or organization or funding from anywhere.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ali, A.A.B., Montasser, A.A. & Mohamed, S.N.A. Histopathological effects of the fruit extract of Citrullus colocynthis on the ovary of the tick Hyalomma dromedarii. Exp Appl Acarol 92, 275–295 (2024). https://doi.org/10.1007/s10493-023-00895-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-023-00895-z