Abstract
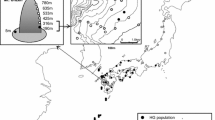
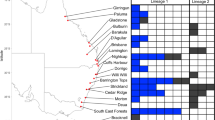
Stigmaeopsis celarius Banks (hereafter Sc) is a spider mite living and feeding on the leaves of various bamboo species such as Moso bamboo [Phyllostachys edulis (=P. pubescens)] and Pleioblastus spp. (Poaceae). A previous phylogenetic study revealed a cryptic, phylogenetic sister species to Sc (hereafter Ss). Although its life type appears to be similar to that of Sc, individuals of Ss make much smaller nests compared with Sc, and the nests have been found mostly on Nezasa bamboo (Pleioblastus argenteostriatus). To investigate whether Sc and Ss are reproductively isolated, we explored their populations in southwestern Japan, and crossed them to examine mating behaviors and fertilization success. Field surveys revealed that the nests of these two species occur on the same leaves and, thus, the individuals of these species may make frequent contact. Reciprocal crosses suggested that the two species are reproductively isolated. Though Sc males have tried to mate with Ss females, copulation seldom occurred because of their long opisthosoma (hind body), which prevented the insertion of the aedeagus into the genitalia of Ss females. In contrast, most Ss males ignored Sc females, and eggs were not fertilized even in the few cases where copulation appeared to occur. These results suggest that strong selection pressure is imposed on body length to prevent interspecific hybridization in the contact area of these species.



Similar content being viewed by others
References
Ben-David T, Gerson U, Morin S (2009) Asymmetric reproductive interference between two closely related spider mites: Tetranychus urticae and T. turkestani (Acari: Tetranychidae). Exp Appl Acarol 48:213–227
Bull CM (1991) Ecology of parapatric distributions. Annu Rev Ecol Syst 22:19–36
Cone WW (1985) Mating and chemical communication. In: Helle W, Sabelis MW (eds) Spider mites: their biology, natural enemies, and control, vol 1A. Elsevier, Amsterdam, pp 243–251
Coyne JA, Orr HA (2004) Speciation. Sinauer Associates, Incorporated Publishers, Sunderland, MA
Fujimoto H, Hiramatsu T, Takafuji A (1996) Reproductive interference between Panonychus mori Yokoyama and P. citri (McGregor) (Acari: Tetranychidae) in peach orchards. Appl Entomol Zool 31:59–65
Futuyma DJ (1998) Evolutionary biology, 3rd edn. Sinauer Associates, Incorporated Publishers, Sunderland, MA
Gröning J, Hochkirch A (2008) Reproductive interference between animal species. Q Rev Biol 83:257–282
Hochkirch A, Gröning J, Bücker A (2007) Sympatry with the devil: reproductive interference could hamper species coexistence. J Anim Ecol 76:633–642
Ito K, Fukuda T (2009) Molecular phylogeny of Stigmaeopsis spider mites (Acari: Tetranychidae) based on the Cytochrome Oxidase subunit I (COI) region of mitochondrial DNA. Appl Entomol Zool 44:343–355
Ito K, Yokoyama N, Hayakawa H, Minamiya Y, Yokoyama J, Fukuda T (2011) Molecular phylogenetic relationship of Stigmaeopsis spider mites (Acari: Tetranychidae) collected from Yamagata Prefecture. Bull Yamagata Univ Nat Sci 17:19–29
Kishi S, Nishida T, Tsubaki Y (2009) Reproductive interference determines persistence and exclusion in species interactions. J Anim Ecol 78:1043–1049
Kuno E (1992) Competitive exclusion through reproductive interference. Res Popul Ecol 34:275–284
Kyogoku D, Nishida T (2012) The presence of heterospecific males causes an Allee effect. Popul Ecol 54:391–395
Kyogoku D, Nishida T (2013) The mechanism of the fecundity reduction in Callosobruchus maculatus caused by Callosobruchus chinensis males. Popul Ecol 55:87–93
Lindquist EE (1985) Anatomy, phylogeny and systematics. 1.1.1 External anatomy. In: Helle W, Sabelis MW (eds) Spider mites: their biology, natural enemies, and control, vol 1A. Elsevier, Amsterdam, pp 3–28
Mori K (2000) Factors causing variation in social systems of spider mites, Schizotetranychus celarius species group (Acari: Tetranychidae), Ph.D. thesis, Hokkaido University, Sapporo
Mori K, Saito Y (2004) Variation in social behavior within a spider mite genus, Stigmaeopsis (Acari: Tetranychidae). Behav Ecol 16:232–238
Mori K, Saito Y (2006) Communal relationships in a social spider mite, Stigmaeopsis longus (Acari: Tetranychidae): an equal share of labor and reproduction between nest mates. Ethology 112:134–142
Mori K, Saito Y (2013) Genetic basis of woven nest size in subsocial spider mites. Exp Appl Acarol 60:463–469
Nagel L, Schluter D (1998) Body size, natural selection, and speciation in sticklebacks. Evolution 52:209–218
Oku K (2014) Sexual selection and mating behavior in spider mites of the genus Tetranychus (Acari: Tetranychidae). Appl Entomol Zool 49:1–9
Oku K, Saito Y (2014) Do males evaluate female age for precopulatory mate guarding in the two-spotted spider mite? J Ethol 32:1–6
Okuzaki Y, Takami Y, Sota T (2010) Resource partitioning or reproductive isolation: the ecological role of body size differences among closely related species in sympatry. J Anim Ecol 79:383–392
Ryan TA (1960) Significance tests for multiple comparison of proportions, variances and other statistics. Psychol Bull 57:318–328
Saito Y (1983) The concept of “life types” in Tetranychinae: an attempt to classify the spinning behaviour of Tetranychinae. Acarologia 24:377–391
Saito Y (1985) Life types of spider mites. In: Helle W, Sabelis MW (eds) Spider mites: their biology, natural enemies, and control, vol 1A. Elsevier, Amsterdam, pp 253–264
Saito Y (1987) Extraordinary effects of fertilization status on the reproduction of an arrhenotokous and sub-social spider mite (Acari: Tetranychidae). Res Popul Ecol 29:57–71
Saitō Y (1990) Two new spider mite species of the Schizotetranychus celarius complex (Acari: Tetranychidae): Study on variation of Schizotetranychus celarius (Banks) III. Appl Entomol Zool 25:389–396
Saito Y (1997) Sociality and kin selection in Acari. In: Choe JC, Crespi BJ (eds) The evolution of social behaviour in insects and arachnids. Cambridge University Press, London, pp 443–457
Saito Y (2010) Plant mites and sociality. Springer, Tokyo
Saitô Y, Takahashi K (1982) Study on variation of Schizotetranychus celarius (Banks) II. Comparison of mode of life between two sympatric forms (Acarina: Tetranychidae). Jpn J Ecol 32:69–78 (in Japanese)
Saito Y, Mori K, Chittenden AR (1999) Body characters reflecting the body size of spider mites in flattened specimens (Acari, Tetranychidae). Appl Entomol Zool 34:383–386
Saito Y, Mori K, Sakagami T, Lin J (2004) Reinstatement of the genus Stigmaeopsis Banks, with descriptions of two new species (Acari: Tetranychidae). Ann Entomol Soc Am 97:635–646
Sato Y, Saito Y (2006) Nest sanitation in social spider mites: interspecific differences in defecation behavior. Ethology 112:664–669
Sato Y, Saito Y, Mori K (2000a) Reproductive isolation between populations showing different aggression in a subsocial spider mite, Schizotetranychus miscanthi Saito (Acari: Tetranychidae). Appl Entomol Zool 35:605–610
Sato Y, Saito Y, Mori K (2000b) Patterns of reproductive isolation between two groups of Schizotetranychus miscanthi Saito (Acari: Tetranychidae) showing different male aggression traits. Appl Entomol Zool 35:611–618
Sato Y, Saito Y, Sakagami T (2003) Rules for nest sanitation in a social spider mite, Schizotetranychus miscanthi Saito (Acari: Tetranychidae). Ethology 109:713–724
Sato Y, Saito Y, Chittenden A (2008) The parapatric distribution and contact zone of two forms showing different male-to-male aggressiveness in a social spider mite, Stigmaeopsis miscanthi (Acari: Tetranychidae). Exp Appl Acarol 44:265–276
Schluter D (1998) Ecological cause of speciation. In: Howard DJ, Berlocher SH (eds) Endress forms. Oxford University Press, New York, pp 114–129
Schluter D, Nagel LM (1995) Parallel speciation by natural selection. Am Nat 146:292–301
Shimizu N, Mori N, Kuwahara Y (2001) Aggregation pheromone activity of the female sex pheromone, beta-acaridial, in Caloglyphus polyphyllae (Acari: Acaridae). Biosci Biotechnol Biochem 65:1724–1728
Suzuki S (1996) Illustrations of Japanese Bambusaceae. Jukai Shorin, Funabashi (in Japanese)
Takafuji A, Kuno E, Fujimoto H (1996) Reproductive interference and its consequences for the competitive interactions between two closely related Panonychus spider mites. Exp Appl Acarol 21:379–391
Tanabe T, Sota T (2008) Complex copulatory behavior and the proximate effect of genital and body size differences on mechanical reproductive isolation in the millipede genus Parafontaria. Am Nat 171:692–699
Uchimura E (2005) Bamboo, bamboo grass picture book: type, features and applications. Soshinsha, Tokyo (in Japanese)
Uesugi R, Kunimoto Y, Osakabe Mh (2009a) The fine-scale genetic structure of the two-spotted spider mite in a commercial greenhouse. Exp Appl Acarol 47:99–109
Uesugi R, Sasawaki T, Osakabe Mh (2009b) Evidence of a high level of gene flow among apple trees in Tetranychus urticae. Exp Appl Acarol 49:281–290
Acknowledgements
We thank Drs. Kotaro Mori, Takane Sakagami, Ken Sahara for their valuable suggestions. We are indebted to Mr. Y. Kumekawa and Ms. K. Tamura, Drs. H. Hayakawa and Y. Minamiya for providing sampling material. This study was supported by JSPS-KAKENHI 24570014, China Recruitment Program of Global Experts (Foreign Experts) (2012-323) and State Administration of Foreign Experts Affairs Key Project for Introduction of Foreign Experts (SZ2013003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chae, Y., Yokoyama, N., Ito, K. et al. Reproductive isolation between Stigmaeopsis celarius and its sibling species sympatrically inhabiting bamboo (Pleioblastus spp.) plants. Exp Appl Acarol 66, 11–23 (2015). https://doi.org/10.1007/s10493-014-9865-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-014-9865-0