Abstract
Varroa destructor Anderson & Trueman (Acari: Varroidae) is of paramount significance in modern beekeeping, with infestations presenting a primary challenge that directly influences colony health, productivity, and overall apicultural sustainability. In order to control this mite, many beekeepers rely on a limited number of approved synthetic acaricides, including the pyrethroids tau-fluvalinate, flumethrin and organophosphate coumaphos. However, the excessive use of these substances has led to the widespread development of resistance in various beekeeping areas globally. In the present study, the occurrence of resistance mutations in the voltage-gated sodium channel (VGSC) and acetylcholinesterase (AChE), the target-site of pyrethroids and coumaphos, respectively, was examined in Varroa populations collected throughout the southeastern and eastern Anatolia regions of Türkiye. All Varroa samples belonged to the Korean haplotype, and a very low genetic distance was observed based on cytochrome c oxidase subunit I (COI) gene sequences. No amino acid substitutions were determined at the key residues of AChE. On the other hand, three amino acid substitutions, (L925V/I/M), previously associated with pyrethroid resistance, were identified in nearly 80% of the Turkish populations. Importantly, L925M, the dominant mutation in the USA, was detected in Turkish Varroa populations for the first time. To gain a more comprehensive perspective, we conducted a systematic analysis of the distribution of pyrethroid resistance mutations across Europe, based on the previously reported data. Varroa populations from Mediterranean countries such as Türkiye, Spain, and Greece exhibited the highest frequency of resistance mutation. Revealing the occurrence and geographical distribution of pyrethroid resistance mutations in V. destructor populations across the country will enhance the development of more efficient strategies for mite management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ectoparasitic honey bee mite Varroa destructor Anderson & Trueman (Acari: Varroidae) is a destructive parasite of the Western honey bee Apis mellifera and is widely recognized to be one of the primary reason for colony collapse disorder (CCD) (Rosenkranz et al. 2010a, b; Le Conte et al. 2010). In addition to directly feeding on the fat body and hemolymph of bees (Ramsey et al. 2019), Varroa mites can transmit several virus diseases, including Deformed Wing Virus (DWV), which can be lethal to honey bee colonies (McMenamin and Genersch 2015; Martin and Brettell 2019). Although organic acids have been increasingly used by a growing number of beekeepers, the main control of Varroa has still largely relied on chemical acaricides worldwide (Rosenkranz et al. 2010a, b; Tutun et al. 2018; Gregorc and Sampson 2019; Brodschneider et al. 2023). It has long been a critical issue to kill mites in-hive without any damage to bees. The acaricides such as tau-fluvalinate, flumethrin, amitraz, and coumaphos are known to offer such selectivity and thus have been used for decades in Varroa control (van der Steen and Vejsnæs, 2021). In the case of pyrethroids, the amino acid variations in the voltage-gated sodium channel (VGSC), leading to differential acaricide binding, have been suggested as the primary factor underlying the selectivity between Varroa and A. mellifera, as well as the common eastern bumblebee Bombus impatiens (O’Reilly et al. 2014; Wu et al. 2017). Repetitive selection, driven by the limited number of available acaricides in the portfolio, has inevitably resulted in resistance development in Varroa populations (Elzen et al. 1999; Spreafico et al. 2001; Martin 2004; Pettis 2004; Mitton et al. 2022). Due to honey bee trade, migratory beekeeping, and the various dispersal strategies of Varroa mites (Sammataro et al. 2000; Rosenkranz et al. 2010a, b; Jack and Ellis 2021), resistance can quickly spread over wide areas once it arises, making the issue even more challenging to address.
Resistance development in Varroa mites has been mainly associated with increased detoxification enzyme activity and a number of point mutations in the target-site of pyrethroids (Van Leeuwen and Dermauw 2016; Mitton et al. 2022; De Rouck et al. 2023). Current knowledge of metabolic aspects of pyrethroid resistance mechanisms is primarily based on biochemical and synergism assays (Hillesheim et al. 1996; Mozes-Koch et al. 2000), with relatively limited information available regarding specific mechanisms. Development of metabolic resistance can be complex in Varroa, as recently demonstrated in the case of coumaphos resistance, which results from the downregulation of a P450 enzyme, leading to decreased activation (Vlogiannitis et al. 2021a). On the other hand, no mutation conferring resistance to coumaphos in Varroa mites has been reported so far, although target-site mutations are known as a common resistance mechanism for other acetylcholinesterase inhibitors (Feyereisen et al. 2015).
Point mutations in the VGSC associated with tau-fluvalinate resistance in V. destructor were first uncovered in the USA (Wang et al. 2002). Specifically, the L1596P (Musca domestica numbering) mutation in the linker connecting domains III and IV in V. destructor was further examined since the proline (P) is conserved in insects and had a potential to be one of the reasons for low toxicity of fluvalinate to bees. Indeed, functional validation in Xenopus oocytes, using a cockroach sodium channel, showed a fivefold increase in sensitivity to fluvalinate when proline was replaced with leucine (L) (Liu et al. 2006). However, this mutation was not detected anymore in following studies. Years later, a L925V mutation in the transmembrane segment 5 of domain II of the VGSC was uncovered in V. destructor populations from the UK and Czechia (González-Cabrera et al. 2013; Hubert et al. 2014). Today, the presence of the L925V mutation has been reported in other countries and continents (Alissandrakis et al. 2017; Panini et al. 2019; Stara et al. 2019; Koç et al. 2021; Vlogiannitis et al. 2021b; Mitton et al. 2021; Ogihara et al. 2021; Hernández-Rodríguez et al. 2021; Millán-Leiva et al. 2021a). Therefore, similar to other arthropods, resistance to pyrethroids in European populations of V. destructor is thought to be primarily driven by the target-site mutation L925V. (González-Cabrera et al. 2018). Other mutations in the same position (L925I and L925M) have also been documented, especially in Varroa samples from the USA (González-Cabrera et al. 2016). More recently, the knockdown resistance-like (kdr-like) mutation M918L in the IIS4—S5 channel linker, a variant of the well-described super-kdr mutation (M918T), in combination with L925V was detected in V. destructor populations from Eastern Spain (Millán-Leiva et al. 2021b). The M918L together with other mutations was also found in insect and other mite species (Carletto et al. 2010; Karatolos et al. 2012; Katsavou et al. 2020). The role of both L925I and M918T (super-kdr) in pyrethroid resistance was functionally validated by electrophysiology and molecular docking analyses (Vais et al. 2003; Usherwood et al. 2007).
Türkiye is the second largest honey-producing country following China (FAOSTAT 2023). However, the expected honey production yield has not been achieved due to several factors. Among these, Varroa mites and their ability to develop resistance, resulting in failure in chemical control, are regarded as a primary and significant threat. Koç et al. (2021) documented the widespread distribution of the L925V/I mutations in Varroa populations from the central Anatolia region. This finding highlights the significance of conducting resistance monitoring in additional geographic areas, such as the eastern Anatolia region, where traditional beekeeping is widespread. Therefore, in the present study, we screened well-known pyrethroid resistance mutations, including L925V/L/M, along with the super-kdr M918T mutation. We also investigated mutations at key positions in the target site of coumaphos, acetylcholinesterase (AChE), in Varroa populations. Next, we reviewed the occurrence and distribution of pyrethroid resistance mutation across the country, as well as in Europe. Last, a haplotype network analysis was performed to analyze the relationship among worldwide Varroa populations.
Materials and methods
Origin of Varroa destructor populations
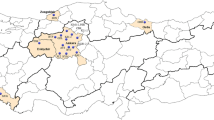
A total of 44 Varroa populations, each from a different apiary, were sampled across 21 cities in southeastern and eastern Anatolia regions of Türkiye during 2022 (Fig. 2B and Table S1). Mites were collected with the powdered sugar method as outlined by Gregorc et al. (2017) and subsequently transferred to the laboratory in 90% ethanol for molecular analysis. Further information regarding the history of pesticide usage, beekeeping practices, and the location of the apiaries are presented in Table S1.
Genomic DNA extraction
The genomic DNA (gDNA) was extracted from pools of 10 adult females per population, using the DNeasy Blood & Tissue Kit (Qiagen, Germany) according to the manufacturer's instructions. In the final stage, DNAs were eluted using 100 μl of elution buffer. The quality and quantity of the gDNA were assessed through agarose gel electrophoresis (1.5%) and a spectrophotometer (NanoDrop™ 2000, Thermo Scientific, USA). The DNA samples were then stored at − 20 °C until needed.
Amplification of target-sites and mtDNAs
The presence of the resistance mutations located at the IIS4–IIS5 region of the VGSC gene was screened as previously described (Alissandrakis et al. 2017). New primers were designed for VdAChE1 (accession number: XP_022645444), the major catalytic enzyme in V. destructor (Kim et al. 2022), using primer3 (Untergasser et al. 2007). These primers allow screening for key residues previously associated with resistance to AChE inhibitors. The primers were as follows: VdAChE1_F1: ATACGCTAAACCGCCGATTG; VdAChE1_R1: TCGTCACATTGTTGGGGTTG and VdAChE1_F2: CCGTTAAGTGCCTTCGACAG; VdAChE1_R2: CTCGACAAAGTCCTCTCGGG (see Fig. S1 for amplified fragments). The Varroa samples (n = 22) collected by Koç et al. (2021) was also included for the screening of AChE mutations. PCR condition for AChE primers were as follows: 5 min at 95 °C, 35 cycles of 30 s at 95 °C, 30 s at 53 °C and 30 s at 72 °C and a final extension of 5 min at 72 °C.
The genetic distance between Varroa mite populations from different locations was determined by analyzing the mtDNA sequences of the cytochrome c oxidase subunit I (COI) (10KbCOIF1-6,5KbCOIR primers) and NADH dehydrogenase subunit 4 (ND4) (ND4F- ND4R primers) genes that were amplified according to Navajas et al. (2010) and Muntaabski et al. (2020).
Each PCR reaction was performed with Promega GoTaq® Flexi kit in a final volume of 50 μL consisting of 2 μL of mite DNA (ranging from 40 to 65 ng/μL), 3 µL of MgCl, 1 µL of dNTP, 10 µL of 5X Buffer, 2.5 µL of each of forward and reverse primer, and 0.25 µL Taq DNA polymerase. The amplified products were visualized on a 1.5% agarose gel stained with ethidium bromide using a UV transilluminator. PCR amplicons were purified with the EZNA Cycle-Pure kit (Omega Biotek, USA) and subsequently sequenced at LGC Genomics (Berlin, Germany).
Data analysis and haplotype network
The presence and frequency of pyrethroid mutations, both in this study and in our previous study (Koç et al. 2021), were evaluated by analyzing the peak heights in sequencing chromatographs, following the methodology described by Van Leeuwen et al. (2008). The maps were generated using mapchart.net with inputs from current and previous studies (see Table S2-S3 for details on the data used and the corresponding references). All previous studies employed individual mites for pyrethroid mutation identification, with the exception of Farjamfar et al. (2018), who used pooled DNA but found no mutations in their study. Therefore, only homozygous mutant individuals were considered resistant when generating the map, given the recessive nature of the pyrethroid resistance mutation (González-Cabrera et al. 2013, 2018).
To explore the genetic diversity among various Varroa populations, we examined the sequences obtained in our study together with sequences retrieved from the GenBank database (see Table S4 for accession numbers of sequences used in analysis). Because we used pooled mites for DNA extraction in this study, we could not determine individual haplotypes. Therefore, we used consensus sequences based on dominant nucleotides as representative haplotypes for each sample. MAFFT v.7 with the ‘Auto’ strategy (Katoh et al. 2019) was utilized to perform a multiple sequence alignment, which was later refined using Bioedit v.7.0.5 software (Hall 1999). Haplotype analysis of COI sequences was conducted using DnaSP v6.12.03 (Rozas et al. 2017) and PopArt v1.7 was used to draw haplotype networks based on the haplotypes generated by DnaSP (Leigh and Bryant 2015). The accession numbers of sequences obtained in this study (both COI and ND4), as well as those retrieved from GenBank for analysis, along with their haplotypes, are provided in Table S2 and Table S4. The genetic distance among all the obtained sequences was assessed using MEGA11 (Tamura et al. 2021).
Results and discussion
Varroa destructor is widely recognized as a significant factor contributing to the decline of honey bee colonies (Traynor et al. 2020). In addition to their considerable economic importance to Türkiye through honey exportation, honey bees play a crucial role in pollinating numerous crops. However, Varroa control seems to be not at the intended level, as 100% infection rates have been previously reported in Turkish apiaries (Yalçınkaya and Keskin 2010; Koç et al. 2021). Similarly, all hives in the study's sampling area were infested with Varroa mites, albeit with varying levels of severity as observed through visual inspection.
Arrhenotokous reproduction together with the dispersal ability of resistant individuals due to their parasite biology, the honey bee trade, and migratory beekeeping all contribute to the rapid development and spread of resistance across large areas (Carrière 2003; Miller and Sappington 2017; Sammataro et al. 2000). In this study, we conducted a molecular screening for well-known pyrethroid resistance mutations in Varroa populations from the southeastern and eastern Anatolian regions of Türkiye. These regions are of significant importance to beekeeping due to being major stops for numerous migratory beekeepers across the country and their diverse and distinct flora, which results in the production of high-quality and multifloral honeys (Akkaya and Alkan 2007; Güler and Demir 2005).
Two highly virulent haplotypes, namely the Korean and Japanese/Thailand haplotypes, of V. destructor shifted their host from the Asian honey bee (Apis cerana) to the European honey bee (Apis mellifera) in the Far East over fifty years ago, and since then, they have extensively spread across the globe (Traynor et al. 2020). This host shift resulted in a genetic bottleneck, leading to reduced genetic variation within V. destructor populations (Solignac et al. 2005; Navajas et al. 2010). The comparison of 332 COI sequences analyzed in the present study revealed very high similarity (only 0.06% variation), supporting the evolutionary history of V. destructor. Similar to previous findings in Türkiye (Warrit et al. 2004; Ayan and Aldemir 2018; Koç et al. 2021), all V. destructor populations investigated in the present study belonged to Korean haplotype. To provide a broader perspective, we conducted a haplotype analysis using over 300 sequences from worldwide populations of Varroa mites (Fig. 1). Hap_1 emerged as the most prevalent haplotype, encompassing all Turkish Varroa sequences. The haplotype network analysis aligns with the genetic bottleneck hypothesis, primarily attributed to host shifting, as the majority of the haplotypes belong to single haplotype (Hap_1) (Fig. 1). Another mitochondrial gene, ND4, has been suggested as a sensitive marker for detecting genetic variations in V. destructor (Muntaabski et al. 2020). In our study, we observed no genetic variation (0%) among ND4 sequences, while a minimal variation of 0.03% was observed in COI sequences among Turkish Varroa populations.
AChE inhibitors including organophosphates (OPs) and carbamates have been long used to control pests or vector species (Sparks and Nauen 2015), although most of them have been banned today. The first case of coumaphos resistance was reported more than 20 years ago in Italian populations of Varroa (Spreafico et al. 2001), and it has since been found on different continents (Pettis 2004; Sammataro et al. 2005; Maggi et al. 2009, 2011; Hernández-Rodríguez et al. 2021). However, the molecular mechanisms underlying the development of resistance remain unknown. A number of target-site mutations have been reported to confer resistance to AChE inhibitors in invertebrates (Lee et al. 2015). Nevertheless, as of now, no such mutations have been reported within Varroa. In the present study, a comprehensive molecular screening, encompassing 66 populations across the country, was conducted. In line with previous reports, no mutations were detected at the key residues of AChE. Since we lack information regarding the phenotypic susceptibility levels to coumaphos, it is challenging to make a definitive statement ruling out target-site mutations as a resistance mechanism in Turkish Varroa populations. A recent study showed that resistance to coumaphos can indeed arise through mechanisms other than target-site mutation. Vlogiannitis et al. (2021a, b) documented the downregulation of a P450 gene, CYP4EP4, which results in reduced activation of coumaphos. More studies are needed to show if this mechanism is shared across Varroa populations from geographical backgrounds.
Target-site mutations have been suggested as the primary mechanism responsible for pyrethroid resistance in V. destructor (Dong et al. 2014; De Rouck et al. 2023), allowing the implementation of molecular screening for resistance (Van Leeuwen et al. 2020). The results of this study showed that approximately 80% of the sampled Varroa populations contained at least one of the mutations (L925V, L925I, or L925M). Specifically, both L925V and L925I mutations appear to be prevalent in the country (Koç et al. 2021; this study). Fifteen out of the 44 samples contained no susceptible wild-type alleles. Among them, five samples exhibited fixation for either L925V or L925I, whereas the rest of the 11 samples had mixed mutations, without any mites having susceptible leucine (L) at position 925. Some beekeepers in the sampled regions have opted to discontinue the application of flumethrin due to the treatment's inefficacy (personal communications). Notably, upon examining the genotyping results, we observed a fixed L925V/I mutation in all areas where flumethrin was not used, highlighting the potential of these mutations as molecular diagnostics for pyrethroid resistance. Conversely, none of the Turkish Varroa populations had M918L mutation, in line with Koç et al. (2021). This study, along with Koç et al. (2021), clearly demonstrates the widespread distribution of pyrethroid resistance mutations within the Turkish Varroa populations; however, the frequencies of these mutations differed significantly (Table S2). In addition to populations with fixed mutations, populations having a low frequency of homozygous resistant individuals can also be easily selected after repeated rounds of selection. Therefore, they may also present a potential risk for Varroa control. Since pooled DNAs were used in both present study and Koç et al. (2021), it was not possible to estimate the exact frequencies of homozygous individuals (i.e. valine in both alleles) or heterozygous individuals (i.e. leucine in one allele, valine in the other allele). This is particularly important as most of the pyrethroid mutations are recessive and thus no or little phenotypic effect is expected at the heterozygous state. Nevertheless, a previous study showed high levels of pyrethroid resistance in aphids although the mutation was heterozygous (Panini et al. 2021). In this case, transposon-mediated monoallelic expression prevented the expression of susceptible alleles and thus caused resistant individuals (Panini et al. 2021). Therefore, molecular screening should be combined with phenotypic assays to reveal phenotypic resistance levels.
L925M mutation has been initially reported in the USA and its presence in Europe was documented only recently (González-Cabrera et al. 2016; Millán-Leiva et al. 2021b). A phylogenetic analysis of VGSC haplotypes of V. destructor suggested a sequential evolution of these mutations: L925M (CTG → ATG) arose first and then L925I (ATG → ATA) (Millán-Leiva et al. 2021b). However, in this study, we found multiple populations with L925V and L925I mutations without L925M, which was suggested to be the origin of L925I mutation, in line with previous reports from Europe (Alissandrakis et al. 2017; Vlogiannitis et al. 2021b). The presence of a putative fitness cost for the L925M mutation has been suggested, which might explain its absence in cases where L925I is present (Millán-Leiva et al. 2021b). However, there is still a lack of experimental data to support this claim. In addition, we determined the L925M mutation in Turkish V. destructor populations, for the first time in Asia, at a prevalence of 14.3%.
Resistance development allows insects to survive exposure to the insecticide, however, it often comes with a trade-off that affects the insect's ability to thrive in other aspects of its life cycle or in its natural environment, a phenomenon referred to as “fitness cost” (Kliot and Ghanim 2012; Ffrench-Constant and Bass 2017). Indeed, a reduction in the number of pyrethroid-resistant Varroa mites in the absence of selection pressure has been documented by Milani and Della Vedova (2002). Later on, a putative fitness cost has been associated with L925V/M mutations, based on the correlation observed between the mutation frequency and prior acaricide use (González‑Cabrera et al. 2018; Millán-Leiva et al. 2021b). In the present study, the susceptible leucine at the position 925 was fixed in nine out of 44 samples. Furthermore, it is noteworthy that susceptible genotypes were present in nearly half of the populations. The presence of these susceptible individuals allows for a decrease in resistance levels following cessation of pyrethroid use, primarily due to the associated fitness cost. Thus, alternative control methods or acaricides with different mode of action should be considered in rotation among Turkish bee farmers.
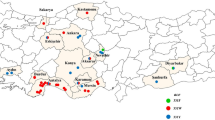
In Europe, pyrethroid resistance in Varroa mites was initially reported in Italy in the early 1990s (Lodesani et al. 1995). After its first appearance, the resistant individuals quickly spread to neighboring countries such as France, Switzerland, and Slovenia, and subsequently to broader regions across the continent (Martin 2004). Subsequently, the L925V mutation has been identified as the primary driving factor for pyrethroid resistance across Europe, although other variants such as L925I and L925M have also been detected (González-Cabrera et al. 2018). We have compiled the available data to provide an overview of the prevalence and frequency of pyrethroid mutations across Europe (Fig. 2A, Table S3). V. destructor populations from Mediterranean countries, namely Türkiye, Spain, and Greece, had the highest frequency of L925V/M/I mutations (Fig. 2A). While pyrethroid usage is not widespread throughout Europe, the percentage of beekeepers in Spain and Greece employing tau-fluvalinate and flumethrin, respectively, is higher than in most other European countries (Brodschneider et al. 2023). Likewise, flumethrin has been extensively employed in Turkish apiaries (Koç et al. 2021; this study). The more frequent use of pyrethroids in these countries has likely contributed to the widespread presence of pyrethroid mutations. Additionally, the substantially faster population growth of Varroa mites in Mediterranean climates, compared to temperate regions, is likely caused by the consistent presence of brood, potentially contributing to resistance development (Kraus and Page Jr 1995). Hence, it is advisable to urgently prioritize alternative control methods, especially in Mediterranean countries with a high mutation frequency. On the other hand, none of the Varroa samples from Hungary, Austria, and Iran and only 1 out of 172 samples from the Netherlands had L925 mutations, indicating low pyrethroid selection pressure in these areas. The dominance of susceptible mites (homozygous susceptible and heterozygous) across Europe except in southern parts indicates the rational use of pyrethroids. Indeed, control practices in Europe have demonstrated the utilization of alternative control measures (Brodschneider et al. 2023). Nevertheless, the presence of pyrethroid resistant individuals must be considered during the design of Varroa management programs, as their frequency can be rapidly increased in case of repetitive use.
A A map showing the distribution of L925V/I/M mutations in Europe Varroa destructor populations. Homozygous resistant individuals were represented by red color. Green color were used to show homozygous susceptible and heterezygous individuals (due to recessive nature of mutation). The size of the circles are proportional to the number of samples (1–200; 200–1000; 1000–2500 and > 2500). B Spread of L925V/I/M mutations across Türkiye. The samples that were obtained in this study were labeled with their codes on the map (see Table S1 for population codes). The other samples belong to Koç et al. (2021)
References
Akkaya H, Alkan S (2007) Beekeeping in Anatolia from the Hittites to the present day. J Apic Res 46(2):120–124
Alissandrakis E, Ilias A, Tsagkarakou A (2017) Pyrethroid target site resistance in Greek populations of the honey bee parasite Varroa destructor (Acari: Varroidae). J Apic Res 56(5):625–630
Ayan A, Aldemir OS (2018) Genetic characterization of Varroa destructor (Family: Varroidae) prevalent in honeybees (Apis mellifera) in the province of Aydin in Turkey. MAKÜ Sag Bil Enst Derg 6(1):26–32
Brodschneider R, Schlagbauer J, Arakelyan I, Ballis A, Brus J, Brusbardis V et al (2023) Spatial clusters of Varroa destructor control strategies in Europe. J Pest Sci 96(2):759–783
Carletto J, Martin T, Vanlerberghe-Masutti F, Brévault T (2010) Insecticide resistance traits differ among and within host races in Aphis gossypii. Pest Manag Sci 66(3):301–307
Carrière Y (2003) Haplodiploidy, sex, and the evolution of pesticide resistance. J Econ Entomol 96(6):1626–1640
De Rouck S, İnak E, Dermauw W, Van Leeuwen T (2023) A review of the molecular mechanisms of acaricide resistance in mites and ticks. Insect Biochem Mol Biol 159:103981
Dong K, Du Y, Rinkevich F, Nomura Y, Xu P, Wang L, Silver K, Zhorov BS (2014) Molecular biology of insect sodium channels and pyrethroid resistance. Insect Biochem Mol Biol 50:1–17
Elzen PJ, Baxter JR, Spivak M, Wilson WT (1999) Amitraz resistance in varroa: new discovery in North America. Am Bee J 139(5):362–362
FAOSTAT (2023) Livestock primary production. http://www.fao.org/faostat/en/#data/QL. Accessed 10 Aug 2023
Farjamfar M, Saboori A, González-Cabrera J, Hernández Rodríguez CS (2018) Genetic variability and pyrethroid susceptibility of the parasitic honey bee mite Varroa destructor (Acari: Varroidae) in Iran. Exp Appl Acarol 76:139–148
Feyereisen R, Dermauw W, Van Leeuwen T (2015) Genotype to phenotype, the molecular and physiological dimensions of resistance in arthropods. Pestic Biochem Physiol 121:61–77
Ffrench-Constant RH, Bass C (2017) Does resistance really carry a fitness cost? Curr Opin Insect Sci 21:39–46
González-Cabrera J, Davies TE, Field LM, Kennedy PJ, Williamson MS (2013) An amino acid substitution(L925V) associated with resistance to pyrethroids in Varroa destructor. PLoS ONE 8(12):e82941
González-Cabrera J, Rodriguez-Vargas S, Davies TE, Field LM, Schmehl D, Ellis JD, Krieger K, William-Son MS (2016) Novel mutations in the voltage-gated sodium channel of pyrethroid-resistant Varroa destructor populations from the Southeastern USA. PLoS ONE 11(5):e0155332
González-Cabrera J, Bumann H, Rodríguez-Vargas S, Kennedy PJ, Krieger K, Altreuther G, Hertel A, Hertlein G, Nauen R, Williamson MS (2018) A single mutation is driving resistance to pyrethroids in European populations of the parasitic mite. Varroa Destructor. J Pest Sci 91(3):1137–1144
Gregorc A, Sampson B (2019) Diagnosis of Varroa Mite (Varroa destructor) and sustainable control in honey bee (Apis mellifera) colonies—a review. Diversity 11(12):243
Gregorc A, Knight PR, Adamczyk J (2017) Powdered sugar shake to monitor and oxalic acid treatments to control varroa mites (Varroa destructor Anderson and Trueman) in honey bee (Apis mellifera) colonies. J Apic Res 56(1):71–75
Güler A, Demir M (2005) Beekeeping potential in Turkey. Bee World 86(4):114–119
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hernández-Rodríguez CS, Marín Ó, Calatayud F, Mahiques MJ, Mompó A, Segura I, Simó E, González-Cabrera J (2021) Large-scale monitoring of resistance to coumaphos, amitraz, and pyrethroids in Varroa destructor. InSects 12(1):27
Hillesheim E, Ritter W, Bassand D (1996) First data on resistance mechanisms of Varroa jacobsoni (Oud.) against tau-fluvalinate. Exp Appl Acarol 20(5):283–296
Hubert J, Nesvorna M, Kamler M, Kopecky J, Tyl J, Titera D, Stara J (2014) Point mutations in the sodium channel gene conferring tau-fluvalinate resistance in Varroa destructor. Pest Manag Sci 70(6):889–894
Jack CJ, Ellis JD (2021) Integrated pest management control of Varroa destructor (Acari: Varroidae), the most damaging pest of (Apis mellifera L.(Hymenoptera: Apidae)) colonies. J Insect Sci 21(5):6
Karatolos N, Gorman K, Williamson MS, Denholm I (2012) Mutations in the sodium channel associated with pyrethroid resistance in the greenhouse whitefly, Trialeurodes vaporariorum. Pest Manag Sci 68:834–838
Katoh K, Rozewicki J, Yamada KD (2019) MAFFT online service: multiple sequence alignment, inter- active sequence choice and visualization. Brief Bioinform 20(4):1160–1166
Katsavou E, Vlogiannitis S, Karp-Tatham E, Blake DP, Ilias A, Strube C, Kioulos I, Dermauw W, Van Leeuwen T, Vontas J (2020) Identification and geographical distribution of pyrethroid resistance mutations in the poultry red mite Dermanyssus gallinae. Pest Manag Sci 76(1):125–133
Kim S, Yoon KA, Cho S, Lee J, Lim Y, Lee SH (2022) Molecular and kinetic properties of three acetylcholinesterases in the Varroa mite, Varroa destructor. Pestic Biochem Physiol 188:105277
Kliot A, Ghanim M (2012) Fitness costs associated with insecticide resistance. Pest Manag Sci 68(11):1431–1437
Koç N, İnak E, Jonckheere W, Van Leeuwen T (2021) Genetic analysis and screening of pyrethroid resistance mutations in Varroa destructor populations from Turkey. Exp Appl Acarol 84(2):433–444
Kraus B, Page RE Jr (1995) Population growth of Varroa jacobsoni Oud in Mediterranean climates of California. Apidologie 26(2):149–157
Le Conte Y, Ellis M, Ritter W (2010) Varroa mites and honey bee health: can Varroa explain part of the colony losses? Apidologie 41(3):353–363
Lee SH, Kim YH, Kwon DH, Cha DJ, Kim JH (2015) Mutation and duplication of arthropod acetylcholinesterase: implications for pesticide resistance and tolerance. Pestic Biochem Physiol 120:118–124
Leigh JW, Bryant D (2015) Popart: full-feature software for haplotype network construction. Methods Ecol Evol 6:1110–1116
Liu Z, Tan J, Huang ZY, Dong K (2006) Effect of a fluvalinate-resistance-associated sodium channel mutation from varroa mites on cockroach sodium channel sensitivity to fluvalinate, a pyrethroid insecticide. Insect Biochem Mol Biol 36(11):885–889
Lodesani M, Colombo M, Spreafico M (1995) Ineffectiveness of Apistan® treatment against the mite Varroa jacobsoni Oud in several districts of Lombardy (Italy). Apidologie 26(1):67–72
Maggi M, Ruffinengo S, Damiani N, Sardella N, Eguaras M (2009) First detection of Varroa destructor resistance to coumaphos in Argentina. Exp Appl Acarol 47(4):317–320
Maggi MD, Ruffinengo SR, Mendoza Y, Ojeda P, Ramallo G, Floris I, Eguaras MJ (2011) Susceptibility of Varroa destructor (Acari: Varroidae) to synthetic acaricides in Uruguay: Varroa mites’ potential to develop acaricide resistance. Parasitol Res 108:815–821
Martin SJ (2004) Acaricide (pyrethroid) resistance in Varroa destructor. Bee World 85(4):67–69
Martin SJ, Brettell LE (2019) Deformed wing virus in honeybees and other insects. Annu Rev Virol 6:49–69
McMenamin AJ, Genersch E (2015) Honey bee colony losses and associated viruses. Curr Opin Insect Sci 8:121–129
Milani N, Della Vedova G (2002) Decline in the proportion of mites resistant to fluvalinate in a population of Varroa destructor not treated with pyrethroids. Apidologie 33:417–422
Millán-Leiva A, Marín Ó, Christmon K, VanEngelsdorp D, González-Cabrera J (2021a) Mutations associated with pyrethroid resistance in Varroa mites, a parasite of honey bees, are widespread across the USA. Pest Manag Sci 77(7):3241–3249
Millán-Leiva A, Marín Ó, De la Rúa P, Muñoz I, Tsagkarakou A, Eversol H, Christmon K, Van Engelsdorp D, González-Cabrera J (2021b) Mutations associated with pyrethroid resistance in the honey bee parasite Varroa destructor evolved as a series of parallel and sequential events. J Pest Sci 94:1505
Miller NJ, Sappington TW (2017) Role of dispersal in resistance evolution and spread. Curr Opin Insect Sci 21:68–74
Mitton GA, Quintana S, Mendoza Y, Eguaras M, Maggi MD, Ruffinengo SR (2021) L925V mutation in voltage-gated sodium channel of Varroa destructor populations from Argentina and Uruguay, with different degree of susceptibility to pyrethroids. Int J Acarol 47(5):374–380
Mitton GA, Meroi Arcerito F, Cooley H, Fernández de Landa G, Eguaras MJ, Ruffinengo SR, Maggi MD (2022) More than sixty years living with Varroa destructor: a review of acaricide resistance. Int J Pest Manag. https://doi.org/10.1080/09670874.2022.2094489
Mozes-Koch R, Slabezki Y, Efrat H, Kalev H, Kamer Y, Yakobson BA, Dag A (2000) First detection in Israel of fluvalinate resistance in the varroa mite using bioassay and biochemical methods. Exp Appl Acarol 24(1):35–43
Muntaabski I, Russo RM, Liendo MC et al (2020) Genetic variation and heteroplasmy of Varroa destructor inferred from ND4 mtDNA sequences. Parasitol Res 119:411–421. https://doi.org/10.1007/s00436-019-06591-5
Navajas M, Anderson DL, De Guzman LI, Huang ZY, Clement J, Zhou T, Le Conte Y (2010) New Asian types of Varroa destructor: a potential new threat for world apiculture. Apidologie 41(2):181–193
Ogihara MH, Kobayashi E, Morimoto N, Yoshiyama M, Kimura K (2021) Molecular analysis of voltage-gated sodium channels to assess τ-fluvalinate resistance in Japanese populations of Varroa destructor (Acari: Varroidae). Appl Entomol Zool 56:277–284
O’Reilly AO, Williamson MS, González-Cabrera J, Turberg A, Field LM, Wallace BA, Davies TE (2014) Predictive 3D modelling of the interactions of pyrethroids with the voltage-gated sodium channels of ticks and mites. Pest Manag Sci 70(3):369–377
Panini M, Reguzzi MC, Chiesa O, Cominelli F, Lupi D, Moores G, Mazzoni E (2019) Pyrethroid resistance in Italian populations of the mite Varroa destructor: a focus on the Lombardy region. Bull Insectol 72(2):227–232
Panini M, Chiesa O, Troczka BJ, Mallott M, Manicardi GC, Cassanelli S et al (2021) Transposon-mediated insertional mutagenesis unmasks recessive insecticide resistance in the aphid Myzus persicae. PNAS 118(23):e2100559118
Pettis JS (2004) A scientific note on Varroa destructor resistance to coumaphos in the United States. Apidologie 35(1):91–92
Ramsey SD, Ochoa R, Bauchan G, Gulbronson C, Mowery JD, Cohen A, Lim D, Joklik J, Cicero JM, Ellis JD, Hawthorne D, vanEngelsdorp D (2019) Varroa destructor feeds primarily on honey bee fat body tissue and not hemolymph. Proc Natl Acad Sci USA 116(5):1792–1801
Rosenkranz P, Aumeier P, Ziegelmann B (2010a) Biology and control of Varroa destructor. J Invertebr Pathol 103:96-S119
Rosenkranz P, Aumeier P, Ziegelmann B (2010b) Biology and control of Varroa destructor. J Invertebr Pathol 103:S96–S119
Rozas J, Ferrer-Mata A, Sanchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-OnsinsSanchez-Gracia SEA (2017) DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol Biol Evol 34:3299–3302
Sammataro D, Gerson U, Needham G (2000) Parasitic mites of honey bees: life history, implications, and impact. Annu Rev Entomol 45(1):519–548
Sammataro D, Untalan P, Guerrero F, Finley J (2005) The resistance of varroa mites (Acari: Varroidae) to acaricides and the presence of esterase. Int J Acarol 31(1):67–74
Solignac M, Cornuet JM, Vautrin D, Le Conte Y, Anderson D, Evans J, Cros-Arteil S, Navajas M (2005) The invasive Korea and Japan types of Varroa destructor, ectoparasitic mites of the Western honeybee (Apis mellifera), are two partly isolated clones. Proc R Soc B 272(1561):411–419
Sparks TC, Nauen R (2015) IRAC: mode of action classification and insecticide resistance management. Pestic Biochem Physiol 121:122–128
Spreafico M, Eördegh FR, Bernardinelli I, Colombo M (2001a) First detection of strains of Varroa destructor resistant to coumaphos. Results of laboratory tests and field trials. Apidologie 32(1):49–55
Stara J, Pekar S, Nesvorna M, Kamler M, Doskocil I, Hubert J (2019) Spatio-temporal dynamics of Varroa destructor resistance to tau-fluvalinate in Czechia, associated with L925V sodium channel point muta- tion. Pest Manag Sci 75(5):1287–1294
Tamura K, Stecher G, Kumar S (2021) MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 38(7):3022–3027
Traynor KS, Mondet F, de Miranda JR, Techer M, Kowallik V, Oddie MA, Chantawannakul P, McAfee A (2020) Varroa destructor: a complex parasite, crippling honey bees worldwide. Trends Parasitol 36(6):592–602
Tutun H, Koç N, Kart A (2018) Plant essential oils used against some bee diseases. TURJAF 6(1):34–45
Untergasser A, Nijveen H, Rao X, Bisseling T, Geurts R, Leunissen JA (2007) Primer3Plus, an enhanced web interface to Primer3. Nucleic Acids Res 35(suppl_2):W71–W74
Usherwood PNR, Davies TGE, Mellor IR, O’reilly AO, Peng F, Vais H, Khambay BPS, Field LM, Williamson MS (2007) Mutations in DIIS5 and the DIIS4–S5 linker of Drosophila melanogaster sodium channel define binding domains for pyrethroids and DDT. Febs Lett 581(28):5485–5492. https://doi.org/10.1016/j.febslet.2007.10.057
Vais H, Atkinson S, Pluteanu F, Goodson SJ, Devonshire AL, Williamson MS, Usherwood PNR (2003) Mutations of the para sodium channel of Drosophila melanogaster identify putative binding sites for pyrethroids. Mol Pharmacol 64:914–922
Van der Steen J, Vejsnæs F (2021) Varroa control: A brief overview of available methods. Bee World 98(2):50–56
Van Leeuwen T, Dermauw W (2016) The molecular evolution of xenobiotic metabolism and resistance in chelicerate mites. Annu Rev Entomol 61:475–498
Van Leeuwen T, Vanholme B, Van Pottelberge S, Van Nieuwenhuyse P, Nauen R, Tirry L et al (2008) Mitochondrial heteroplasmy and the evolution of insecticide resistance: non-Mendelian inheritance in action. PNAS 105(16):5980–5985
Van Leeuwen T, Dermauw W, Mavridis K, Vontas J (2020) Significance and interpretation of molecular diagnostics for insecticide resistance management of agricultural pests. Curr Opin Insect Sci 39:69–76
Vlogiannitis S, Jonckheere W, Laget D, de Graaf DC, Vontas J, Van Leeuwen T (2021a) Pyrethroid target-site resistance mutations in populations of the honey bee parasite Varroa destructor (Acari: Varroidae) from Flanders, Belgium. Exp Appl Acarol 85(2–4):205–221
Vlogiannitis S, Mavridis K, Dermauw W, Snoeck S, Katsavou E, Morou E, Harizanis P, Swevers L, Hemingway J, Feyereisen R, Van Leeuwen T, Vontas J (2021b) Reduced proinsecticide activation by cytochrome P450 confers coumaphos resistance in the major bee parasite Varroa destructor. PNAS 118(6):e2020380118
Wang R, Liu Z, Dong K, Elzen PJ, Pettis J, Huang Z (2002) Association of novel mutations in a sodium channel gene with fluvalinate resistance in the mite. Varroa Destr J Apic Res 41(1–2):17–25
Warrit N, Hagen TA, Smith DR, Çakmak I (2004) A survey of Varroa destructor strains on Apis mellifera in Turkey. J Apic Res 43(4):190–191
Wu S, Nomura Y, Du Y, Zhorov BS, Dong K (2017) Molecular basis of selective resistance of the bumblebee BiNav1 sodium channel to tau-fluvalinate. PNAS 114(49):12922–12927
Yalçınkaya A, Keskin N (2010) The investigation of honey bee diseases after colony losses in Hatay and Adana provinces of Turkey. Mellifera 10(20):24–31
Acknowledgements
The authors wish to acknowledge the Turkish Beekeepers Association for their invaluable support in facilitating communication with the beekeeping community. Gratitude is extended to the beekeepers who willingly provided us with Varroa samples and shared details regarding the last acaricide treatments.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This study was supported by Sirnak University Research Fund under grant number 2021.FNAP.13.02.01.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. EE and MR collected the samples. NKİ and Eİ performed the molecular analysis and analyzed the data. NKİ and Eİ wrote the original draft with inputs from EE. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Erdem, E., Koç-İnak, N., Rüstemoğlu, M. et al. Geographical distribution of pyrethroid resistance mutations in Varroa destructor across Türkiye and a European overview. Exp Appl Acarol 92, 309–321 (2024). https://doi.org/10.1007/s10493-023-00879-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-023-00879-z