Abstract
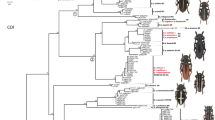
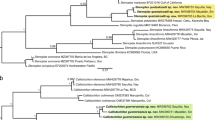
Species of the genus Steganacarus are soil-living oribatid mites (Acari, Phthiracaridae) with a ptychoid body. The phylogeny and species status of the species of Steganacarus are not resolved, some authors group all ten German species of Steganacarus within the genus Steganacarus whereas others split them into three subgenera, Steganacarus, Tropacarus and Atropacarus. Additionally, two species, S. magnus and T. carinatus, comprise morphotypes of questionable species status. We investigated the phylogeny and species status of ten European Steganacarus species, i.e. S. applicatus, S. herculeanus, S. magnus forma magna, S. magnus forma anomala, S. spinosus, Tropacarus brevipilus, T. carinatus forma carinata, T. carinatus forma pulcherrima, Atropacarus striculus and Rhacaplacarus ortizi. We used two molecular markers, a 251 bp fragment of the nuclear gene 28S rDNA (D3) and a 477 bp fragment of the mitochondrial COI region. The phylogeny based on a combined analysis of D3 and COI separated four subgenera (Steganacarus, Tropacarus and Atropacarus, Rhacaplacarus) indicating that they form monophyletic groups. The COI region separated all ten species of the genus Steganacarus and showed variation within some species often correlating with the geographic origin of the species. Resolution of the more conserved D3 region was limited, indicating that radiation events are rather recent. Overall, our results indicate that both genes alone cannot be used for phylogeny and barcoding since variation is too low in D3 and too high in COI. However, when used in combination these genes provide reliable insight into the phylogeny, radiation and species status of taxa of the genus Steganacarus.



Similar content being viewed by others
References
Aoki J (1980) A revision of the oribatid mites of Japan. I. The families Phthiracaridae and Oribotritiidae. Bull Inst Environ Sci Technol Yokohama 6:1–88
Avanzati AM, Bernini F (1989) Notulae Oribatologicae L. The redescription of Steganacarus (S.) spinosus (Sellnick, 1920) (Acarida, Oribatida). Redia 62:149–167
Avanzati AM, Baratti M, Bernini F (1994) Notulae Oribatologicae LIX. Taxonomy and biogeography of the genus Steganacarus Ewing, 1917 (Acari, Oribatida) in Italy. Redia 77:313–347
Berlese A (1883) Sopra due nuovi generi di Acari italiani. Atti R Accad Padova 33:45–52
Berlese A (1887) Acari, Myriapoda et Scorpiones hucusque in Italia reperta, Portici et Padova. Fasc. 36
Bernini F, Avanzati AM (1988a) Taxonomic revision of Steganacarus (Steganacarus) magnus (Nicolet, 1855) (Acari, Oribatida). J Nat Hist 22:435–464
Bernini F, Avanzati AM (1988b) Notulae Oribatologicae XLVII. Intraspecific variability in Tropacarus: the example of Steganacarus (Tropacarus) pulcherrimus (Berlese, 1887) junior synonym of S. (T.) carinatus (Koch, 1841) (Acarida, Oribatida). Int J Acarol 14:107–114
Bernini F, Avanzati AM (1989) Notulae Oribatologicae XLVIII. The taxonomic position of Steganacarus brevipilus (Berlese, 1923) and Tropacarus in the Steganacaridae system (Acarida, Oribatida). Int J Acarol 15:5–16
Bernini F, Avanzati AM (1991) A new approach to the systematics of the genus Steganacarus. In: Schuster R, Murphy PW (eds) The acari. Reproduction, development and life-history strategies, Chapman and Hall, London, pp 335–342
Bernini F, Avanzati AM, Petrucci R (1988) Genetic variation in the soil phthiracarid mite, Steganacarus (S.) magnus (Nicolet, 1855) (Acari, Oribatida) (Notulae Oribatologicae XL). Z Zool Syst Evol-Forsch 26:104–113
Collins RA, Cruickshank RH (2013) The seven deadly sins of DNA barcoding. Mol Ecol Res 13:969–975
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772
Deagle BE, Jarman SN, Coissac E, Pompanon F, Taberlet P (2015) DNA metabarcoding and the cytochrome c oxidase subunit I marker: not a perfect match. Biol Lett. doi:10.1098/rsbl.2014.0562
Domes K, Norton NA, Maraun M, Scheu S (2007) Reevolution of sexuality breaks Dollo’s law. Proc Natl Acad Sci USA 104:7139–7144
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotech 3:294–299
Gilyarov MS, Krivolutskij AD (1975) Opredelitel obitayushchikh v pochve kleshchej Sarcoptiformes. Izd. Nauka (Key for the identification of soil mites Sarcoptiformes, Izdatelstvo Nauka) Moskva, 492 pp (In Russian)
Guindon S, Gascuel O (2003) A simple, fast and accurate method to estimate large phylogenies by maximum-likelihood. Syst Biol 52:696–704
Hall T (2011) BioEdit: an important software for molecular biology. GERF Bull Biosci 2:60–61
Hebert PDN, Ratnasingham S, deWaard JR (2003) Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related Species. Proc R Soc Lond B (Suppl.) 270:S96–S99
Hebert PDN, Stoeckle MY, Zemlak TS, Francis CM (2004) Identification of birds through DNA barcodes. PLoS Biol 2(10):e312
Heethoff M, Domes K, Laumann M, Maraun M, Norton RA, Scheu S (2007) High genetic divergences indicate ancient separation of parthenogenetic lineages of the oribatid mite Platynothrus peltifer (Acari, Oribatida). J Evol Biol 20:392–402
Hogg ID, Hebert PDN (2004) Biological identification of springtails (Hexapoda: collembola) from the Canadian Arctic, using mitochondrial DNA barcodes. Can J Zool 82:749–754
Huang D, Meier R, Todd PA, Chou LM (2008) Slow mitochondrial COI sequence evolution at the base of the metazoan tree and its implications for DNA barcoding. J Mol Evol 66:167–174
Illig J, Norton RA, Langel R, Scheu S, Maraun M (2005) Where are the decomposers? Uncovering the soil food web of a tropical montane rain forest in Southern Ecuador using stable isotopes (15 N). J Trop Ecol 21:589–593
Kamill BW, Baker AS (1980) The genus Atropacarus (Acari: Cryptostigmata). Bull Br Mus Nat Hist 39:189–204
Kempson D, Lloyd M, Ghelardi R (1963) A new extractor for woodland litter. Pedobiologia 3:1–21
Laumann M, Norton RA, Weigmann G, Scheu S, Maraun M, Heethoff M (2007) Speciation in the parthenogenetic oribatid mite genus Tectocepheus (Acari, Oribatida) as indicated by molecular phylogeny. Pedobiologia 51:111–122
Litvaitis MK, Nunn G, Thomas WK, Kocher TD (1994) A molecular approach for the identification of meiofaunal turbellarians (Platyhelminthes, Turbellaria). Mar Biol 120:437–442
Maraun M, Heethoff M, Scheu S, Weigmann G, Norton RA, Thomas RH (2003) Radiation in sexual and parthenogenetic oribatid mites (Oribatida, Acari) as indicated by genetic divergence of closely related species. Exp Appl Acarol 29:265–277
Maraun M, Heethoff M, Schneider K, Scheu S, Weigmann G, Cianciolo J, Thomas RH, Norton RA (2004) Molecular phylogeny of oribatid mites (Oribatida, Acari): evidence for multiple radiations of parthenogenetic lineages. Exp Appl Acarol 33:183–201
Niedbala W (1986) Système des Phthiracaroidea (Oribatida, Euptyctima). Acarologia 27:61–84
Niedbala W (1992) Phthiracaroidea, Acari, Oribatida. Elsevier PWN Polish Scientific Studies, Amsterdam
Niedbala W (2011) Ptyctimous mites (Acari: Oribatida) of the Palaearctic Region. Systematic part. Fauna Mundi, vol 4. Museum and Institute of Zoology Polish Academy of Sciences. Natura optima dux Foundation Press, Warszawa, p 472
Norton RA, Behan-Pelletier VM (2009) Suborder Oribatida. In: Krantz GW, Walter DE (eds) A manual of acarology. Texas Tech University Press, Lubbock, USA pp 430–564
Pachl P, Domes K, Schulz G, Norton RA, Scheu S, Schaefer I, Maraun M (2012) Convergent evolution of defense mechanisms in oribatid mites (Acari, Oribatida) shows no “ghosts of predation past”. Mol Phylogen Evol 65:412–420
Pérez-Inigo C (1970) Bioespeleología de la cueva de Ojo Guareña. Ácaros oribátidos. Bol R Soc Esp Hist Nat (Biol) 67:143–160
Pigliucci M, Avanzati AM, Bernini F, Petrucci R (1990) Genetic structure in populations and species of Steganacarus soil mites (Acarida, Oribatida, Phthiracaroidea). Int J Acarol 16:67–76
Pollierer MM, Langel R, Körner C, Maraun M, Scheu S (2007) The underestimated importance of belowground carbon input for forest soil food webs. Ecol Lett 10:729–736
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Rosenberger M, Maraun M, Scheu S, Schaefer I (2013) Pre- and post-glacial diversifications shape genetic complexity of soil-living microarthropod species. Pedobiologia 56:79–87
Salomone N, Frati F, Bernini F (1996) Investigation on the taxonomic status of Steganacarus magnus and Steganacarus anomalus (Acari: Oribatida) using mitochondrial DNA sequences. Exp Appl Acarol 20:607–615
Salomone N, Emerson BC, Hewitt GM, Bernini F (2002) Phylogenetic relationships among the Canary Island Steganacaridae (Acari, Oribatida) inferred from mitochondrial DNA sequence data. Mol Ecol 11:79–89
Schaefer I, Norton RA, Scheu S, Maraun M (2010) Precambrian mites colonized land and formed parthenogenetic clusters. Mol Phylogen Evol 57:113–121
Stamatakis SA, Ludwig T, Meier H (2005) RAxML-III: a fast program for maximum likelihood-based inference of large phylogenetic trees. Bioinformatics 21:456–463
Subias LS (2014) Listado sistematico, sinonimico y biogeografico de los acaros oribatidos (Acariformes, Oribatida) del Mundo (1758-–002). Graellsia 60:3–305. http://escalera.bio.ucm.es/usuarios/bba/cont/docs/RO_1.pdf
Weigmann G (2006) Hornmilben (Oribatida). Die Tierwelt Deutschlands. Goecke & Evers, Keltern
Young MR, Behan-Pelletier VM, Hebert PDN (2013) Revealing the hyperdiverse mite fauna of subarctic Canada through DNA barcoding. PLoS One 7(11):e48755
Acknowledgments
We thank Patrick Pachl and Garvin Schulz for help in the molecular laboratory and during phylogenetic analysis, and Roy A. Norton and Gerd Weigmann for valuable comments on an earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10493_2015_9905_MOESM1_ESM.pptx
Figure 4. Maximum likelihood tree of ten species of the genus Steganacarus based on the protein sequence of the mitochondrial COI gene. Numbers on nodes are bootstrap support and posterior probabilities
10493_2015_9905_MOESM2_ESM.pptx
Figure 5. Maximum likelihood tree of ten species of the genus Steganacarus based on the nucleotide sequences of the mitochondrial COI gene, the 3rd codon positions were excluded from the analysis. Numbers on nodes are bootstrap support and posterior probabilities
Rights and permissions
About this article
Cite this article
Kreipe, V., Corral-Hernández, E., Scheu, S. et al. Phylogeny and species delineation in European species of the genus Steganacarus (Acari, Oribatida) using mitochondrial and nuclear markers. Exp Appl Acarol 66, 173–186 (2015). https://doi.org/10.1007/s10493-015-9905-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-015-9905-4