Abstract
The present study was conducted to evaluate sublethal effects of B-azolemiteacrylic on the two-spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae). Female adults of T. urticae were exposed to LC10 and LC30 of the acaricide, and the effects on treated females and their offspring were evaluated. The results showed that the fecundity of F0 female adults treated with LC10 and LC30 of B-azolemiteacrylic was reduced by 30.9 and 39.2%, respectively. Longevity and oviposition period of the females were significantly reduced as well. The developmental duration of egg and deutonymph stage of the F1 generation were not significantly different from that of the control. The protonymph stage after LC30 treatment lasted significantly longer, whereas the larva, deutonymph and female stage were significantly shorter than the control. The oviposition period of the F1 generation was significantly shortened, the fecundity of each female decreased significantly, and the ratio of female-to-male was reduced too. Moreover, the average generation period of T. urticae after LC10 and LC30 treatments was shorter than that of the control, and the net production rate (R0), intrinsic rate of increase (rm) and finite rate of increase (λ) were all reduced by 33.3, 7.5 and 1.9% (LC10 treatment) and by 51.3, 14.8 and 3.6% (LC30 treatment), respectively. The population doubling time was prolonged by 7.5 and 14.8% after LC10 and LC30 treatments, respectively, compared with the control. These results indicate that B-azolemiteacrylic may effectively inhibit the development rate of the F0 and F1 populations of T. urticae, which will help design integrated strategies for the comprehensive control of T. urticae and rational use of pesticides in the field.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The two-spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae), is a destructive pest that causes serious damage to a wide range of crops and its host plants are over 140 families and 1100 species, including field crops, vegetables, fruits, and ornamental plants such as cotton, peach, strawberry, cucumber, soybean, eggplant, etc. (Maleknia et al. 2016; Mollaloo et al. 2017; Najafabadi et al. 2019). Tetranychus urticae uses its mouthparts to penetrate host cells and ingest cell contents (Wang et al. 2016), causing the leaves to lose green quickly until they wither and fall off. The population of T. urticae can be easily expanded because of its short life cycle and high reproductive potential (Saito et al. 2010; Nauen et al. 2001).
Using acaricides is the most common method to control T. urticae in recent years. However, the wide application of acaricides not only enables T. urticae to develop resistance (Brattsten et al. 1986; Van Leeuwen et al. 2010) but also leads to side effects on humans (García-Marí and González-Zamora 1999) and non-target organisms (Croft 1990), as well as the outbreak of secondary pests (Elzen 2001). One of the most used methods to manage resistance development and the conservation of biological agents is reduction of applied concentration (He et al. 2013; Song et al. 2013). Sublethal effects can be very delicate and affect populations at lower concentrations than the traditional ones (Stark and Banks 2003). In some cases, sublethal effects of pesticides can be integrated into pest control (Wang et al.2016). For instance, sublethal concentrations may increase pest developmental duration and reduce adult fecundity and longevity (Wang et al. 2016; Elzen 2001). Sublethal concentrations have also been applied to assess the selectivity of pesticides to beneficial mites (Alinejad et al. 2016, 2020; Bozhgani et al. 2019; Shahbaz et al. 2019). So, it is important to understand the sublethal effects and risks of acaracide application.
B-azolemiteacrylic shows excellent inhibition effects on mitochondrial respiratory chain complexes II, which mainly kills mites through contact and gastric toxicity. It also has quick effect, long duration of efficacy, broad spectrum of pests and low toxicity to non-target organisms such as bees, silkworms, fish and birds, and no interactive resistance to conventional acaricides such as abamectin and cypermethrin. It is safe for crops and environmentally friendly, and can meet the needs of integrated pest control (Song et al. 2017; Gong et al. 2017; Li 2016).
After application in the field, its toxicity will gradually decrease to sublethal doses with the extension of time and the change of environment. In addition to directly killing the target mites, some individuals will survive due to uneven application of the acaricide and other reasons, and suffer sublethal effects. As a result, the structure and size of the mite population will change again, and secondary pests will probably rise to become the primary ones (Quan et al. 2016; Han 2011). Therefore, understanding the sublethal effects of acaricides is key to evaluating their efficacy and acaricide risk management. Besides, there have been no reports on the sublethal effect of B-azolemiteacrylic on T. urticae. In the present study, the LC10 and LC30 of B-azolemiteacrylic were applied to T. urticae to investigate sublethal effects using the life-table method, and the related parameters were analyzed, aiming to evaluate the influence of sublethal effects on the development and reproduction of T. urticae, and to provide practical information for the rational use of B-azolemiteacrylic and comprehensive control of T. urticae in the field.
Material and methods
Mite colony maintenance and host plant
The stock population of T. urticae was originally obtained from Xinglong Mountain, Gansu Province, China, in May 2012, and it is known as a susceptible strain. Mites were reared on bean leaves (Phaseolus vulgaris L.) under acaricide-free conditions in an incubator at 25 ± 1 °C,75 ± 5% RH, and L16:D8 photoperiod.
Acaracide preparation
In this research a commercial formulation of B-azolemiteacrylic was used (SYP-9625, suspension concentrate 30%), produced by Baozhuo, Sinochem Crop Protection Products, China.
Concentration–response bioassay
Toxicity of pesticides to adults of two-spotted mites was tested using the leaf-dipping method (Meng 2002). A bean leaf was placed on a wet sponge in a Petri dish (7 cm diameter) and was surrounded with wet cotton to prevent the escape of mites. Thirty female adult spider mites were transferred to the leaf and prepared for bioassay. The control bean leaf was dipped with distilled water. B-azolemiteacrylic was diluted with distilled water, and five concentrations were prepared for testing: 0.8, 0.4, 0.2, 0.1 and 0.05 mg L−1. Every bean leaf with 30 adult spider mite females as mentioned above was dipped into each of the five B-azolemiteacrylic solution for 5 s, and then they were put in Petri dishes after blotting the spare pesticides. Concentration–response bioassay, comprising five concentrations and control, was carried out in four replications, with 180 females per replication and total sample size 720 females. The mortality covered the range of 10–90%. The LC50 value has a 95% confidence limit.
The mortality of adult females was recorded after 48 h of applying B-azolemiteacrylic. Mites were considered as dead if they did not show any reaction when touched by a brush. The Petri dishes were stored in a cabinet at 25 ± 1 °C, 75 ± 5% RH, and L16:D8 photoperiod.
Assessment of sublethal effects on F0 and F1 generations
Pre-ovipositional adult females from the stock population were transferred to fresh bean leaf discs (20 mites per 7-cm-diameter disc), each of which placed on wet cotton on a sponge in a Petri dish. After about 30–60 min, the discs were dipped for 5 s in distilled water (control) or B-azolemiteacrylic at LC10 or LC30. The sample size was 600 females. After 48 h, each survived female mite (F0 generation) was carefully moved to a new, fresh bean leaf disc with one adult male, which ensure that the pair could mate. Each concentration included 60 pairs. The females’ longevity and fecundity were recorded every 12 h until death. Eggs (F1 generation) laid by F0 generation were collected and transferred to new leaf discs, and each leaf disc only contained one egg. Each concentration included 60 eggs. Hatching rate and development of F1 generation were observed every 12 h. After they entered the adult stage, the sex ratio of F1 was calculated. Then all the females were subjected to further rearing, each paired with one male in a disc for 1 day. The longevity and fecundity were monitored until all females died.
Statistical analysis
In order to determine the LC values and sublethal concentrations, we used IBM SPSS v.24.0. The data obtained from F1 T. urticae were analyzed by one-way ANOVA followed by Tukey's honestly significant difference (HSD) test. Development duration, longevity, fecundity and demographic parameters of F1 T. urticae individuals were analyzed according to the two-sex life table procedure by using the Bootstrap method with 100,000 resamplings (Chi and Liu 1985; Chi 1988; Huang and Chi 2012). The paired bootstrap test was used to compare differences (Chi 2018). The computer program TWOSEX-MSChart (Chi 2018) was used to analyze the raw data. The survival rate curve was constructed using Kaplan–Meier test in IBM SPSS v.24.0.
Results
Estimation of LC10 and LC30 of B-azolemiteacrylic to Tetranychus urticae
The LC50 values of B-azolemiteacrylic on T. urticae was estimated to be 0.127 mg L−1 based on the leaf-dipping method, and then sublethal concentrations (LC10 and LC30) were calculated to be 0.043 and 0.009 mg L−1, respectively (Table 1).
Sublethal effects of B-azolemiteacrylic on F0 generation
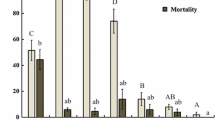
After being treated with B-azolemiteacrylic at sublethal doses LC10 and LC30 for 48 h, the influence on their longevity and oviposition period was recorded. Longevity and oviposition period of adult females were significantly shortened after being treated with LC10 and LC30 of B-azolemiteacrylic (Fig. 1). Compared to the control’s 23.4 days, the longevity was reduced by 13.4% (LC10) and 17.1% (LC30); the oviposition period dropped from 11.46 days (control) to 8.95 days (LC10) and 8.05 days (LC30). Besides, the longevity and oviposition period at LC30 treatment were significantly shorter than at LC10 (Fig. 1).
The total and daily fecundity of the treated mites were significantly lower than of the control (Table 2). Total fecundity for untreated mites was 76.1 eggs/individual, whereas this was reduced by 30.9% (LC10) and 39.2% (LC30) after treatment. Compared with the control, the daily fecundity of each female dropped by 11.7% (LC10) and 16.6% (LC30). Total and daily fecundity at LC30 treatment were significantly lower than at LC10 (Table 2). The hatching rate (χ2 = 1.604, d.f. = 2, P = 0.45) and sex ratio (χ2 = 1.343, d.f. = 2, P = 0.51) of the F1 generation did not differ among the three treatments.
Sublethal effects of B-azolemiteacrylic on F1 generation
The larva and adult periods and the average female longevity of the treated mites were significantly shortened (Table 3); at the LC10 treatment they were decreased by 5.4,13.3 and 8.0% respectively, whereas at the LC30 treatment reduction was 11.3, 17.4 and 9.4%, respectively. There were no significant differences in duration of the egg and deutonymph stages among all the treatments (Table 3).
The pre-oviposition period of the F1 generation in treatment had no significant difference from that of control, whereas the oviposition period was reduced by 20.1 and 20.7% at LC10 and LC30, respectively (Table 4). The post-oviposition period was significantly prolonged relative to the control, by 6.5% (LC10) and 10.6% (LC30). The total fecundity after LC10 and LC30 treatment was significantly lower than that of the control; it was decreased by 11 and 20.2%, respectively. Compared to the control, the sex ratio of F2 generation was also decreased (Table 4).
The survival curves of F1 generation were similar with that of the control (χ2 = 1.627, d.f. = 2, P = 0.44), all of type I (arched curve) (Fig. 2). The survival rate of both treatments were lower than that of the control except for the egg stage, and treated mites lived shorter than mites of the control group.
Fecundity (Mx, the average number of females produced by a female mite) earliest at LC30 treatment (on day 13), then at LC10 (day 14) and latest at the control (day 16). The peak was highest for the control, and lowest for the LC30 treated mites (Fig. 3), indicating that the capability of each adult to produce females decreased after being with a sublethal dose of B-azolemiteacrylic.
The net reproductive rate (R0) of both treatments was significantly lower than that of the control group—compared to the control, R0 was 33.3% (LC10) and 51.3% (LC30) lower (Table 5), indicating that the B-azolemiteacrylic had a great impact on the fecundity of the F1 generation. Compared with the control group, the mean generation time (T), the intrinsic rate of increase (rm), the finite rate of increase (λ), and the population doubling time for mites treated with both sublethal concentrations of B-azolemiteacrylic did not differ significantly (Table 5).
Discussion
In the present study, the biological parameters and demographic data related to different generations of T. urticae were investigated by applying sublethal concentrations of B-azolemiteacrylic. In recent years, a number of studies have been conducted for evaluating the lethal and sublethal effects of various pesticide groups such as tetrazine, tetronic acid, pyrazolium, pyrethroid, organophosphate, pyridine azomethines, and neonicotinoid derivatives on two-spotted spider mites, as well as its predatory mites (Hamedi et al. 2010, 2011; Lima et al. 2013; Alinejad et al. 2016; Bozhgani et al. 2018; Havasi et al. 2021). As one of the effective acrylonitrile group acaricides, however, no sublethal effects of B-azolemiteacrylic on biological parameters of T. urticae were known.
Our study indicated that when treated by B-azolemiteacrylic at LC30, the protonymph stage was significantly prolonged, and the larvae stage, adult stage and average life span were shortened. In addition, the oviposition period, fecundity and sex ratio from mites of the F1 generation treated at LC10 and LC30 were also decreased. These results corresponded with those of Havasi et al. (2018), in which the experimental concentration of diflovidazin played a negative role during all pre-adult developmental stages such as the egg, larva, protonymph, and deutonymph among males. Regarding females, no significant difference was observed between the immature stages for all the tested concentrations, except in egg and protonymph stages. Similar results were also seen in other investigations (Fan 2015; Tian 2017; Gao 2018). On the contrary, an increase in the concentration caused a significant difference during immature stages of T. urticae in males and females when treated by sublethal concentrations of bifenazate (Li et al. 2017). This might be caused by a different working mechanism of the two agents.
The results of the present study indicated the sublethal concentration had a certain inhibitory influence on the population growth of F0 generation, which was specifically displayed in decreases of longevity, oviposition period, fecundity and hatching rate, sex ratio of the next generation; the higher the concentration, the greater the degree in reduction. Negative sublethal effects of a variety of acaricides on, for instance, fecundity, life span, and oviposition period of pest mites have been reported by many researchers (Yong et al. 2011; Tao and Wu 2006; Xin et al. 2019; Li et al. 2016; Bozhgani et al. 2019; Havasi et al. 2020). Our results were consistent with those of Alinejad et al. (2015), in which a significant decrease happened in longevity after being treated with sublethal concentrations of fenazaquin. Similarly, a significant decrease occurred in the longevity for mites treated with azadirachtin at 64 and 128 ppm (Martínez-Villar et al. 2005), the reduction in fecundity was shown after treatment with a sublethal dose of spiromesifen (Marcic 2005). Reduction of the oviposition period can decrease the next-generation population size. Shortening of the life span would not only restrain fecundity, but also lower the potential damage caused by pest mites to their hosts.
Life-table parameters play a vital role in the comprehensive evaluation of the controlling effect of pesticides against mites. It is recommended to evaluate the sublethal effect of agents on target pests with the instantaneous rate of increase (r) or intrinsic rate of increase (rm) of the population, and conduct a comprehensive study with the life table technology (Stark and Wennergren 1995, Stark and Banks 2003). In this study, the net reproductive rate (R0) following the treatment of females from F0 generation with sublethal concentrations of B-azolemiteacrylic was significantly lower than that of the control group, but the intrinsic rate of increase (rm) and finite rate of increase (λ) were not significantly different from the control. The results were congruent with those of Wang et al. (2014a, b) and Marcic (2007), in which the sublethal doses of bifenthrin (LC10 and LC25) and spirodiclofen (6, 12, 24, 48, and 96 mg L−1) were examined on the two-spotted spider mite, respectively. Similar results about the effect of triflumuron on T. urticae were also seen in the study of Sáenz-de-Cabezón et al. (2006).
Based on the results of the present study, the exposure to sublethal concentrations of B-azolemiteacrylic had a negative effect on biological parameters of T. urticae (i.e., lower R0). B-azolemiteacrylic sublethal doses could effectively inhibit the developmental rates of F0 and F1 populations of T. urticae, and the higher the concentration, the stronger the inhibition effect. Besides, no proliferation effect was found in T. urticae population, which suggests that T. urticae may not easily develop resistance to B-azolemiteacrylic. This advantage is of positive significance to the formulation of integrated management strategies for T. urticae. Consequently, it is recommended that applying B-azolemiteacrylic at lower rates could lead to effective control of T. urticae. Nevertheless, most of the similar experiments including ours carried out under laboratory conditions may not be fully representative of a natural field, because environmental complexity, different plants and other natural characteristics cannot be 100% replicated in a small room. Further experiments carried out under greenhouse and field conditions are therefore needed.
References
Alinejad M, Kheradmand K, Fathipour Y (2015) Sublethal effects of fenazaquin on biological performance of the two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae): application of age-stage, two-sex life tables. Acarina 23:172–180
Alinejad M, Kheradmand K, Fathipour Y (2016) Assessment of sublethal effects of spirodiclofen on biological performance of the predatory mite, Amblyseius swirskii. Systematic Appl Acarol 21:375–384
Alinejad M, Kheradmand K, Fathipour Y (2020) Demographic analysis of sublethal effects of propargite on Amblyseius swirskii (Acari: Phytoseiidae): Advantages of using age-stage, two sex life table in ecotoxicological studies. Systematic Appl Acarol 24:906–917
Bozhgani NSS, Ghobadi H, Riahi E (2018) Sublethal effects of chlorfenapyr on the life table parameters of two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae). Systematic Appl Acarol 23(7):1342–1351
Bozhgani NSS, Kheradmand K, Talebi A (2019) The effects of Spiromesifen on life history traits and demographic parameters of predatory mite Neoseiulus californicus (Acari: Phytoseiidae) and its prey Tetranychus urticae Koch (Acari: Tetranychidae). Systematic Appl Acarol 24:1512–1525
Chi H (1988) Life-table analysis incorporating both sexes and variable development rates among individuals. Environ Entomol 17:26–34
Chi H (2018) TWOSEX-MSChart: a computer program for the age-stage, two-sex life table analysis. National Chung Hsing University, Taichung
Chi H, Liu H (1985) Two new methods for the study of insect population ecology. Bull Inst Zool Acad Sin (taipei) 24:225–240
Croft BA (1990) Arthropod biological control agents and pesticides. Wiley, New York
Elzen GW (2001) Lethal and sublethal effects of insecticide residues on Orius insidiosus (Hemiptera: Anthocoridae) and Geocoris punctipes (Hemiptera: Lygaeidae). J Econ Entomol 94:55–59
Fan X (2015) Study on the sublethal effect of bifenazate and etoxazole on Tetranychus urticae and Neoseiulus barkeri. Southwest University, Chongqing, pp 17–29
Gao JT (2018) Sublethal effect of scopoletin on the detoxification enzyme system of Tetranychus truncates. Gansu Agricultural University, Lanzhou, pp 28–34
García-Marí F, González-Zamora JE (1999) Biological control of Tetranychus urticae (Acari: Tetranychidae) with naturally occurring predators in strawberry plantings in Valencia, Spain. Exp Appl Acarol 23:487–495
Gong YJ, Chen JC, Jiang JY, Wang ZH, Cao LJ, Wei SJ (2017) Toxicity and field control effect of a new acaricide, B-azolemiteacrylic, on Tetranychus urticae. Pesticide 56(8):561–563
Hamedi N, Fathipour Y, Saber M (2010) Sublethal effects of fenpyroximate on life table parameters of the predatory mite Phytoseius plumifer. Biocontrol 55:271–278
Han WS, Wang LH, Sun H, Gao XW (2011) Progress in studies on sublethal effects of insecticides on insects. Plant Protect Guide China 31(11):15–20
Havasi M, Alsendi A, Bozhgani NSS, Kheradmand K, Sadeghi R (2021) The effects of bifenazate on life history traits and population growth of Amblyseius swirskii Athias-Henriot (Acari: Phytoseiidae). Systematic Appl Acarol 26(3):610–623
Havasi M, Kheradmand K, Mosallanejad H, Fathipour Y (2020) Life history traits and demographic parameters of Neoseiulus californicus McGregor (Acari: Phytoseiidae) treated with the Biomite (R). Systematic Appl Acarol 25(1):125–138
Havasi M, Kheradmand K, Mosallanejad H, Fathipour Y (2018) Sublethal effects of diflovidazin on life table parameters of two-spotted spider mite Tetranychus urticae (Acari: Tetranychidae). Int J Acarol 44(2–3):115–120
He YX, Zhao JW, Zheng Y, Weng QY, Biondi A, Desneux N, Wu KM (2013) Assessment of potential sublethal effects of various insecticides on key biological traits of the tobacco whitefly, Bemisia tabaci. Int J Biol Sci 9(3):246–255
Huang YB, Chi H (2012) Age-stage, two-sex life tables of Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae) with a discussion on the problem of applying female age specific life tables to insect populations. Insect Sci 19:263–273. https://doi.org/10.1111/j.1744-7917.2011.01424
Li B, Yu HB, Luo YM, Wu HF (2016) Synthesis of B-azolemiteacrylic and its acaricidal activity. Modern Pestic 15(6):15–16
Li YY, Fan X, Zhang GH, Liu YQ, Chen HQ, Liu H, Wang JJ (2017) Sublethal effects of bifenazate on life history and population parameters of Tetranychus urticae (Acari: Tetranychidae). Systematic Appl Acarol 22:148–158
Lima DB, Monteiro VB, Guedes RNC, Siqueira HAA, Pallini A, Gondim MGC (2013) Acaricide toxicity and synergism of fenpyroximate to the coconut mite predator Neoseiulus barkeri. Biocontrol 58:595–605
Maleknia B, Fathipour Y, Soufbaf M (2016) How greenhouse cucumber cultivars affect population growth and two-sex life table parameters of Tetranychus urticae (Acari: Tetranychidae). Int J Acarol 42:70–78
Marcic D (2005) Sublethal effects of tebufenpyrad on the eggs and immatures of two-spotted spider mite Tetranychus Urticae. Exp Appl Acarol 36(3):177–185
Marcic D (2007) Sublethal effects of spirodiclofen on life history and life-table parameters of two-spotted spider mite (Tetranychus urticae). Exp Appl Acarol 42:121–129
Martínez-Villar E, Sáenz-De-Cabezón FJ, Moreno-Grijalba F, Marco V, Pérez-Moreno I (2005) Effects of azadirachtin on the two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae). Exp Appl Acarol 35:215–222
Meng HS (2002) Comparison of the effects of two biological methods on the determination results of acaricide toxicity. Plant Prot 28(3):49–51
Mollaloo MG, Kheradmand K, Sadeghi R, Talebi AA (2017) Demographic analysis of sublethal effects of spiromesifen on Neoseiulus californicus (Acari: Phytoseiidae). Acarologia 57:571–580
Najafabadi SSM, Bagheri A, Hooei MAS (2019) Cucumber cultivar responses to two tetranychid mites, two-spotted spider mite and strawberry spider mite in greenhouses. Systematic Appl Acarol 24:1383–1393
Nauen R, Stumpf N, Elbert A, Zebitz CP, Kraus W (2001) Acaricide toxicity and resistance in larvae of different strains of Tetranychus urticae and Panonychus ulmi (Acari: Tetranychidae). Pest Manag Sci 57:253–261
Quan LF, Zhang HJ, Sun LN, Li YY, Yan WT, Yue Q, Qiu GS (2016) Advances in studies on the sublethal effects of insecticides on pests. Chinese J Agron 6(05):33–38
Sáenz-de-Cabezón FJ, Martínez-Villar E, Moreno F, Marco V, Pérez-Moreno I (2006) Influence of sublethal exposure to triflumuron on the biological performance of Tetranychus urticae Koch (Acari: Tetranychidae). Span J Agric Res 4:167–172
Shahbaz M, Khoobdel M, Khanjani M, Hosseininia A, Khederi SJ (2019) Sublethal effects of acetamiprid on biological aspects and life table of Amblyseius swirskii (Acari: Phytoseiidae) fed on Aleuroclava jasmini (Hemiptera: Aleyrodidae). Systematic Appl Acarol 24:814–824
Song YQ, Dong JF, Sun HZ (2013) Chlorantraniliprole at sublethal concentrations may reduce the population growth of the Asian corn borer, Ostrinia furnacalis (Lepidoptera: Pyralidae). Acta Entomol Sin 56(4):446–451
Song YQ, Feng C, Liu SW, Zhang JL, Li B (2017) Bioactivity and application of B-azolemiteacrylic, a new acaricide. Pesticide 56(09):628–631
Stark JD, Wennergren U (1995) Can population effects of pesticides be predicted from demographic toxicological studies. J Econ Entomol 88(5):1089–1096
Stark JD, Banks JE (2003) Population level effects of pesticides and other toxicants on arthropods. Annu Rev Entomol 48:505–519
Tao SQ, Wu F (2006) Effect of the sublethal dose of chlorpyrifos on the experimental population dynamics of Tetranychus cinnabarinus. Chin J Appl Ecol 07:1351–1353
Saito T, Tabata K, Kohnot S (1983) Mechanisms of acaricide resistance with emphasis on dicofol. In: Georghiou GP, Saito T (eds) Pest Resistance to Pesticides. PlenumPress, New York, pp 429–444
Van Leeuwen T, Vontas J, Tsagkarakou A, Tirry L (2010) Acaricide resistance mechanisms in the two-spotted spider mite Tetranychus urticae and other important Acari: a review. Insect Biochem Mol Biol 40:563–572
Tian YJ (2017) Study on the sublethal effect of B-azolemiteacrylic and Cyflumetofen on Tetranychus cinnabarinus and the Neoseiulus californicus. Sichuan Agricultural University, Chengdu, pp 24–46
Wang S, Tang X, Wang L, Zhang Y, Wu Q, Xie W (2014a) Effects of sublethal concentrations of bifenthrin on the two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae). Systematic Appl Acarol 19:481–490
Wang SL, Tang XF, Wang L, Zhang YJ, Wu QJ, Xie W (2014b) Effects of sublethal concentrations of bifenthrin on the two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae). Syst Appl Acarol 19(4):481–490
Wang LZ, Xie YJ, Wu W, Wang QJ, Shao L (2016) Sublethal effects of spinetoram on the two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae). Pestic Biochem Physiol 132:102–107
Xin TR, Lian LIXE, Wang J, Zou ZW, Xia B (2019) Effects of sublethal concentration of diflubenzuron on the growth and reproduction of Tetranychus cinnabarinus. Appl Entomol 56(04):736–743
Yong XJ, Zhang YQ, Ding W (2011) Sublethal effect of scopoletin on the experimental population of Tetranychus cinnabarinus. Acta Entomol Sin 54(12):1377–1383
Acknowledgements
We would like to express our appreciation to Dr. Zhi-Qiang Zhang (Landcare Research and the University of Auckland, New Zealand) for his reading the early draft of this manuscript and providing some valuable comments. This research is supported by the Fund for key course construction of postgraduate of Gansu Agricultural University (no. GSAU-ZDKC-2016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted (the study was approved).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shang, S., Chang, Y., Li, WZ. et al. Effects of B-azolemiteacrylic on life-history traits and demographic parameters of two-spotted spider mite, Tetranychus urticae (Acari: Tetranychidae). Exp Appl Acarol 86, 61–71 (2022). https://doi.org/10.1007/s10493-021-00678-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-021-00678-4