Abstract
Epilepsy is a chronic neurological disorder that may be diagnosed and monitored using routine diagnostic tests like Electroencephalography (EEG). However, manual introspection and analysis of EEG signals is presently difficult and repetitive task even for experienced neuro-technologists with high false-positive rates and inter- and intra-rater reliability. Software advancements using Artificial Intelligence (AI) algorithms have the potential to early detect and predict abnormal patterns observed in EEG signals. The present review focuses on systematically reporting software advancements and their implementation using hardware systems in automatic epilepsy diagnosis and seizure detection for the past 10 years. Traditional, hybrid, and end-to-end AI-based pipelines and associated EEG datasets have been discussed. The review summarizes and compares reported articles, datasets, and patents through various subjective and objective parameters in this field. Latest advancements demonstrate that AI-based pipelines can reduce the introspection time by at least 50% without compromising the diagnostic accuracy or abnormal event detection. A significant rise in hardware implementation of software-based pipelines, end-to-end deep learning architectures for real-time analysis, and granted patents has been noticed since 2011. More than twenty-eight datasets have been developed to automatically diagnose epileptic EEG signals from 2001 to 2023. Extensive analysis using explainability tools, cross-dataset generalizations, reproducibility analysis, and ablation experiments can further improve the existing AI-based pipelines in this field. There is a need for the development of standardized protocols for data collection and its AI pipeline for a robust, inter- and intra-rater reliability-free, and real-time automatic epilepsy diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Epilepsy is a chronic neurological disorder marked by sudden recurrent episodes of sensory disturbance, motor activity, or loss of consciousness associated with abnormal electrical discharges in the brain (Dumanis et al. 2017; Gotman 2011). It is a non-communicable disorder affecting one in twenty-six individuals worldwide (Dumanis et al. 2017). More than 80% Persons With Epilepsy (PWE) are living in low-middle-income countries (Dumanis et al. 2017; Amudhan et al. 2015). With an increasing no. of PWE, their diagnosis, treatment, and management have become a burden for the community (Boubchir et al. 2017; Boonyakitanont et al. 2020).
According to the International League Against Epilepsy (ILAE) classification of Epilepsies (2021), there are three steps to epilepsy diagnosis i.e., seizure type classification, epilepsy type classification, and specific epilepsy syndrome diagnosis (Pressler et al. 2021). It depends on the etiology, and the prognosis during seizure type and epilepsy type classification (Pressler et al. 2021).
A patient with a history of unconsciousness or seizure-like symptoms is recommended to undergo scalp Electroencephalography (EEG) to confirm or rule out epilepsy. EEG is a cost-effective method to record and monitor the activities of the human brain. It is attached to hardware and software systems to view the real-time activity of the brain. The software screen shows a continuous real-time video of EEG signals which are fetched from the electrodes attached to the patient’s scalp. In day-to-day clinical practice, a routine scalp EEG takes about thirty minutes. It generates about one-eighty pages of EEG recording for a paper speed of 3 cm for these thirty minutes of recording (Deuschl 1999). The length of EEG recording may vary and may be done for a short-term to long-term period (Xinghua et al. 2020; Smith 2005).
Seizure patterns like pre-ictal, peri-ictal, pro-ictal, and inter-ictal EEG spike discharges, high-frequency oscillations, low voltage fast activity, blood-oxygenation level-dependent response, high free oscillations, electrical discharges, micro-seizures, and seizure threshold, etc, observed on long-term EEG recording with varied settings are considered potential electrophysiological biomarkers of epilepsy (Jerome et al. 2013; Staba et al. 2014).
Detection of seizure or epileptic activity on a short-term scalp EEG recording may be infrequent due to various factors. It may be dependent on the patient’s behavior, a close approximation of the EEG electrodes during an abnormal event, and the ability of the technician to detect the abnormal patterns (Dumanis et al. 2017). Sometimes, it is not properly visualized due to the poor placement of the electrodes which may result in artifacts. Hence, it is further ascertained using various multi-modalities such as computed tomography, and magnetic resonance imaging scans to further localize seizure foci precisely (Dumanis et al. 2017). The manual introspection of the EEG pages is not only burdensome for a skilled health expert but also time-consuming, and prone to human error (Deuschl 1999; Santhosh et al. 2014; Acharya et al. 2018; Abdulhay et al. 2020; Subasi 2007; Handa et al. 2021). Automation of this introspection can help in early diagnosis, and aid the burden of caretakers.
Research in Artificial Intelligence (AI) has focused on finding meaningful solutions for the betterment of PWE. The idea of automatic epilepsy detection and analysis of EEG spikes started in 1965 wherein features extracted from band-pass filtering, match filtering, and cross-spectral analysis etc were considered (Smith 1974). Computer-aided systems were developed for detecting ictal and interictal activities in epilepsy using EEG recording in 1982 (Gotman 1982). Since then, different signal processing methods, machine learning, and now, deep learning, and transfer learning have been explored and paved the way to early diagnosis and management for PWE (Shoeibi et al. 2021). The field has slowly moved from shallow learning to deep learning for feature extraction and detection of normal and abnormal EEG signals. Several state-of-the-art algorithms have also been done to detect different types of seizures, and epilepsies (Boubchir et al. 2017; Saini and Dutta 2017; Supriya et al. 2020; Kaur et al. 2021).
Several reviews on automatic epilepsy diagnosis and seizure detection have been published in the last 40 years (Boubchir et al. 2017; Boonyakitanont et al. 2020; Shoeibi et al. 2021; Saini and Dutta 2017; Supriya et al. 2020; Kaur et al. 2021; Farooq et al. 2023; Ein Shoka et al. 2023a; Saminu et al. 2021; Sharmila and Geethanjali 2019; Rasheed et al. 2020; Orosco et al. 2013; Ahmad et al. 2022). Thousands of results are generated for the keyword ‘automatic epilepsy diagnosis’ on the Google Scholar database. These results show active participation from the scientific community to combat challenges faced in the early diagnosis and management of epilepsy and provide effective solutions for PWE. This also directs to a need for an organized review of software advancements and their implementation using hardware systems in this field.
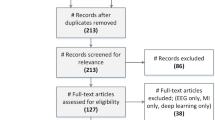
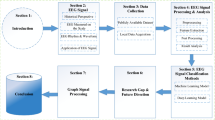
The present review focuses on systematically reporting a review of software advancements and their implementation using hardware systems in automatic epilepsy diagnosis and seizure detection for the past 10 years. The research articles, datasets, and patents have been collected from Google Scholar, Google Patents, pub med, and science direct by searching keywords like ‘EEG datasets for epilepsy diagnosis’. ‘automatic detection of epilepsy’, ‘automatic detection of seizures’, ‘EEG epilepsy diagnosis’, ‘epilepsy detection’, and ‘EEG seizure detection’ from the years 2014 to 2023. Figure 1 depicts the flow diagram followed to identify related articles in this review. About 1020 articles were identified in searching the above-mentioned keywords on popular databases. After removing the duplicates, the articles were screened for titles and abstracts. The irrelevant articles were removed during the screening stage. 532 articles were then screened for full-text checkup wherein 211 articles were found in-eligible due to the use of multi-modalities, lack of experimental work, and missing information. Finally, 321 articles were included in the present review. A similar process was followed to collect hardware articles based on software implementations and granted patents. Only patents published in the English language were considered. Figure 2 depicts the distribution of the reviewed articles according to their publications. Figure 3 shows the taxonomy developed for both software advancements and their implementation using hardware systems in this field for the past 10 years. Figure 4 shows the advancements and evolution of shallow learning to deep learning techniques.
Section 1 discusses the need for automatic detection of epileptic patterns using EEG signals. It is followed by its comparative analysis with the existing reviews in Sect. 1.1 and an overview of the existing datasets and their shortcomings in Sect. 1.2 for AI applications in epilepsy diagnosis and seizure detection. In Sects. 2 and 3, software advancements and their implementation using hardware systems in this field have been discussed. Traditional and recent advancements in the field of signal processing, machine learning (shallow learning), deep learning, transfer learning, and meta-learning for automatic epilepsy diagnosis, and seizure detection have been covered in software advancements. AI algorithms deployed on hardware systems have been covered in the hardware advancements. Existing patents in this field have been covered in Sect. 4. Finally, evaluation metrics utilized in this research field have been discussed in Sect. 5. It is followed by a conclusion and future directions in Sect. 6.
1.1 Comparative analysis with existing reviews in this field
Extensive reviews have timely addressed the need for AI algorithms in epilepsy diagnosis and seizure detection (Boubchir et al. 2017; Boonyakitanont et al. 2020; Shoeibi et al. 2021; Saini and Dutta 2017; Kaur et al. 2021; Farooq et al. 2023; Saminu et al. 2021; Sharmila and Geethanjali 2019; Rasheed et al. 2020; Orosco et al. 2013; Ahmad et al. 2022; Liu et al. 2021; Ein Shoka et al. 2023b). The reviews have also mentioned the lack of publicly available databases, the development of universal classifiers, the curse of dimensionality, black box problem in deep learning and its interpretability, security issues, and EEG localization models etc (Boubchir et al. 2017; Rasheed et al. 2020; Andrzejak et al. 2001). Increasing scientific advancements in signal processing methods, machine learning and transfer learning, and deep learning in seizure-type and epilepsy-type classification have been observed (Shoeibi et al. 2021; Supriya et al. 2020; Rasheed et al. 2020).
Table 1 shows a comparative analysis of the existing review papers with the present review. It is based on factors including the year of release, no. of papers reviewed, software, hardware, patent, and dataset coverage in this field. The major limitation in Boonyakitanont et al. (2020), Kaur et al. (2021), Ahmad et al. (2022), Liu et al. (2021) include the absence of analysis based on available datasets for automatic epilepsy diagnosis and discussion on future direction in this field. The review conducted in Farooq et al. (2023), Sharmila and Geethanjali (2019), Rasheed et al. (2020), Liu et al. (2021) has narrow research coverage. Review provided in Saini and Dutta (2017), Saminu et al. (2021) lacks coverage based on datasets and hardware developments. Several reviews have not mentioned their literature coverage, and keywords are chosen for a systematic review (Supriya et al. 2020; Sharmila and Geethanjali 2019; Rasheed et al. 2020; Liu et al. 2021). Evaluation metrics used to check the efficacy of the automated systems have also not been discussed in several reviews (Shoeibi et al. 2021; Kaur et al. 2021; Orosco et al. 2013; Ein Shoka et al. 2023b). Recent concepts in short learning and meta-transfer learning have not been explored in this field. Interested readers can refer to Rasheed et al. (2020) for timeline-based advancement in this field since 1970. To the best of the author’s knowledge, no review has covered patents and the implementation of algorithms on hardware systems in this research field.
1.2 Open source and private datasets in this field
High-quality EEG data is paramount in the development of AI algorithms for the early detection and prediction of epileptic signals. Table 2 discusses twenty-eight open source and private datasets in this field since 2001.
Bonn EEG time series database is the first dataset, developed to investigate the non-linearity of epileptic time-series EEG data with reference to normal data. In 2010, the second dataset, the Children’s Hospital Boston (CHB) Massachusetts Institute of Technology (MIT) scalp EEG database was released in the work done by Ali Shoeb to estimate feature engineering applications for seizure onset detection (Goldberger et al. 2000). Then the Bern Barcelona dataset was released to study the focal and non-focal EEGs in epilepsy diagnosis (Andrzejak et al. 2012). The Temple University Hospital (TUH) EEG data corpus was generated to substantially accelerate research progress in this field (Obeid and Picone 2016; Harati et al. 2014). It has more than 20,000 EEGs with physicians’ EEG reports and classification labels needed for supervised machine learning experiments. It is freely available and can be collected by sending a four Terra-byte drive to TUH.
Researchers of project EPILEPSIAE have generated the largest private database of different multi-modalities (Ihle et al. 2012). University of Bonn, Freiburg, and Bern Barcelona databases have been a part of this project since 2018. Helsinki University Hospital, Australia generated multi-channel EEG data from 79 term neonates admitted to their hospital unit. The developed dataset can be used as a reference dataset for neonatal seizures in the development of automated methods of seizure detection (Stevenson et al. 2019). Multiple research institutes and hospitals across globe have contributed to developing private databases and AI pipelines. Multiple Indian research institutes and hospitals have helped in developing a private database for different seizure and epilepsy-type patients. Interested readers can read more about these datasets in Handa et al. (2021).
It has been noticed from Table 2 that information about the placing of EEG electrodes is missing in all publically available databases. None of the databases have mentioned a change of impedance with the time of the EEG electrodes. This information may help in deciding the quality of the EEG signal. Fewer studies have been done on the variation observed in the EEG bands and sub-bands of the available databases (Zhong et al. 2008). Raw data is not available for several EEG data like the Epileptic Seizure Recognition Data Set, Neurology and Sleep Center, New Delhi EEG dataset, Bonn EEG time series database, and Bern-Barcelona EEG database.
Most of the available datasets have been recorded for a short duration of time with minimal information given about the stage of epilepsy and the nature of abnormality present in the epileptic EEG signals like spikes, wave complexes, sharp waves, slow waves, etc. Bonn dataset, Neurology and Sleep center, New Delhi EEG dataset (Swami et al. 2016a), and Bern-Barcelona EEG database do not contain information about montages, specific epilepsy types, proper information about the patients, and seizure-affected brain area. However, both CHB-MIT and Bonn data have become a benchmark for research due to their availability since 2001 and 2010. They have helped in escalating research in this domain.
CHB-MIT database is a heavy and complex database and may occupy about 2–40 GB depending on the number of patients taken into consideration. Age, gender, the occurrence of a seizure, and its duration have been mentioned. Discussion about artifact present, noise, and seizure-affected areas of the brain has not been clearly mentioned. Work done in Rout et al. (2022) has provided the seizure category and its brain position of the original CHB-MIT EEG scalp data. Neurology and Sleep Center databases have not disclosed about patient’s age, gender, the occurrence of the seizure, and its duration and discussion about the artifact present, noise, and seizure-affected area of the brain. Only TUH data contains both research and clinical grade data. However, due to its size and variations in settings, it requires state-of-the-art processing, computational power, and storage.
Newer datasets such as Siena scalp (Detti 2020; Detti et al. 2020), multi-center intracranial EEG, and neonatal datasets, signal-to-image seizure EEG datasets like Sachdeva et al. (2022) and others Stevenson et al. (2019), Nejedly et al. (2020), Nasreddine (2021), Cserpan et al. (2021), Panwar et al. (2019), Panwar (2020), Kuhlmann et al. (2018), Jeffry Howbert et al. (2014) need exploration by the research community. Meta-data of CHB-MIT and Siena EEG like Gupta et al. (2022a), Gupta et al. (2022b), Handa and Goel (2021a), Deepa and Ramesh (2021) has also been released. Several private databases have also been released such as the Das et al. (2020), Yedurkar and Metkar (2020), Bilal et al. (2019), Cao et al. (2019), Choi et al. (2019), Yan et al. (2019), Avcu et al. (2019), Duanpo et al. (2019), Raghu et al. (2019), Selvaraj et al. (2014), Hunter et al. (2005).
Most available databases have different clinical settings hence evaluating them on similar parameters such as signal processing, a feature used and AI pipelines may produce inaccurate results. Reported methods have evaluated their results using different EEG window lengths hence an effective comparison becomes difficult for the further hardware implementation process.
2 Software developments
This section details the software advancements in automatic epilepsy diagnosis and seizure detection from 2014 to 2022. Section 2.1 discusses EEG signal processing and feature extraction methods. Section 2.2 discusses machine learning algorithms used for automatic epilepsy diagnosis and seizure detection.
2.1 EEG signal processing and feature extraction based pipelines
The signal processing technology is a vast field that has been used for artifact removal, montage selection, noise detection, rejection, Power Spectral Density (PSD) analysis, and selection of frequency band range etc in this field. Advanced signal processing techniques like Tunable Q factor Wavelet Transform (TQWT), Continuous Wavelet Transform (CWT), Empirical Model Decomposition (EMD) and its Intrinsic Mode Function (IMF), Variational Modal Decomposition (VMD), Wavelet Packet Decomposition (WPD), Wavelet Transform (WT), Fourier Transform (FT), Short-Time FT (STFT), etc have played a significant role in extracting relevant information present in EEG signals in frequency, frequency–time, and spatial domains (Krishnan and Athavale 2018).
Feature estimation is a supreme task in this field as it helps in finding distinguishing characteristics between epileptic and non-epileptic EEG signals which are non-stationary, casual, non-Gaussian, and time-variant in nature. Generally, an EEG signal consists of five frequency bands namely delta, theta, alpha, beta, and gamma which denote the activities of the human brain. Interest readers are referred to Dumanis et al. (2017), Boubchir et al. (2017), Boonyakitanont et al. (2020), Deuschl (1999) for a detailed exploration of this. Table 3 shows the list of features that have been popularly used in this field. Optimization techniques like Genetic Algorithm (GA), Simulated Annealing (SA), different types of searches, Independent Component Analysis (ICA), Principal Component Analysis (PCA), etc., have also been applied to optimize features in this field.
Moctezuma and Molinas (2020) used a Non-dominated Sorting GA (NSGA) for selecting specific channel(s) to maximize the accuracy of their AI model. Their method showed the feasibility of detecting epileptic activity using fewer EEG channels. El-Gindy et al. (2021) compared Coifets, Haar, Symlets, and Daubechies wavelets using the CHB-MIT dataset. They found Daubechies (db4) yielded the highest sensitivity up to 100%. Romney and Manian (2021) suggested using a patient-specific channel reduction method named Model Agnostic Meta-Learning (MAML) with Ensemble EMD and Sequential Feature Selection (SFS). The work yielded an average sensitivity of 91% using the CHB-MIT dataset.
Emara et al. (2022) proposed a work introducing three different frameworks for EEG signal processing. The frameworks were based on Scale-Invariant Feature Transform (SIFT), Fast FT, and Artificial Neural Network (ANN) for extraction, prediction, and channel selection respectively. Amiri et al. (2023) proposed an automated seizure identification method. Their method utilized a Sparse Common Spatial Pattern (SCSP) to select suitable channels and segregate the seizure states. In their proposed model, adaptive FT was used to enhance the time–frequency representation of EEG signals while minimizing noise and interference. The frequency spectrum was segmented into distinct sections to optimize seizure detection. It was achieved by EEG signal decomposition to the rhythmic component of seizures. Their proposed method demonstrated that the EEG interpretation workload for specialists can be significantly reduced and may serve as a valuable tool in epilepsy diagnosis.
Sopic et al. (2022) introduced a technique that involved identifying a group of seizure signatures with similar characteristics across two different EEG channels, from two randomly selected seizures from each individual through visual inspection. The entire recording from the same patient was scanned using Dynamic Time Warping (DTW) as a similarity metric to locate the same seizure signature. Their approach demonstrated high sensitivity, low false alarm rate, easy interpretability, and low computational complexity, making it suitable for application in wearable devices. Tuncer et al. (2020) proposed a feature extraction way using Local Graph Structure (LGS), and ReliefF and CA for feature selection. They found that the LGS method gave a higher classification rate than the One-dimensional (1D) Convolutional Neural Network (CNN).
Supriya et al. (2021) proposed an innovative way of converting the EEG signals to a new weighted complex network and using a new feature edge weight fluctuation. They used the machine learning classification method at Bern Barcelona and Bonn University and got an accuracy of 99% and 100% respectively. Göksu (2018) used wavelet packet analysis and extracted log energy entropy, norm entropy, and energy from the Bonn dataset. These features were given to a Multilayer Perceptron (MLP) classifier which gives an accuracy of 100%. The authors also suggested a way to detect epileptiform patterns during and outside the seizure states. Houssein et al. (2019) used Whale Optimization Algorithm Support Vector Machine (WOA-SVM) with features extracted using DWT on the Bonn dataset. The accuracy without WOA was recorded as 94.6%, and with WOA, the accuracy increased up to 100%. Another work, Sunaryono et al. (2023) utilized a combination of 1D CNN with WOA methodologies to improve the detection of seizures. The signals were first segmented, followed by the extraction of features using DWT. The CNN configuration was then optimized using WOA. Due to the addition of WOA, an impressive accuracy rate of 99.76% was achieved.
Dash et al. (2021) extracted features like variance, Katz fractal dimension features which were derived from wavelet packet decomposition coefficients. By utilizing these features, the authors were able to accurately identify and distinguish seizures from normal EEG signals. K-Nearest Neighbours (KNN) classifier was used for classification which gave 98.99% accuracy. Şeker and Özerdem (2021) proposed a classification approach for focal or non-focal EEG detection using Cepstral Analysis (CA). EEG epochs were used to calculate Mel Frequency Cepstral Coefficients (MFCC). Using Quadratic Discriminant Analysis (QDA), Logistic Regression (LR), Cubic SVM, and Weighted KNN, all focal and non-focal EEG pairings were categorized with specificity, and F1-scores of 100% and AUC of 1.
Tuncer et al. (2021) proposed an automated EEG signal classification using a chaotic Local Binary Pattern (LBP). In order to reduce the EEG matrix dimensionality, Abdulla et al. (2022) proposed a determinant of the Covariance matrix (Cov-Det) model. A set of statistical features were retrieved from each period to create a feature vector. The Kolmogorov–Smirnov (KST) and Mann–Whitney U (MWUT) tests were combined to minimize redundant characteristics. A dual-index approach called Maximum Fuzzy Membership Difference Entropy (MFMDE) was suggested in Zhang et al. (2021a) to characterize the randomness and complexity of an EEG signal. Thirteen different types of synthetic signals were utilized to investigate MFMDE’s properties, three real-world datasets were used to validate MFMDE’s performance, and MFMDE was finally compared to other six nonlinear analysis methods.
A chaotic 1D LBP and WPD technique was proposed to create an abnormal EEG detection model in Tuncer et al. (2021). Using an SVM classifier with a tenfold cross-validation technique, the created model achieved detection accuracies ranging from 93.84 to 98.19% for 24 EEG multi-channels. A Riemannian geometry-based kernel was used by Shariat et al. (2021) to obtain symmetric and positive definite matrices that contain extra information and high dimensions. Features were selected using the sequential forward feature selection method and then used a support vector machine with Radial Basis Function (RBF) and got an accuracy of 98.14%. Mishra et al. (2023) proposed a classification technique that classifies EEG brain signals for epilepsy identification. The proposed technique was based on DWT and Moth Flame Optimization-based Extreme Learning Machine (DM-ELM). In their proposed model, the DM-ELM performed better than the basic ELM. A single state seizure estimation was made using one binary and one continuous feature which were then used to produce a combined seizure state estimation using square-root Kalman filter in Steele et al. (2021). The combined seizure state estimation gave average accuracy, sensitivity, and specificity of 92.7%, 92.8%, and 93.4%, respectively.
2.2 Machine learning based pipelines
Machine learning techniques are changing the world and saving lives in many ways. It focuses on recognizing and extracting vital patterns from a given data. A manual analysis of long EEG recording to find traces of epilepsy is not only time taking but also requires skilled technicians (Pachori and Bajaj 2011; Acharya et al. 2018; Sethy et al. 2021; Schmidhuber 2015; Pandey et al. 2019; Shaikh et al. 2019; Palak et al. 2023; Lopez et al. 2016). Hence, an automated process with the help of machine learning algorithms can be of great clinical significance. Section 2.2 is further bifurcated to Sects. 2.2.1, 2.2.2, and 2.2.3 wherein traditional machine learning-based pipelines, hybrid, and end-to-end pipelines have been discussed.
a Evolution of shallow learning to deep learning techniques (Schmidhuber 2015). b Representative diagram to show traditional (shallow) learning and deep learning pipelines. Traditional approaches often called shallow learning utilize one hidden layer, require domain knowledge data, and learn from a pre-defined set of features. Whereas deep learning utilizes multiple layers and performs automatic feature extraction using complex mathematical computations. SOM self organizing maps, LSTM long short term memory, DBN deep belief network, ReLU rectified linear unit, NAS neural architecture search, GRU gated recurrent unit, GAN generative adversarial network, CNN convolutional neural network, RNN recurrent neural network
In the traditional machine learning-based pipelines, studies that have used advanced signal processing techniques along with traditional machine learning classifiers like SVM, Random Forest (RF), Naive Bayes (NB), Decision Tree (DT), KNN, LR, Deep Forest (DF), Hidden Markov Model (HMM), linear classifiers, boosting algorithms, etc have been discussed.
In hybrid pipelines, studies in which features extracted from advanced signal processing methods are fed to deep learning architectures such as CNN, Recurrent Neural Networks (RNN), Long-term Short-term Memory (LSTM), Deep Neural Networks (DNN), Generalized Regression Neural Networks (GRNN), Stochastic Gradient Descent (SGD), etc. Several transfer learning architectures like Visual Geometric Group (VGG), Residual networks (Resnet), Dense networks (Densenet), etc have been discussed. They may be used in 1D, Two-Dimensional (2D), Three-Dimensional (3D), or Four-Dimensional (4D) formats. Pipelines that have not used hand-crafted features, and automatically extracted features and classified or detected abnormal and normal EEG signals have been discussed under ‘end-to-end pipelines’.
The collected articles have been summarized through Table 4 in chronological order for eight publicly available EEG datasets in this field. The datasets include the University of Bonn data, CHB-MIT EEG Scalp data, Epileptic Seizure Recognition data, Juntendo University hospital data, TUH data, Neurology and Sleep Centre data, Karunya Institute of Technology and Sciences (KITS) database, and Bern Barcelona data. The articles have been compared based on their signal processing technique, features extracted from the EEG signals, classification methods, and accuracy achieved from their AI model. Comparison of other evaluation metrics like Area Under the Receiver Operating Characteristic (AUC-ROC) curve, precision, recall, Root Mean Square (RMSE), False Alarm Rate (FAR), sensitivity, Geometric Mean (GM), specificity, F1-score, etc has been done in the subsequent sections.
2.2.1 Traditional pipelines
Gupta and Pachori (2019a) used weighted multiscale Renyi permutation entropy as a feature from Fourier–Bessel series expansion of the EEG signals from Bonn dataset and recorded a 99.50% with Least Squares SVM (LS-SVM) as a classifier. Yu et al. (2019) used DWT on signals obtained from the Bonn dataset. They combined the features and reduced their dimensions using PCA and then used SVM which gave an accuracy of 98.4%. One-way analysis of variance and forward sequential feature selection was used to select features from eight entropies (Chen et al. 2019). They were then classified by using six different machine learning classifiers, out of which the Least-Square SVM (LS-SVM) gave the highest accuracy of 99.5%. Dash et al. (2020) used an iterative filtering-based decomposition of EEG signals with Dynamic Mode Decomposition (DMD) power, 2D PSD, and variance features to improve upon the accuracy of seizure detection. All India Institute of Medical Sciences (AIIMS) Patna and CHB-MIT datasets were classified using HMM classifiers which gave an accuracy of 99.74% and 99.6% respectively. A Cluster-based KNN (CKNN) algorithm was proposed with a distance-based features selection algorithm on Bern Barcelona, Bonn University, and CHB MIT dataset (Syed et al. 2021). Thor method yielded a 100% accuracy in classifying seizure and normal EEG signals. By using CKNN, the complexity was found reduced when compared with the traditional KNN classifier.
Tzimourta et al. (2019) used an RF classifier with features extracted from DWT. The accuracy was above 95% for both Bonn and University Hospital of Freiburg datasets. Anand et al. (2019) used discrete stationary wavelet-based Stockwell transform on EEG signals from the Bonn dataset. The work extracted temporal features, spectral features, and amplitude distribution estimation, and applied a modified filter bank-based particle swarm optimization to the data. With the help of hybrid K-nearest SVM (Kn-SVM), the work reported accuracy up to 100%. Using wavelet feature extraction and a gradient-boosting decision tree, Akbari and Sadiq (2021) proposed an automated method for focal and non-focal EEG signal identification. The study paradigm was linked to the invention of the EWT, which decomposes EEG data into the delta, theta, alpha, beta, and gamma rhythms. Several statistical tests were used to identify the significant features. SVM and KNN classifiers were implemented with a tenfold cross-validation, which provided an average classification of 82.6% and 93% for entire and small datasets. Zarei et al. (2019) used the Douglas–Peucker (DP) algorithm and PCA to extract information for the Bonn dataset. DP algorithm gives out the most relevant samples which were then given to PCA which reduced the dimensionality of the samples. Machine learning classifier namely RF yielded the best performance with an accuracy of 99.85%. The study reported a limitation that as the size of the model increased, the computational time also increased substantially.
Anuragi et al. (2021) used Fourier–Bessel series expansion-based EWT with half-quarter frame-size time-segmented EEG signal and three accumulated norm-entropy, log-energy-entropy, and line-length as features which were selected by using relief ranking method. By using SVM, KNN, and ensemble learning, the authors achieved an accuracy of 100% on the Bonn EEG dataset and 99.84% on the CHB-MIT dataset. Another study by Anuragi et al. (2022) proposed a framework for the automated detection of epileptic seizures in EEG signals using a combination of Empirical Wavelet Transform (EWT) and ensemble learning classifiers. The framework extracted non-linear features from the phase-space representation of EEG signals and applied feature ranking to reduce the feature space. Mehla et al. (2021) used the Fourier decomposition method on CHB-MIT and Bonn datasets and then used the Kruskal–Wallis (KW) test to select features from Fourier intrinsic band functions. They further used SVM to classify and record accuracies of 99.94% and 99.96% on CHB-MIT and Bonn respectively.
Sethy et al. (2021) used thirteen features namely mean, standard deviation, variance, skewness, entropy, RMS value, minimum, maximum, energy, median absolute deviation, interquartile range, kurtosis, and semi-quartile range. Then they compared twenty-four machine learning classifiers using the CHB-MIT dataset and found KNN to be the best. Li and Chen (2021) presented a method for seizure classification for EEG recordings that employs a DT-CWT and FFT classifier in combination with an LS-SVM classifier. In this procedure, each EEG wave was separated into four parts, and sections were subdivided into smaller sub-segments. The method showcased an average accuracy of 96.8% and 97.7% for the Bern and Bonn databases, respectively. The results demonstrated that the suggested DT-CWT and FFT-based feature extraction method is an excellent method for extracting discriminative information from brain signals. Deivasigamani et al. (2021) proposed a proficient technique to classify epileptic EEG signals based on DT-CWT and SVM classifiers. DT-CWT processing method was applied to TUH EEG signals. A sensitivity, specificity, and accuracy of up to 98%, 98%, and 96% respectively was obtained from their proposed technique
Abdulbaqi et al. (2022) aimed to use EEG signals to diagnose neurological disorders, specifically epilepsy. The authors extracted features from EEG signals using DWT, KNN, and SVM classifiers to categorize the EEG signals as normal or pathological. They evaluated the performance of both classifiers using accuracy, sensitivity, and specificity measures and found that SVM performed better than KNN in terms of accuracy and specificity. The proposed method by Kavitha et al. (2022) for detecting epileptic seizures involved disintegrating EEG signals into six frequency sub-bands using DWT and extracting twelve statistical functions. The best seven features were then fed into various machine learning classifiers such as SVM, KNN, NB, and DT for two-type and three-type classifications. The performance of these classifiers was measured using six statistical parameters.
Nishad and Pachori (2020) used a TQWT-based filter bank and cross-information potential features and fed the information to the RF classifier. The work resulted in an accuracy of 99% on the Bern Barcelona dataset which was 0.4% more than (Bhattacharyya et al. 2017) who used TQWT-based multi-scale KNN entropy with SVM. Instead of using the previous seizure detection methods which focused on analyzing whole spectrum frequencies, Sameer and Gupta (2020) proposed a system using only the alpha band. Such a change made its hardware implementation to be less complex. KNN, NB, DT, SVM, AdaBoost, and RF algorithms were used and RF was found to give the most optimized performance. Chen et al. (2020) proposed a novel classifier based on pairwise one-class SVM which produced an accuracy of 93% on Bern Barcelona data and 94% on CHB-MIT data. Thomas George et al. (2020) used the KITS EEG database and TUH dataset and characterized signals using TQWT, features like log energy, Shannon entropy, and sure entropy were extracted. The informative part was fed into the ANN classifier using PSO. This resulted in a maximum of 100% accuracy. Sadiq et al. (2021) proposed a method using TQWT, along with binary feature selection algorithms and machine learning classifiers to distinguish focal and non-focal EEG signals. The method achieved an average classification accuracy of 97.68%.
KNN and Random-under sampling (RuS) boosting classifier was used on extracted time-domain features from Bonn and CHB-MIT dataset and achieved an overall accuracy of 94–98% (Banerjee et al. 2019). Albaqami et al. (2021) proposed an automatic detection of abnormal EEG signals. Categorical Boosting (CatBoost), Extreme Gradient boosting (XGBoost), and Light Gradient-Boosting (LightGBM) were used to classify features. CatBoost classifier achieved 87.68% binary classification accuracy and surpassed state-of-the-art approaches on the same dataset by more than 3% in sensitivity and more than 1% in accuracy. Shen et al. (2022) presented a real-time method for detecting epilepsy seizures using EEG. Their approach used DWT and eight eigenvalues algorithms to extract features. SVM was then utilized to classify the EEG signals into three categories: seizure-free, seizure-active, and health control. Real-time seizure onset detection was performed using the RUBSboot tree ensemble method. Complete Ensemble Empirical Mode Decomposition with adaptive noise Extreme Gradient Boosting (CEEMD-XGBoost) was proposed in Jiang et al. (2020). They first processed the EEG signals using CEEMD and used multi-domain features from IMF, its residue, and the EEG signal itself to give to XGBoost for classification. This resulted in an accuracy of 99% on the Bonn dataset and 95.79% on the CHB MIT dataset.
Liu et al. (2022) introduced a method for detecting seizures that involved utilizing VMD and a DF-based model. VMD was used to generate functions from EEG recordings. EEG features were derived from the log-Euclidean covariance matrix to represent the properties of the EEG. The DF model was utilized to classify the EEG signal. Additional post-processing techniques such as moving average filtering and adaptive collar expansion were employed to enhance the classification accuracy and generate discriminant results. Zhang et al. (2022a) introduced a novel method for epileptic seizure classification based on a combination of signal decomposition and statistical techniques. Specifically, the VMD algorithm was employed to extract IMF components from the signals. Statistical methods were then used to calculate eight key features (variance, skewness, maximum, minimum, kurtosis, coefficient of variation, average, and volatility index) for each extracted IMF component. The best combinations of these features were then input into a Non-Linear Twin SVM (NLTWSVM) for epileptic signal classification. The results indicate that the proposed model has the potential to assist experienced neurophysiologists in accurately classifying epileptic seizure types in a clinical setting.
Logistic regression, ANNs, and support vector machines were used in Guerrero et al. (2021) to analyze EEG signals. A comparison of measurements and performance revealed that ANNs, with an accuracy of 86%, were the best classification tool for characterizing epileptic patients. Rajinikanth et al. (2022) proposed an EEG classification pipeline using hybrid features and machine learning classifiers. They transformed EEG into RGB-scaled images and implemented DWT to enhance image textures. Grey Level Correlation Matrix (GLCM) and entropy features were extracted. Binary classifier implementation and fivefold cross-validation were done. Among Adaptive Neuro-Fuzzy Inference System (ANFIS), KNN, NB, LS-SVM, and RBF wherein KNN gave the highest accuracy of 95%. Sunaryono et al. (2022) presented a methodology aimed at the identification of epilepsy through the analysis of EEG signals. Their method incorporates the utilization of DFT and DWT for feature extraction, in conjunction with a combination of 2-class and 3-class Gradient Boosting Machines (GBM) for the classification of EEG signals. The integration of 2-class and 3-class GBM models has demonstrated a notable enhancement in classification performance when compared to a singular GBM approach, resulting in the achievement of a 100% accuracy rate. The authors have shown the importance of comparing computational time of AI models in addition to other evaluation metrics like accuracy.
Pipelines mentioned in Table 4 have computed their features from the reconstructed signal matrix of advanced signal processing methods like DWT, CWT, Ramanujan transform, ICA, and EMD etc. It has been found that WDT and EMD (Das and Bhuiyan 2016) is a well-liked choices among existing EEG signal decomposition techniques (Das and Bhuiyan 2016). TQWT (Reddy and Rao 2017; Gupta et al. 2017, 2018a; Patidar and Panigrahi 2017; Reddy et al. 2015), EWT (Gupta et al. 2017, 2019b; Bajaj and Pachori 2011) and VMD (Rout and Biswal 2020; Chakraborty and Mitra 2021) have also gained popularity and are used as signal decomposition techniques.
It has been found that SVM-based pipelines are the most popular choice amongst the classification of binary and trio sets of seizure-free and seizure-containing EEG signals. The studies in Handa and Goel (2021a), Gupta et al. (2017, 2018a, 2018b, 2018c, 2019b), Bajaj and Pachori (2011), Mitha et al. (2014), Fasil and Rajesh (2019), Sharma et al. (2018, 2020), Borowska (2021), Senthilpari et al. (2021), Saputro et al. (2019), Li et al. (2019a), Balamareeswaran and Ebenezer (2015), Sairamya et al. (2019, 2021), Rajendran et al. (2019), Geethu and Santhoshkumar (2020), Ahmad et al. (2014), Islam et al. (2015), Xiang et al. (2015), Samiee et al. (2017), Kai et al. (2014), Jiang et al. (2021), Al-Salman et al. (2022) have utilized SVM in their pipeline. Different kernels of SVM have been utilized wherein a classic 100% accuracy has been achieved. RF classifier and KNN classifier have also been found popular in this field (Reddy and Rao 2017; Lopez et al. 2015; Bhattacharyya and Pachori 2017; Mursalin et al. 2017; Naro et al. 2014; Swami et al. 2016b; Brari and Belghith 2021; Zhang et al. 2021b). No study has focused on exploring the best kernel for SVM, nor hyper-parameters of RF and KNN in this field. Hyper-parameter tuning and cross-validation should also be done (Handa and Goel 2021a).
Research works have used machine learning techniques in channel detection, and selection of bands of EEG (Rasheed et al. 2020). It is still debatable whether a single feature matrix or hybrid matrix has the potential to generate 100% accuracy. Several studies have focused on generating features from EEG signals in this field (Sairamya et al. 2019; Arunkumar et al. 2018; Prasanna et al. 2019; Ahmedt-Aristizabal et al. 2019; Priyanka et al. 2017; Swami et al. 2017; Hadiyoso et al. 2021; Namazi and Jafari 2018; Li et al. 2019b; Shriram et al. 2019; Simozo et al. 2014; Iqbal et al. 2015; Muhammed Shanir et al. 2015; Gill et al. 2015; Wang et al. 2014; Jaiswal and Banka 2017; Aghababaei et al. 2021; Peng et al. 2021; Hussain and Qaisar 2022). ReliefF feature selection method has been used in many research papers to help select the features (Tuncer et al. 2020; Anuragi et al. 2021; Nishad and Pachori 2020; Praveena et al. 2021). Studies have suggested that a mixture of time–frequency-based features produces better results than separate features (Saminu et al. 2021). No one feature has been universally accepted and only a few are supported by performance-based statistical testing like Analysis of Variance (ANOVA), Kruskal Wallis test etc. More research can be done in this domain. However, with the advent of deep learning methods in this domain, more studies are adapting to model-extracted ‘deep features’ in comparison to hand-picked features.
2.2.2 Hybrid pipelines
Recent studies carried out using deep learning such as CNN, LSTM, residual CNN, DNN, and transfer learning architectures etc., have been trained on raw datasets instead of statistically developed features. Such techniques do not require manual intervention and hold a promising future in the detection of epilepsy (Acharya et al. 2018). Reported works in Sects. 2.2.2 and 2.2.3 have achieved better model performances and reported fully or semi-automated (hybrid) pipelines. Several studies have utilized CNN architectures (Ahmedt-Aristizabal et al. 2019; Madhavan et al. 2019; Golmohammadi et al. 2018; Sriraam et al. 2019; Shankar et al. 2021) and deep features (Siddharth et al. 2019; Shekokar and Dour 2021; Yildiz et al. 2021; Khan et al. 2021; Tuncer 2021; Ilakiyaselvan et al. 2022) to perform automatic epilepsy diagnosis and seizure detection. However, these studies present suffer from a lack of interpretability i.e., they are black boxes and can’t be easily understood at each step of the pipeline. They require more training time, and data quantity, which leads to a significant increase in computational complexity during hardware development and marketing purposes.
Recent trends have utilized 2D images representing 1D EEG signals. It comparatively reduces the time to transform 1D EEG signals to appropriate 2D images. Various 1D to 2D transformations have been proposed by researchers such as the use of spectrograms, variograms, scalograms, periodograms, henon maps, EEG time frames, Divided Difference Approximation (DDA) feature-based histograms, power or intensity-based histograms, line graphs, and Eigen visualizations etc. 2D frequency–time scalograms were extracted by using CWT in Türk and Özerdem (2019) whose properties were then learned by a CNN model. This yielded an accuracy of 99.5% on Bonn University’s normal v/s ictal data. Jana et al. (2020) used a spectrogram feature matrix and 1D-CNN-based scheme for seizure detection. This approach resulted in an accuracy of 77.8%. They used the feature matrix obtained from the spectrogram as input for their CNN pipeline. Another study developed a generic CNN of 5 layers for epileptic seizure detection using one-sided rhythmicity spectrograms (Handa and Goel 2021b). The model achieved validation of 88.17%, the training set accuracy of 91.89%, and test set accuracy of 61% respectively.
The study by Saneesh Cleatus and Thungamani (2022) proposed an innovative computer-aided approach to detect pre-ictal and ictal activity in a multichannel EEG signal. Their method involved inputting an image database into a machine-learning algorithm for classification. To achieve this, they converted a time domain EEG signal into an image by extracting EEG signal features. The processed EEG waveform, represented as images, was then utilized to train a CNN for classification. The CNN successfully categorized input signals into three classes: seizure, normal, and pre-ictal. This breakthrough in EEG signal analysis could pave the way for a more accurate and efficient diagnosis of epileptic seizures. Gao et al. (2022) used Generative Adversarial Networks (GAN) to create more seizure data to balance three datasets namely CHB-MIT, Bonn, and private data from Sir Ganga Ram Hospital, New Delhi. The authors then used 1D-CNN to classify seizure and non-seizure and reported it to be more efficient in terms of classification report than 2D-CNN.
Ein Shoka et al. (2023a) presented an encrypted EEG data classification and recognition system that utilized algorithms like chaotic baker map and Arnold transform in combination with CNNs to improve efficiency. The EEG time series was transformed into a 2D spectrogram image, and encrypted using the before-mentioned algorithms. The resulting data was then used as input to CNN-based models. Currey et al. (2021) trained three CNN models namely 1D CNN, 2D CNN-spectrogram, and CNN-combined on CHB MIT, and tested it on the University of Wisconsin dataset. Their study is one of the first works to report the inter-hospital generalization effect of CNN models.
Wang et al. (2021a) also used 1D-CNN with a reduced feature strategy to balance the samples of CHB-MIT scalp EEG and private intracranial EEG dataset. The work achieved an accuracy of 99.54% and 99.73% for the before-mentioned datasets, for the segment-based level. 97.52% sensitivity was achieved for the event-based level method. Wang et al. (2021b) used 1D and 2D CNN on the University of Bonn dataset by using sliding window and CWT techniques. They reported the highest accuracy of 99.92% in two-class classification between normal v/s ictal, while normal vs. interictal vs. ictal had the overall best results in three-classification. Zhao et al. (2020) used the Bonn dataset and introduced the batch normalization and dropout layers in addition to the traditional convolutional blocks which increased the accuracy to 97.63%. Another seizure detection approach was introduced by Sameer and Gupta (2022) by using a 1D CNN. Their proposed method extracted features automatically followed by a traditional ML classifier. No preprocessing of the EEG signal was required and that reduced the training time by 94% as compared to end-to-end DL models. The proposed detection system has the potential to assist neurologists in making more accurate decisions. Furthermore, this model can be utilized to develop both cloud-based and standalone seizure detection systems.
Dhar et al. (2022) proposed an epileptic seizure detection approach using the feature extraction method and CNN-RNN model. Different feature extraction techniques, such as LBP, FFT, DWT, and EMD were analyzed. Another technique to detect normal and epileptic signals was proposed using an adaptive ANN (Munirathinam et al. 2022). The proposed classification technique used frequency, time, frequency–time, and non-linear features. Kumar et al. (2022) proposed an intelligent system that integrated two powerful signal processing techniques, VMD and HT, to extract useful features from EEG signals. The extracted features were then fed into a stacked NN for epilepsy seizure detection. Ghazali et al. (2022) developed an ML technique optimized for epilepsy. DWT was used to decompose the EEG signals, proceeded by the extraction of features. The model was trained as a Feed-Forward Neural Network (FFNN) using the extracted features.
Sivasaravanababu et al. (2022) proposed an efficient seizure detection framework based on unsupervised diagnosis for epileptic seizures. Feature extraction and pre-processing were done via Deep Convolutional Variational Autoencoder (DCVAE) and TQWT. The stacked Bi-Directional LSTM model was then applied for automatic seizure detection. Notably, this model was able to bypass the overhead of feature extraction while achieving a high specificity of 99.67%, accuracy of 99.79%, and sensitivity of 99.52%. Hurst Exponent and Auto-regressive Moving Average (ARMA) features were extracted from each signal and the LSTM classifier was used by Abbasi et al. (2019). The proposed approach improved the binary classification accuracy by 2% from the previous SVM classifier and gave an accuracy of 94% in the case of multi-class classification on the Bonn dataset. Praveena et al. (2021) tried to resolve the curse of dimensionality issue using reliefF feature selection method to reduce the total features. The overall accuracy was increased by 0.6–16% using LSTM as a classifier and was recorded as 98.78% for the TUH dataset, 98.89% for the Bern Barcelona dataset, and 98.73% for the University of Bonn dataset.
Anila Glory et al. (2021) proposed Adaptive Haar Wavelet-based Binary Grasshopper Optimization Algorithm (AHW-BGOA) DNN, a way to classify EEG data. They also compared this method to DNN without using AHW-BGOA on Bern Barcelona, Bonn University, and CHB MIT data and found this method to be superior. Dominguez et al. (2020) used the KITS dataset and converted the EEG signal to a 2D brain map using Anatomical Mapping using Intrinsic Correlations through Alignment (AMICA) and used ANN on the features extracted which yield an accuracy of 99.53%. Hassan et al. (2022) proposed a method using a combination of EMD, a Mutual Information-based Best Individual Feature (MIBIF) selection algorithm, along with a MLP Neural Network (MLPNN). The most significant features were selected and fed into the MLPNN classifier.
Prathaban and Balasubramanian (2021) used sparsity-based EEG reconstruction to remove artifacts with 3D-optimized CNN. They used phase transition-based Kullback–Leibler divergence which helped them obtain an optimal seizure prediction horizon of about 1.1 h before the seizure onset. Zhang et al. (2021c) used the Pearson correlation coefficient to calculate correlation matrices, which were used to classify between preictal and interictal signals. They used CHB-MIT and reported an accuracy of 89.98% and an alarm 15 min in advance using a CNN architecture.
Peachap and Tchiotsop (2019) used Laguerre polynomials to construct wavelets, SVM and ANNs for classification which yielded an accuracy of more than 95% in most cases. Features from DWT and Haar wavelets were extracted and given to feed-forward and GRNN (Vijay Anand and Shantha Selvakumari 2019). The GRNN gave better results than FFNN, with 100% accuracy on the University of Bonn dataset. Pan et al. (2022) introduced a deep learning approach for detecting epileptic seizures using a combination of different input formats, including short-time Fourier transform of EEG signals, Fourier transforms of EEG signals, and wavelet transform of EEG signals. To generate a more reliable feature for seizure detection, they implemented a feature fusion mechanism that combined the learned features. The hybrid method was particularly successful in improving seizure detection performance in scenarios where there was limited data available. The method was able to leverage the unique strengths of time-domain, frequency-domain, and time–frequency-domain properties for more comprehensive analysis.
Guo et al. (2022) proposed a hybrid system integrating an Unsupervised Learning (UL) module and a Supervised Learning (SL) module. The UL synthesizes Amplitude-integrated EEG (aEEG) extraction, isolation forest-based anomaly detection, adaptive segmentation, and silhouette coefficient-based anomaly detection evaluation and serves to quickly locate the determinate subjects and the indeterminate subjects. Then more robust seizure detection was performed by the SL using an easy ensemble algorithm which could potentially decrease the generalization error of the seizure-free segments. The proposed method can significantly reduce the workload of data labeling while guaranteeing satisfactory performance. Escorcia-Gutierrez et al. (2022) proposed an automated deep learning-enabled brain signal classification for epileptic seizure detection. The technique consisted of three stages: pre-processing, Improved Teaching and Learning-Enabled Optimization (ITLBO)-based feature selection, and classification using the Swallow Swarm Optimization Algorithm (SSA) and Deep Belief Network (DBN). The hyper-parameters of the DBN model were optimized using the SSA to achieve the effective classification of EEG signals. Their proposed technique was found to be an effective tool for classifying brain signals and detecting and classifying epileptic seizures.
Hilal et al. (2022) proposed an intelligent deep canonical sparse autoencoder-based epileptic seizure detection and classification model. The feature selection and classification were done by a new Coyote Optimization Algorithm (COA) and autoencoder-based classifier respectively to detect different types of epileptic seizures.
Huang and Duan conducted a feature extraction utilizing Graph Convolutional Networks (GCN), followed by integration into a Graph-regularized Fuzzy Broad Learning System (GFBLS) (Huang and Duan 2023). Their approach effectively tackled the challenge of overlooking the locally invariant nature of data, resulting in enhanced performance and superior outcomes.
Zhao et al. presented the Linear LGCN (LGCN) as a solution to the issue of feature extraction from single-channel EEG data, with a specific focus on overlooking the spatial correlation between various EEG channels (Zhao et al. 2021). The primary goal was to enhance the feature representation of unprocessed EEG signals during both seizure and non-seizure phases.
Jia et al. presented a methodology for seizure prediction that leverages GCN, enabling the analysis of the connections between various brain regions depicted in a graphical manner (Jia et al. 2022). Their model was distinguished by its effectiveness and ease of use, making it well-suited for implementation on portable devices. Additionally, it offered valuable insights into the accurate identification and attributes of abnormal brain activities.
2.2.3 End-to-end pipelines
Zhang et al. (2018) proposed a Temporal CNN-based end-to-end detection of epileptic EEG signals using Bonn data and achieved an accuracy of up to 100%. Seizurenet, an end-to-end deep learning-based network for automatic seizure detection was proposed in Asif et al. (2019). The framework consisted of two modules i.e., 3D structures developed from divergence encoded spectrograms and dense feature learning (Asif et al. 2019). It is the first framework that has attempted to classify seven different types of seizures. The framework achieved an overall weighted F1 score of up to 0.88 and performed best amongst conventional classifiers like KNN, SGD classifier, XGBoost, AdaBoost, and generic CNN for seizure-type classification. DWTnet was proposed for the automatic detection of epileptic events using TUH data. The network was based on multiple feature extraction using a CNN structure (Zhang et al. 2020a). Sensitivity of up to 59% was obtained with a FAR of 12/24 h. WTRPnet was proposed for the automatic classification of epileptic and non-epileptic EEG signals in Xin et al. (2021). The network was comprised of graph features along with CNN. Their method utilized a recurrence plot in the wavelet domain to obtain the graph feature in the EEG signal.
Bajpai et al. (2021) proposed a deep learning approach for an automated EEG disease identification based on multiple CNN models. Three well-known transfer learning techniques namely DenseNet, Inception-ResNet v2, and SeizureNet were employed. Their proposed SeizureNet-SVM-based system delivered cutting-edge performance. Svantesson et al. (2021) invented the concept of virtual EEG electrodes. They used CNN as a method for up-sampling and restoring channels. The results were compared to those obtained using spherical spline interpolation. Overall, the generative networks outperformed the control networks. Furthermore, network performance improved as the number of included individuals increased, with the highest effect reported in the 5–100 subject range.
Multi-branch-based CNN has also been proposed in this field (Wang et al. 2021c). The multi-branch architecture trained on the Bern-Barcelona database yielded a high sensitivity of up to 97.78%, accuracy of up to 97.60%, and specificity of up to 97.42%. Good intra-dataset accuracy was also achieved in their work. Toraman (2021) proposed 1D caps network to classify epileptic signals from non-epileptic signals. Caps networks were able to understand structural relationships between EEG channels. A lightweight deep-learning model, for real-time epileptic seizure detection, was proposed by Qiu et al. (2022). Lu and Triesch (2019) used Bonn University and Bern Barcelona dataset and proposed an architecture that used CNN with residual connections, batch-normalization, and drop-out layers. This gave 99% accuracy on the Bonn dataset and 91.8% on Bern Barcelona.
Yao et al. (2019) proposed an Independent RNN (IndRNN) for an automatic epilepsy diagnosis. The authors compared their proposed model with the LSTM model mentioned by Hussein et al. (2018) and the CNN model by Rajendran et al. (2019) on CHB-MIT dataset. The IndRNN model outperformed the before-mentioned models. Zhang et al. (2022b) proposed an automatic seizure detection method based on a Bidirectional Gated Recurrent Unit (Bi-GRU) neural network. Tuncer and Bolat (2022) presented a method to classify EEG data for epilepsy detection using a Bi-LSTM architecture. The study focused on determining the optimal architectural hyper-parameters (number of neurons, optimization algorithms, and initial learning rate) of the Bi-LSTM model for the accurate classification of epileptic and non-epileptic activity. The proposed model achieved a success rate of 97.78% for classification, highlighting the importance of parameter optimization in the Bi-LSTM model for the accurate classification of EEG data.
Qiu et al. (2023) proposed a model to overcome weaknesses such as limited epilepsy seizure information, and characteristic information extraction in the feature map, distracting the attention of the deep learning model. They proposed a model using the Difference Attention ResNet-LTSM network (DARLNet) to capture spatial correlations and temporal dependencies. This developed a different layer to automatically mine additional epileptic seizure information. The proposed model provided superiority of DARLNet on the two-category and five-category epileptic seizure detection tasks. Parija et al. (2021) introduced an approach for classifying epileptic EEG signals using a Deep LSTM (DLSTM) based minimum variance kernel random vector functional link network. The proposed method directly applied the EEG signal to the DLSTM to obtain compressed and significant features, resulting in good classification accuracy, superior detection ability, faster speed, a simpler structure, and the ability to distinguish between seizure and non-seizure signals with a negligible false positive rate per hour.
Pre-trained networks such as VGG16, Alexnet, Inceptionv3, VGG19, Squeezenet, Googlenet, Resnet18, Densenet201, Resnet50, and Resnet101 have proposed for automatic seizure detection (Raghu et al. 2020). The authors compared the pre-trained networks with conventional feature extraction and SVM-based pipelines and reported the latter to be more efficient in terms of evaluation metrics. A compressive sensing-based network was proposed for automatic seizure prediction in Wu et al. (2021). In this network, resnet architecture was utilized to extract features from the EEG signals. Their proposed network yielded an accuracy and sensitivity up to 96.04% and 97.80% respectively. Zhang et al. (2020b) compared CNN models based on ResNet50, VGG19, and VGG16 and found VGG19 to be the most accurate for CHB-MIT data. Similarly, Gao et al. (2020) extracted features from power spectrum density energy diagrams using CHB MIT data and applied transfer learning architecture to it. Chou et al. (2023) developed a fast seizure detection model utilizing video EEG data through a CNN-based approach. Epileptic patients were recruited at video-EEG examination beds, where the four stages of EEG were labeled, confirmed by neurologists, and transformed into second-order Poincare difference plots. The authors employed four types of CNN, specifically, GoogLeNet, AlexNet, DenseNet201, and ResNet50, in their model.
Several works have been published on attention networks with data fusion. Priyasad et al. (2021) sent the EEG signal from each channel directly through a deep convolutional network composed of sinc network and 1D convolutional network layers, which learned robust features directly from the input signals, enhancing its model interpretability. The proposed method obtained an average F1 score up to 96.7%. Sun et al. (2021) proposed a method to overcome the use of single-domain analysis of vital information from EEG signals using the CHB-MIT dataset. The authors extracted both features from raw EEG and spatial-temporal features obtained from STFT. These were fed to a deep learning framework called Channel Attention Dual-input CNN. This yielded a sensitivity of 97.1%. EEG spectrogram images were developed by a novel proposed architecture called Deep Convolutional Generative Adversarial Network (DCGAN) in Rasheed et al. (2021). For data validation, one-class SVM and convolutional epileptic seizure predictor were used which yielded an accuracy of 88.21% on CHB-MIT data. The work also used transfer learning VGG16, VGG19, Inceptionv3, and ResNet50, out of which Inceptionv3 produced the best accuracy in epileptic seizure detection.
Sui et al. (2021) proposed an iEEG classification technique called the Time–Frequency Hybrid Network (TF-HybridNet) which utilizes feature fusion. This method uses STFT to process iEEG in parallel and 1d convolution layers to extract feature maps and time–frequency domain features. These are combined and then input into a two-dimensional CNN, which achieves a classification accuracy of 94.3%. Ma et al. (2023) developed Transformers for Seizure Detection, a deep learning architecture based on encoder–decoder structure and on attention mechanisms. This was applied on the brain signals. When the input domain provided was time–frequency, the model yielded the best results. Qin et al. (2020) also used 13 layers of multi-scale fusion CNN, on the Bonn dataset, with PSD and a combination of dilated convolution kernels and common convolution kernels which lead to an accuracy of 98.67%. Einizade et al. (2023) presented a model-fusion approach called Frequency-adaptive Transform-based Temporal Network (FATTNet) that can assign dynamic weights to different views in order to improve seizure detection. The model integrated EEG signals from multiple channels, WPD, and hand-engineered features as three different views. The authors also proposed a technique to eliminate the unwanted signals that do not directly originate from the brain. The proposed method is interpretable for medical professionals, which can aid clinicians in determining seizure-occurring regions of the brain. Zhao et al. (2023a) presented an approach for detecting seizures automatically, utilizing the fusion of local and global features via a coupling block called the Feature Coupling Block (FCB) in a hybrid CNN and Transformer architecture. The FCB combined the information in an interactive way and improved the feature representation, which was then passed to the classifier for the classification of normal and seizure EEG. By leveraging the strengths of both models, the proposed method achieved convergence within a short time frame of 15 min.
Islam et al. (2022) proposed an innovative method for detecting epileptic seizures using a deep learning model called Epileptic Network. Their model utilized various components, such as Dense Convolutional Blocks (DCB), Feature Attention Modules (FAM), Residual Blocks (RB), and Hypercolumn Technique (HT) to extract and analyze essential features from EEG samples. DCB was used to obtain discriminative features, FAM extracted essential features, RB learned important parts, and HT retained local features extracted from different levels of the model. The authors have demonstrated that the proposed Epileptic-Net model performs exceptionally well with data augmentation, and its performance is slightly reduced without augmentation.
Zhao et al. (2023b) proposed a data augmentation approach called Random Channel Ordering (RCO), which generated new images by adjusting the channel order. To interpret the models, the Gradient-weighted class activation mapping (Grad-CAM) and attention layer methods were used. The proposed model exhibited excellent performance in the seizure detection task, and the Grad-CAM and attention layer methods provided a clear explanation of the model’s detection basis and computed a value to measure the seizure degree. Moreover, the RCO method effectively addressed the imbalance problem commonly encountered in clinical practice.
The proposed method by Peh et al. (2023) is a patient-independent seizure detector that can automatically detect seizures in both scalp EEG and intracranial EEG (iEEG). The method used CNN to detect seizures and extract regional features, and applied post-processing filters to determine the start and end points of the seizures. The minimum overlap evaluation scoring was used to evaluate the performance of the seizure detector. The method showed good performance in terms of sensitivity and precision and had low false positive rates per hour. The proposed seizure detector can be used for adult EEGs and has a fast detection time of fewer than 15 s for 30-min EEG signals.
3 Development in algorithmic implementations using hardware systems
This section discusses the reported algorithmic implementations using hardware systems in this research field. Table 5 summarizes the below-mentioned developments in chronological order since 2013. The signal processing techniques, features used, classification method, hardware implementations, and the achieved accuracy have been utilized as parameters to summarize the existing studies. In addition to traditional evaluation metrics, it is imperative to consider additional performance metrics in hardware implementations of machine learning algorithms. In particular, inference speed, Frames Per Second (FPS), and processing speed are emerging as vital parameters that significantly impact the efficiency and effectiveness of hardware-based solutions. These metrics reflect the ability of the hardware to process data swiftly and efficiently, which is crucial for real-time applications and resource-constrained environments. As hardware-based machine learning technologies continue to evolve, it is recommended that future research endeavors incorporate the evaluation of these metrics alongside traditional measures to provide a comprehensive assessment of processing speed and efficiency in hardware implementations.
Qidwai et al. (2013) proposed a method to classify normal, pre-seizure, and seizure using a micro-controller by feeding the EEG signals from wireless body area network (version 7) headset to data-acquisition software via LabView. In this method, the initial algorithms were first simulated and tested on controlled data. Shakir et al. (2014) proposed a wearable system to classify EEG signals into normal or seizure for a real-time clinical setting. The authors implemented a rule-based classification method on LilyPad-micro-controller using Arduino and a Bluetooth device. Kusmakar et al. (2018) developed an automated seizure detection system using a single wrist-worn device. The developed system was trained and tested for seventy-nine convulsive seizure patients. The authors concluded that data-explicit learning techniques are more vital to seizure detection systems rather than using multiple sensors.
Ictal and seizure-free EEG signal classification was implemented on the Field Programmable Gate Arrays (FPGA) of the Second-Order Difference Plot (SODP) in Singh and Amalin (2015). The developed hardware sampled the IMF of an EEG signal as input. Zhang and Parhi (2015) proposed a patient-specific algorithm for detecting seizures in epileptic patients using spectrograms and feature selection techniques. The algorithm was designed to be of low complexity and low power. Such a combination made it suitable for implementation on portable devices such as smartphones or wearable devices. The algorithm achieved high sensitivity, specificity, and an average AUC of 0.9818 on the UPenn and Mayo Clinic’s Seizure Detection Challenge database. The work achieved the highest sensitivity and specificity regarding Bajaj and Pachori (2013), and Yuan et al. (2012). Wang et al. (2018) developed a real-time seizure detection technique based on STFT and SVM, as well as its FPGA implementation. The experimental results demonstrated that their method may speed up the non-linear SVM when compared with existing methods.
Feng et al. (2018) proposed a hardware implementation of the second-order difference plot (SODP) technique using a Xilinx FPGA for classifying epileptic EEG signals. The SODP technique is a signal-processing algorithm commonly used in the analysis of EEG signals to detect epileptic seizures. The authors demonstrated that FPGAs can improve the speed and efficiency of epileptic seizure detection compared to traditional hardware-based approaches like the use of micro-controllers and Arduino. The results from the FPGA implementation were consistent with MATLAB simulations, indicating the potential of FPGAs for real-time seizure detection.
Chatterjee et al. (2019) proposed a separation principle for designing controllers and estimators for closed-loop neuro-technology using discrete-time Fractional-Order Dynamical Systems (FODS). FODS are capable of capturing spatiotemporal relations with non-Markovian properties, which are common in neuro-biological systems. The proposed separation principle allows for the decoupling of the design of the controller and estimator, leading to improved closed-loop performance. As a proof-of-concept, their proposed method demonstrates the application of the separation principle to EEG data. The results indicated that the proposed approach may provide better control of brain states in closed-loop neuro-technology applications for epilepsy and seizure patients.
Dutta et al. (2019) used Ferroelectric FET (FeFET) to perform classification directly on analog sensor signals. By using FeFET, they reduced the energy and the area previously used and got an accuracy of 98.46%. With the collection of frontal and front-temporal lobe EEG signals, Zhan et al. (2019) proposed work with better results in comparison to traditional systems. They used low-power FPGA for lower power and detection latency. Zhan et al. (2019) designed a wireless self-contained mini-printed circuit board. The hardware architecture was used for patient-specific seizure detection. The seizure detection was done accurately using quickly mountable dry-electrode ambulatory EEG headsets. The presented model provided a seizure detection sensitivity and specificity of up to 92.5% and 80.1%.
Teijeiro et al. (2019) suggested the use of low-cost hardware for seizure detection. Syed Rafiammal et al. (2019) developed an intellectual property core integrated design. Their model was implemented using a system-on-chip FPGA. Five statistical parameters namely range, root mean square value, standardized moment, coefficient of variance, and energy of the EEG signals were used to extract features for classification. The model achieved an accuracy of up to 97.2% on the University of Bonn database. Burrello et al. (2020) proposed an intracranial EEG signals-based algorithm for detecting epileptic seizures with short latency.
Elhosary et al. (2019) used SVM with sequential minimal optimization for efficient classification of epileptic EEG signals. The work compared FPGA and Application-Specific Integrated Circuit (ASIC)-based implementation wherein ASIC was more power-efficient in comparison to FGPA. The sensitivity of their proposed work using linear kernel function was found to be better than Liu et al. (2012), Yuan et al. (2011) and Shoaran et al. (2018) which used RBF instead. A light seizure detection algorithm was proposed using an ensemble of gradient-boosted decision tree classifiers (Shoaran et al. 2016). The algorithm was able to compete with more complex learning models proposed for on-chip implementation. A Real-Time Wearable FPGA-based seizure detection processor was proposed in Marni et al. (2018) using metropolis–hastings. The pipeline gave an average sensitivity of 90%, an average seizure prediction accuracy of 81.47%, and an onset sensitivity of 100%.
Huang et al. (2019) developed an SVM processor to support seizure detection and on-chip model adaptation for epileptic seizure control. The SVM classifier-based training was carried out using the Alternating Direction Method of Multipliers (ADMM) for highly parallel computing. The hardware complexity was reduced by 87% through a hardware-shared configurable CORDIC-based processing element array. The processor achieved the shortest detection latency compared to other seizure detectors and supports real-time model adaptation. According to their study, the processor has the potential to improve the clinical application of seizure detection and control in epileptic patients. Altaf and Yoo (2015) implemented a non-linear SVM-based epilepsy classification on a Complementary Metal-Oxide Semiconductor (CMOS) processor. Time-division multiplexing was also utilized. The authors showed that the use of a log-linear Gaussian function was found to consume less energy and area in comparison to the linear Gaussian function. Another work implemented SVM on FPGA and achieved an accuracy of 96.8% (Feng et al. 2017). An automatic seizure detection algorithm was proposed by Syed Rafiammal et al. (2020) which included the use of a linear binary SVM classifier.
Taghavi and Shoaran (2019) compared the hardware complexities between deep neural networks and decision trees for neural data classification like seizure and reported that decision trees consumed more energy and were found to be more area efficient. Classification of generalized and focal epileptic seizure was done using a feed-forward multi-layer neural network architecture by Sarić et al. (2020). The proposed model confirmed that an automated system based on a machine learning algorithm with high accuracy can be successfully implemented on FPGA chips which makes them portable and easy to use in everyday diagnosis situations in healthcare institutions. Using a hybrid approach, Page et al. (2016) proposed a wearable seizure detection system. The work utilized max-pooling CNNs to perform end-to-end learning. Sahani et al. (2021) proposed a model for recognizing epileptic seizures using EEG signals. The authors combined optimized variational mode decomposition reduced deep convolutional neural network, and multi-kernel random vector functional link network to extract discriminative features and classify EEG signals. The proposed model achieved high classification accuracy, early recognition capability, and negligible false positive rate.
In order to improve the detection of epileptic seizures, Beeraka et al. (2022) proposed a method using deep learning models with FPGA implementation of the short-time Fourier transform block. EEG segments were analyzed using STFT to extract frequency bands and features of interest in a time–frequency manner. The detection of seizures was performed using a combination of CNN and Bi-LSTM. The proposed approach was not specific to epileptic seizures, and could potentially be applied to classify other physiological signals. Bahr et al. (2021) proposed a CNN architecture for epileptic seizure detection capable of running on an ultra-low-power microprocessor. The proposed approach provided better results in terms of power consumption by a factor of six. The proposed approach is applicable to epilepsy with recurring and comparable seizure events. Ayodele et al. (2020) proposed a domain-generalized approach to combine data from two different datasets for training and then testing on a third dataset. In their proposed approach, 3D electrode coordinates were transformed into 2D space. The generated vectors were a sequence of seventeen ten-layered \(16\times 16\) raster arrays. They were then used to train a recurrent CNN. The proposed model was tested on CHB-MIT and TUSZ datasets and provided a sensitivity of up to 74.5%
Work has also been done to address the cyber-security issues in this field (Pandey et al. 2019). The authors used a multistage residual NN and the concept of under-sampling for the class imbalance problem. Their proposed method did not require any signal processing and showed efficient results on a small duration of the signal. Internet of Medical Things (IoMT) based systems have also been proposed for seizure detection (Sayeed et al. 2019). Lin et al. (2018) proposed a system platform that involved the design of a smart headband for epileptic seizure detection and the creation of smart device applications for healthcare that are integrated with a cloud computing platform.
Crispin-Bailey et al. (2019) proposed an integrated circuit device using a novel ‘xor-log2-sub-band’ compression scheme to compress EEG data utilized for automatic seizure detection. Their proposed design achieved a low power consumption and low silicon area cost in comparison to state-of-the-art compression algorithms in this field. El Halabi et al. (2019) proposed a jacket-based seizure detection system. The jacket consists of Arduino and raspberry pi. Alzghoul (2023) developed a wearable device that can detect epilepsy seizures which included an Arduino processor. To overcome the challenges of noise and power replacement, a work done by Zaghloul and Bayoumi (2019) proposed a brain–computer-interface-based disposable and wireless band system. The authors used Cauchy-based filters to remove the noise followed by a prediction method that resulted in an average seizure prediction time of up to 14.56 s. To overcome high input impedance, low-input-referred noise, and high Common-Mode Rejection Ratio (CMRR), a neural signal-specific analog-to-digital converter was proposed with an instrumentation amplifier in Tohidi et al. (2019a).
The latest works have focused on developing an in-memory memristive crossbar-based accelerator simulator (Li et al. 2023). The training was done with a Python-based code. Xu et al. (2023) proposed a deep learning-based framework to obtain short latency in real-time EEG-based epileptic seizure detection. Multi-scale 3D CNN architecture was used to accurately capture predictive probabilities of samples. The authors proposed a rectified weighting strategy to enhance predictive probabilities. In order to achieve short detection latency, the authors developed an accumulative decision-making rule that allowed for the efficient processing of EEG samples in real time.
4 Existing patents in this field
This section discusses existing granted patents in the field of automatic epilepsy diagnosis and seizure detection using EEG signals since 2011. Both software algorithms implemented using a hardware system and EEG-related patents related to this field have been discussed. Table 6 summarizes the below-mentioned patents in chronological order. The dataset used, signal processing methods, features used, detection methods, and their benefits have been discussed.
The first work by Nandor et al. (2011) focused on proposing a seizure detector headset, a hardware system that included a head mounting arrangement, and a plurality of electrodes disposed on the arrangement of the setup. An installed image-capturing device was installed in the headset which clicks pictures of the brain activity. Upon any variation, an alarm was raised to alert the patient’s family. Osorio and Frei (2017) proposed a software-based pipeline model to detect seizures in patients using a medical device by analyzing electrographic data. Tohidi et al. (2019b) developed a wearable low-power and low-noise epileptic seizure detection system. Epileptic seizures were detected with a sensitivity of 100% and about 2 uW of power was found to be reduced. Another study done in Xianji (2021) focused on developing a wearable system to automatically diagnose epileptic abnormalities in EEG signals using Kalman filtering.
Yun et al. (2019) invented a portable adjustable head-mounted epilepsy monitor-based system to detect the pre-onset of epileptic activity on EEG. The designed monitor collected EEG signals through the head-mounted EEG cap and then transmitted them to the calculation module for analysis and processing. If a seizure was detected, the result was sent to the patient’s mobile application via Bluetooth. Their work showed an accuracy of up to 99.98%. Kamousi et al. (2020) proposed a hardware implementation for seizure detection based on EEG signals received over different channels for a subject. The proposed method included preprocessing the EEG signals, extracting features, and applying a machine learning algorithm for classification. Additionally, a separate system was employed to determine the seizure burden, and if it exceeded a threshold, a notification was generated for healthcare practitioners to assess the subject’s seizure risk.
Aquino et al. (2022) proposed a hardware system including video cameras and a processor system. The processor detected and identified objects in a received video frame classified objects by applying a facial recognition algorithm to identify the person, and determine the posture of the person. The processor determines a change in motion, of the person based on the determined posture and the change in motion and determines that the person has experienced a defined event. It may be useful for immediate emergency events. Narayan (2021) proposed a system to detect abnormal signals for neurological diseases like epilepsy. The authors utilized a catheter which may be used to position through the use of a controller. The controller was guided by the movement of the catheter.
Mishra et al. (2022) proposed a system that consisted of electrodes capable of identifying the brain activity connected to the micro-controller. The micro-controller was capable of executing machine learning algorithms and deep learning algorithms. It was designed to convert the EEG signals into relevant images and perform histogram-of-oriented gradients feature-based models to predict different categories of infected and non-infected seizures due to different brain injuries. Further, images were preprocessed by the Modified Water Cycle Algorithm (MWCA) and wavelet transform-based segmentation technique for detection of seizure from converted images that may inform the doctor about the details of seizure due to tumor or accident. HECOX and Kurt (2021) proposed a seizure detection system that utilized circuits to receive EEG signals from a patient’s brain and determined metrics based on the non-linear features of the signal. These metrics indicated a candidate seizure. The change in the non-linear features was used to determine the physiological force that gave rise to the seizure activities.
A software-based work granted in 2016, focused on using conventional signal processing methods like wavelet and genetic algorithms. The authors in Li et al. (2016) focused on reducing the problems of low accuracy, poor universality, and functional singleness for automatic seizure detection. About 99.3% accuracy was achieved using the SVM classifier. Saminu et al. (2019) proposed a low computational cost system that extracted time–frequency, statistical, and non-linear features from EEG signals. The authors then utilized SVM and feed-forward NN to classify ictal and interictal signals. Their method yielded an accuracy of up to 99.6%. Xiaofeng et al. (2019) proposed an implementation of an SVM-based pipeline using a lower-power circuit for an epilepsy diagnosis. The authors extracted the wavelet coefficients from the EEG signals and fed them to a linear SVM classifier for classification purposes.
Chan et al. (2021) proposed a software-pipeline-based method for concurrent detection and early detection of epileptic seizure episodes using scalp EEG data collected from patients in between normal activities. They developed a machine learning-based early detection model that was trained and retrained at predetermined intervals on the collected data to issue an early warning of an upcoming seizure episode. The proposed method offers a promising approach for patients with epilepsy to take necessary preparatory actions to mitigate the impact of the seizure episodes. Another work by Chakrabarti et al. (2021) focused on epileptic seizure detection which was independent of channels. The authors utilized the classic traditional pipelines in which wavelet packet transform was utilized as a feature extractor. The classification was done using a tree-based algorithm. Based on the classification output, an alarm was triggered.
Besson and Bandt (2022) proposed a system that utilized geometric deep-learning techniques to predict clinically relevant brain features. They used graph convolutional neural networks and auto-encoder networks to analyze brain surface morphology. Another software-based work (Ullah et al. 2018) proposed a seizure detection system using pyramidal 1D CNN. Hyper-parameter tuning of SVM-based pipeline for automatic seizure detection was proposed in Handa et al. (2023). The invention focused on classifying peri-ictal and non-seizure EEG signals for CHB-MIT EEG signals using a hardware implementation of the SVM and KNN-based pipeline. The latest methods have also utilized GAN to generate epileptic seizure EEG data (Ling et al. 2019).
5 Evaluation metrics used in this field
Since 1965, several evaluation metrics have been used to show the efficacy of AI models in automatic epilepsy diagnosis and seizure detection. Each metric mentioned in Table 7 has its significance in this research field. Evaluation metrics vary for software and hardware-based implementations. Signal quality, sampling frequency, speed of operation, and CMRR play an important role in hardware-related systems. The true positives, true negatives, false positives, and false negatives are the basic metrics that can be cumulatively represented through the confusion matrix. Parameters like accuracy, and the error rate are paramount in both software pipelines and their implementation on hardware systems which show how accurate or error-free the model is while predicting or detecting a seizure event. Evaluation metrics focusing on the false rate or speed with which a seizure event is classified or detected or predicted are also important.
Model accuracy is an important parameter but can’t be considered the only reliable factor for analysis as class imbalance is an inevitable case for real-time epilepsy diagnosis and seizure detection scenarios. Hence, discriminating-based evaluations such as the AUC-ROC curve, sensitivity, specificity, and positive and negative predictive values etc play a paramount role in such cases. Geometric mean also helps in evaluating the AI model when time series are not independent of each other. The Kappa score represents the extent to which the data collected show correct representations of the variables measured or not. Recall value helps in measuring the accuracy of a particular AI model as to whether it is able to identify the relevant data or not. ROC and AUC curves show a graphical representation of any possible connection between sensitivity and specificity for every possible cut-off for a test set. They are based on probability curves. Root mean square and R square value along with residual plot visualization plays an integral role in evaluating regression-based seizure signal forecasting. R square values help in estimating the relationship between the movements of a dependent variable based on an independent variable’s movements. Interested readers may study the advantages and biases of the evaluation metrics from Hossin and Sulaiman (2015), Chicco and Jurman (2020), García et al. (2014).
These metrics are often used as a source of comparison between research works. However, to date, there is no standardized protocol to compute a set of evaluation metrics in this field. Guidelines and quality criteria set for prediction models in healthcare, strongly suggest discrimination-based evaluation followed by mathematical and experimental analysis (de Hond et al. 2022).
6 Conclusion and future directions
Epilepsy is a chronic neurological disorder that has affected more than fifty-million individuals worldwide. It is characterized by unprovoked and recurring seizures that leave their distinct impressions in EEG signals. However, their interpretation is presently difficult and is a repetitive task that requires the attention of skilled neuro-technologists. AI algorithms are trying to aid in the interpretation of EEG signals for an effective and early epilepsy diagnosis.
The present review focused on systematically reporting software advancements and their implementation using hardware systems in automatic epilepsy diagnosis and seizure detection for the past 10 years. More than 300 articles have been summarized, compared, and discussed with the help of various subjective and objective parameters and the use of datasets in this comprehensive review. Recent articles and patents of 2023 have also been discussed.
This research field has shifted from the use of traditional signal processing methods, manual feature extraction, and the use of classical machine learning algorithms to the implementation of end-to-end detection and prediction of epileptic EEG signals using deep learning methods in the past 10 years. In machine learning based algorithms, a data split of training and testing have been considered. With the evolution of deep learning algorithms, training, validation and testing with different splits or cross-validation have been followed. This trend is similar for software pipelines, their hardware implementations, and patents granted in this field. The hardware implementations started with the use of micro-controllers, Arduino, raspberry pi, and Bluetooth technology and have now shifted to the use of CMOS, ASIC, and FPGA technology. The VLSI-based implementations offer low power, area, and high speed of operation and promote miniaturization. These advantages help in the development of easy-to-carry detection systems which can detect signals from the human brain and alert the caretakers before and during a seizure event.
Through this comprehensive review, it is evident that the AI-based pipelines can reduce the introspection time by at least 50% without compromising the diagnostic accuracy or abnormal event detection. Improvement in false-positive rates and inter-rater reliability has been observed in the studies using cross-datasets. Studies based on IOMT, Bluetooth technology, brain–computer interfacing, and quantum computing are slowly emerging (Svensson et al. 2019; Duun-Henriksen et al. 2020). Recent concepts in meta-learning, one-short learning, and meta-transfer learning are yet to be explored in this field.
A rise in hardware implementations of software-based algorithms and the release of free EEG datasets has been observed after 2018. Researchers have come forward and developed more than twenty-eight EEG datasets for the development of AI pipelines in this field. However, different approaches have been taken up to develop these datasets and thus require a need of standardization. Different methods have been used to select the window length of EEG signals fed to the AI models at a time. There is no standardized data reduction, noise removal, and optimization method followed in this field. Several data recordings using portable EEG, e-wearable bands, sub-scalp ultra-long term EEG and EEG head caps have been explored. They do not require the patient to stay in a hospital for a long time, are hassle-free, and promote normal living for PWE.
With the increasing computational complexity of the AI models in this field, more focus is required on interpreting the reported AI pipelines. AI models must address the reproducibility, generalizability, and security-related challenges in this field. Advanced analysis like ablation study, weight study, dissection analysis, hyper-parameter tuning, causality, class activation maps, feature maps, and statistical visualization should also be introduced to further interpret the AI models. Dataset development and classification of epilepsy-specific and seizure-specific are required. Fewer studies have focused on cross-patient analysis which should be considered as a benchmark during the analysis of AI models. Studies still lack transparency in the data partitioning methods utilized. A generalized protocol should be set for the pre-processing and post-processing steps of large EEG datasets, and evaluation metrics utilized in this field. With the active participation from the scientific community, real-time epilepsy diagnosis and seizure detection for routine clinical use is a near-reality.
Data availability
Not applicable.
Code availability
Not applicable.
References
Abbasi MU, Rashad A, Basalamah A, Tariq M (2019) Detection of epilepsy seizures in neo-natal EEG using LSTM architecture. IEEE Access 7:179074–179085
Abdulbaqi AS, Younis MT, Younus YT, Obaid AJ (2022) A hybrid technique for EEG signals evaluation and classification as a step towards to neurological and cerebral disorders diagnosis. Int J Nonlinear Anal Appl 13(1):773–781
Abdulhay E, Elamaran V, Chandrasekar M, Balaji VS, Narasimhan K (2020) Automated diagnosis of epilepsy from EEG signals using ensemble learning approach. Pattern Recogn Lett 139:174–181
Abdulla S, Diykh M, Alkhafaji SKD, Greena JH, Al-Hadeethi H, Oudah AY, Marhoon HA (2022) Determinant of covariance matrix model coupled with AdaBoost classification algorithm for EEG seizure detection. Diagnostics 12(1):74
Acharya UR, Oh SL, Hagiwara Y, Tan JH, Adeli H (2018) Deep convolutional neural network for the automated detection and diagnosis of seizure using EEG signals. Comput Biol Med 100:270–278
Aghababaei MH, Azemi G, O’Toole JM (2021) Detection of epileptic seizures from compressively sensed EEG signals for wireless body area networks. Expert Syst Appl 172:114630
Ahmad MA, Khan NA, Majeed W (2014) Computer assisted analysis system of electroencephalogram for diagnosing epilepsy. In: 2014 22nd international conference on pattern recognition. IEEE, pp 3386–3391
Ahmad I, Wang X, Zhu M, Wang C, Pi Y, Khan JA, Khan S, Samuel OW, Chen S, Li G (2022) EEG-based epileptic seizure detection via machine/deep learning approaches: a systematic review. Comput Intell Neurosci 2022:6486570
Ahmedt-Aristizabal D, Fernando T, Denman S, Petersson L, Aburn MJ, Fookes C (2019) Neural memory networks for robust classification of seizure type. arXiv Preprint http://arxiv.org/abs/1912.04968
Akbari H, Sadiq MT (2021) Detection of focal and non-focal EEG signals using non-linear features derived from empirical wavelet transform rhythms. Phys Eng Sci Med 44(1):157–171
Albaqami H, Hassan GM, Subasi A, Datta A (2021) Automatic detection of abnormal EEG signals using wavelet feature extraction and gradient boosting decision tree. Biomed Signal Process Control 70:102957
Al-Salman W, Li Y, Wen P, Miften FS, Oudah AY, Al Ghayab HR (2022) Extracting epileptic features in EEGs using a dual-tree complex wavelet transform coupled with a classification algorithm. Brain Res 1779:147777
Altaf MAB, Yoo J (2015) A 1.83 μJ/classification, 8-channel, patient-specific epileptic seizure classification SoC using a non-linear support vector machine. IEEE Trans Biomed Circuits Syst 10(1):49–60
Alzghoul SEMF et al (2023) A scoring framework and apparatus for epilepsy seizure detection using a wearable belt. J Med Signals Sens 12(4):326–333
Amiri M, Aghaeinia H, Amindavar HR (2023) Automatic epileptic seizure detection in EEG signals using sparse common spatial pattern and adaptive short-time Fourier transform-based synchrosqueezing transform. Biomed Signal Process Control 79:104022
Amudhan S, Gururaj G, Satishchandra P (2015) Epilepsy in India I: epidemiology and public health. Ann Indian Acad Neurol 18(3):263
Anand S, Jaiswal S, Ghosh PK (2019) Epileptic seizure detection in EEG signal using discrete stationary wavelet-based Stockwell transform. Majlesi J Electr Eng 13(1):55–63
Andrzejak RG, Lehnertz K, Mormann F, Rieke C, David P, Elger CE (2001) Indications of nonlinear deterministic and finite-dimensional structures in time series of brain electrical activity: dependence on recording region and brain state. Phys Rev E 64(6):061907
Andrzejak RG, Schindler K, Rummel C (2012) Nonrandomness, nonlinear dependence, and nonstationarity of electroencephalographic recordings from epilepsy patients. Phys Rev E 86(4):046206
Anuragi A, Sisodia DS, Pachori RB (2021) Automated FBSE-EWT based learning framework for detection of epileptic seizures using time-segmented EEG signals. Comput Biol Med 136:104708
Anuragi A, Sisodia DS, Pachori RB (2022) Epileptic-seizure classification using phase-space representation of FBSE-EWT based EEG sub-band signals and ensemble learners. Biomed Signal Process Control 71:103138
Aquino V, Bennis M, Comlekoglu T, Griscavage J, Hildebrandt C (2022) System and methods for safety, security, and well-being of individuals, March 22. US Patent 11,282,367
Arunkumar N, Ram Kumar K, Venkataraman V (2018) Entropy features for focal EEG and non focal EEG. J Comput Sci 27:440–444
Asif, Roy S, Tang J, Harrer S (2019) SeizureNet: a deep convolutional neural network for accurate seizure type classification and seizure detection. arXiv
Avcu MT, Zhang Z, Chan DWS (2019) Seizure detection using least EEG channels by deep convolutional neural network. In: ICASSP 2019-2019 IEEE international conference on acoustics, speech and signal processing (ICASSP). IEEE, pp 1120–1124
Ayodele KP, Ikezogwo WO, Komolafe MA, Ogunbona P (2020) Supervised domain generalization for integration of disparate scalp EEG datasets for automatic epileptic seizure detection. Comput Biol Med 120:103757
Bahr A, Schneider M, Francis MA, Lehmann HM, Barg I, Buschhoff AS, Wulff P, Strunskus T, Faupel F (2021) Epileptic seizure detection on an ultra-low-power embedded RISC-V processor using a convolutional neural network. Biosensors 11(7):203
Bajaj V, Pachori RB (2011) Classification of seizure and nonseizure EEG signals using empirical mode decomposition. IEEE Trans Inf Technol Biomed 16(6):1135–1142
Bajaj V, Pachori R (2013) Epileptic seizure detection based on the instantaneous area of analytic intrinsic mode functions of EEG signals. Biomed Eng Lett 03:17–21
Bajpai R, Yuvaraj R, Prince AA (2021) Automated EEG pathology detection based on different convolutional neural network models: deep learning approach. Comput Biol Med 133:104434
Balamareeswaran M, Ebenezer D (2015) Denoising of EEG signals using discrete wavelet transform based scalar quantization. Biomed Pharmacol J 8(1):399–406
Banerjee S, Alur V, Shah D (2019) Patient nonspecific epilepsy detection using eeg. In: Wang J, Ram Mohana Reddy G, Kamakshi Prasad V, Sivakumar Reddy V (eds) Soft computing and signal processing, soft computing and signal processing: proceedings of ICSCSP 2018, vol 1
Beeraka SM, Kumar A, Sameer M, Ghosh S, Gupta B (2022) Accuracy enhancement of epileptic seizure detection: a deep learning approach with hardware realization of STFT. Circuits Syst Signal Process 41:461–484
Besson PA, Bandt SK (2022) Brain feature prediction using geometric deep learning on graph representations of medical image data, April 21. https://patents.google.com/patent/US20220122250A1. US Patent App. 17/505,465
Bhattacharyya A, Pachori RB (2017) A multivariate approach for patient-specific EEG seizure detection using empirical wavelet transform. IEEE Trans Biomed Eng 64(9):2003–2015
Bhattacharyya A, Pachori RB, Upadhyay A, Acharya UR (2017) Tunable-Q wavelet transform based multiscale entropy measure for automated classification of epileptic EEG signals. Appl Sci 7(4):385
Bilal M, Rizwan M, Saleem S, Khan MM, Alkatheir MS, Alqarni M (2019) Automatic seizure detection using multi-resolution dynamic mode decomposition. IEEE Access 7:61180–61194
Boonyakitanont P, Lek-Uthai A, Chomtho K, Songsiri J (2020) A review of feature extraction and performance evaluation in epileptic seizure detection using EEG. Biomed Signal Process Control 57:101702
Borowska M (2021) Multiscale permutation Lempel-Ziv complexity measure for biomedical signal analysis: interpretation and application to focal EEG signals. Entropy 23(7):832
Boubchir L, Daachi B, Pangracious V (2017) A review of feature extraction for EEG epileptic seizure detection and classification. In: 2017 40th international conference on telecommunications and signal processing (TSP). IEEE, pp 456–460
Brari Z, Belghith S (2021) A novel machine learning model for the detection of epilepsy and epileptic seizures using electroencephalographic signals based on chaos and fractal theories. Math Probl Eng 2021:2107113
Burrello A, Benatti S, Schindler K, Benini L, Rahimi A (2020) An ensemble of hyperdimensional classifiers: hardware-friendly short-latency seizure detection with automatic iEEG electrode selection. IEEE J Biomed Health Inform 25(4):935–946
Cao J, Zhu J, Hu W, Kummert A (2019) Epileptic signal classification with deep EEG features by stacked CNNs. IEEE Trans Cogn Dev Syst 12(4):709–722
Chakrabarti S, Swetapadma A, Pattnaik PK (2021) A channel independent generalized seizure detection method for pediatric epileptic seizures. Comput Methods Progr Biomed 209:106335
Chakraborty M, Mitra D et al (2021) Epilepsy seizure detection using kurtosis based VMD’s parameters selection and bandwidth features. Biomed Signal Process Control 64:102255
Chan CK, Chan A, Chung JM, Xu L, Tam FK (2021) Method of early detection of epileptic seizures through scalp EEG monitoring, September 16. US Patent App. 17/195,328
Chatterjee S, Romero O, Pequito S (2019) A separation principle for discrete-time fractional-order dynamical systems and its implications to closed-loop neurotechnology. IEEE Control Syst Lett 3(3):691–696
Chen S, Zhang X, Chen L, Yang Z (2019) Automatic diagnosis of epileptic seizure in electroencephalography signals using nonlinear dynamics features. IEEE Access 7:61046–61056
Chen Z, Lu G, Xie Z, Shang W (2020) A unified framework and method for EEG-based early epileptic seizure detection and epilepsy diagnosis. IEEE Access 8:20080–20092
Chicco D, Jurman G (2020) The advantages of the Matthews correlation coefficient (MCC) over F1 score and accuracy in binary classification evaluation. BMC Genomics 21:1–13
Choi G, Park C, Kim J, Cho K, Kim TJ, Bae H, Min K, Jung KY, Chong J (2019) A novel multi-scale 3D CNN with deep neural network for epileptic seizure detection. In: 2019 IEEE international conference on consumer electronics (ICCE). IEEE, pp 1–2
Chou CH, Shen TW, Tung H, Hsieh PF, Kuo CE, Chen TM, Yang CW (2023) Convolutional neural network-based fast seizure detection from video electroencephalograms. Biomed Signal Process Control 80:104380
Crispin-Bailey C, Dai C, Austin J (2019) A 65-nm CMOS lossless bio-signal compression circuit with 250 FemtoJoule performance per bit. IEEE Trans Biomed Circuits Syst 13(5):1087–1100
Cserpan D, Boran E, Rosch R, Pietro Lo Biundo S, Ramantani G, Sarnthein J (2021) Dataset of EEG recordings of pediatric patients with epilepsy based on the 10-20 system. OpenNeuro. https://doi.org/10.18112/openneuro.ds003555.v1.0.0
Currey D, Hsu D, Ahmed R, Venkataraman A, Craley J (2021) Cross-site epileptic seizure detection using convolutional neural networks. In: 2021 55th annual conference on information sciences and systems (CISS). IEEE, pp 1–6
Das AB, Bhuiyan MI (2016) Discrimination and classification of focal and non-focal EEG signals using entropy-based features in the EMD-DWT domain. Biomed Signal Process Control 29:11–21
Das K, Daschakladar D, Roy PP, Chatterjee A, Saha SP (2020) Epileptic seizure prediction by the detection of seizure waveform from the pre-ictal phase of EEG signal. Biomed Signal Process Control 57:101720
Dash DP, Kolekar MH, Jha K (2020) Multi-channel EEG based automatic epileptic seizure detection using iterative filtering decomposition and hidden Markov model. Comput Biol Med 116:103571
Dash DP, Kolekar MH, Jha K (2021) Surface EEG based epileptic seizure detection using wavelet based features and dynamic mode decomposition power along with KNN classifier. Multimed Tools Appl 81:42057–42077
de Hond AAH, Leeuwenberg AM, Hooft L, Kant IMJ, Nijman SWJ, van Os HJA, Aardoom JJ, Debray T, Schuit E, van Smeden M et al (2022) Guidelines and quality criteria for artificial intelligence-based prediction models in healthcare: a scoping review. npj Digit Med 5(1):1–13
Deepa B, Ramesh K (2021) Preprocessed CHB-MIT scalp EEG database. https://doi.org/10.21227/awcw-mn88
Deivasigamani S, Senthilpari C, Yong WH (2021) Machine learning method based detection and diagnosis for epilepsy in EEG signal. J Ambient Intell Humaniz Comput 12(3):4215–4221
Detti P (2020) Siena scalp EEG database (version 1.0.0). https://doi.org/10.13026/5d4a-j060
Detti P, Vatti G, Zabalo Manrique de Lara G (2020) EEG synchronization analysis for seizure prediction: a study on data of noninvasive recordings. Processes 8(7):846
Deuschl G (1999) Recommendations for the practice of clinical neurophysiology. Guidelines of the International Federation of Clinical Neurophysiology
Dhar P, Garg VK, Rahman MA (2022) Enhanced feature extraction-based CNN approach for epileptic seizure detection from EEG signals. J Healthc Eng 2022:3491828
Dominguez EC, Subathra MSP, Sairamya NJ, Thomas George S (2020) Detection of focal epilepsy in brain maps through a novel pattern recognition technique. Neural Comput Appl 32(14):10143–10157
Duanpo W, Zimeng W, Lurong J, Fang D, Xunyi W, Shuang W, Yao D (2019) Automatic epileptic seizures joint detection algorithm based on improved multi-domain feature of cEEG and spike feature of aEEG. IEEE Access 7:41551–41564
Dumanis SB, Riley EAU, Latese B, Yoori K, Erik L, Danielle S, Melissa S (2017) Opportunities for epilepsy: outcomes of the Milken Institute State of the Science Retreat. Epilepsy Behav 68:174–176
Dutta S, Chakraborty W, Gomez J, Ni K, Joshi S, Datta S (2019) Energy-efficient edge inference on multi-channel streaming data in 28nm HKMG FeFET technology. In: 2019 symposium on VLSI technology. IEEE, pp T38–T39
Duun-Henriksen J, Baud M, Richardson MP, Cook M, Kouvas G, Heasman JM, Friedman D, Peltola J, Zibrandtsen IC, Kjaer TW (2020) A new era in electroencephalographic monitoring? Subscalp devices for ultra-long-term recordings. Epilepsia 61(9):1805–1817
Ein Shoka AA, Dessouky MM, El-Sayed A, El-Din Hemdan E (2023a) An efficient CNN based epileptic seizures detection framework using encrypted EEG signals for secure telemedicine applications. Alex Eng J 65:399–412
Ein Shoka AA, Dessouky MM, El-Sayed A, El-Din Hemdan E (2023b) EEG seizure detection: concepts, techniques, challenges, and future trends. Multimed Tools Appl 82:42021–42051
Einizade A, Nasiri S, Mozafari M, Sardouie SH, Clifford GD (2023) Explainable automated seizure detection using attentive deep multi-view networks. Biomed Signal Process Control 79:104076
El Halabi N, Daou RAZ, Achkar R, Hayek A, Börcsök J (2019) Monitoring system for prediction and detection of epilepsy seizure. In: 2019 fourth international conference on advances in computational tools for engineering applications (ACTEA). IEEE, pp 1–7
El-Gindy SA-E, Hamad A, El-Shafai W, Khalaf AAM, El-Dolil SM, Taha TE, El-Fishawy AS, Alotaiby TN, Alshebeili SA, Abd El-Samie FE (2021) Efficient communication and EEG signal classification in wavelet domain for epilepsy patients. J Ambient Intell Human Comput 12:9193–9208
Elhosary H, Zakhari MH, Elgammal MA, Abd El Ghany MA, Salama KN, Mostafa H (2019) Low-power hardware implementation of a support vector machine training and classification for neural seizure detection. IEEE Trans Biomed Circuits Syst 13(6):1324–1337
Emara HM, Mohamed E, Taha Taha E, El-Fishawy AS, El-Rabaie El-Sayed M, Walid E-S, El Banby GM, Turky A, Alshebeili SA, Abd El-Samie FE (2022) Efficient frameworks for EEG epileptic seizure detection and prediction. Ann Data Sci 9(2):393–428
Engel E Jr, Pitkänen A, Loeb JA, Edward DF, Bertram EH III, Cole AJ, Moshé SL, Wiebe S, Jensen FE, Mody I et al (2013) Epilepsy biomarkers. Epilepsia 54:61–69
Escorcia-Gutierrez J, Beleno K, Jimenez-Cabas J, Elhoseny M, Alshehri MD, Selim MM (2022) An automated deep learning enabled brain signal classification for epileptic seizure detection on complex measurement systems. Measurement 196:111226
Farooq MS, Zulfiqar A, Riaz S (2023) Epileptic seizure detection using machine learning: taxonomy, opportunities, and challenges. Diagnostics 13(6):1058
Fasil OK, Rajesh R (2019) Time-domain exponential energy for epileptic EEG signal classification. Neurosci Lett 694:1–8
Feng L, Li Z, Wang Y (2017) VLSI design of SVM-based seizure detection system with on-chip learning capability. IEEE Trans Biomed Circuits Syst 12(1):171–181
Feng L, Li Z, Wang Y (2018) VLSI design of SVM-based seizure detection system with on-chip learning capability. IEEE Trans Biomed Circuits Syst 12(1):171–181
Fu K, Qu J, Chai Y, Dong Y (2014) Classification of seizure based on the time-frequency image of EEG signals using HHT and SVM. Biomed Signal Process Control 13:15–22
Gao Y, Gao B, Chen Q, Liu J, Zhang Y (2020) Deep convolutional neural network-based epileptic electroencephalogram (EEG) signal classification. Front Neurol 11:375
Gao B, Zhou J, Yang Y, Chi J, Yuan Q (2022) Generative adversarial network and convolutional neural network-based EEG imbalanced classification model for seizure detection. Biocybern Biomed Eng 42(1):1–15
García V, Mollineda RA, Sánchez JS (2014) A bias correction function for classification performance assessment in two-class imbalanced problems. Knowl Based Syst 59:66–74
Geethu V, Santhoshkumar S (2020) An efficient FPGA realization of seizure detection from EEG signal using wavelet transform and statistical features. IETE J Res 66(3):315–325
George ST, Subathra MS, Sairamya NJ, Susmitha L, Premkumar MJ (2020) Classification of epileptic EEG signals using PSO based artificial neural network and tunable-Q wavelet transform. Biocybern Biomed Eng 40(2):709–728
Ghazali SM, Alizadeh M, Mazloum J, Baleghi Y (2022) Modified binary salp swarm algorithm in EEG signal classification for epilepsy seizure detection. Biomed Signal Process Control 78:103858
Gill AF, Fatima SA, Usman AM, Khawaja SG, Awan SE (2015) Analysis of EEG signals for detection of epileptic seizure using hybrid feature set. In: Theory and applications of applied electromagnetics. Springer, pp 49–57
Glory HA, Vigneswaran C, Jagtap SS, Shruthi R, Hariharan G, Sriram VS (2021) AHW-BGOA-DNN: a novel deep learning model for epileptic seizure detection. Neural Comput Appl 33(11):6065–6093
Göksu H (2018) EEG based epileptiform pattern recognition inside and outside the seizure states. Biomed Signal Process Control 43:204–215
Goldberger AL, Amaral LA, Glass L, Hausdorff JM, Ivanov PC, Mark RG, Mietus JE, Moody GB, Peng CK, Stanley HE (2000) PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation 101(23):e215–e220
Golmohammadi M, Ziyabari S, Shah V, Obeid I, Picone J (2018) Deep architectures for spatio-temporal modeling: automated seizure detection in scalp EEGs. In: 2018 17th IEEE international conference on machine learning and applications (ICMLA). IEEE, pp 745–750
Gotman J (1982) Automatic recognition of epileptic seizures in the EEG. Electroencephalogr Clin Neurophysiol 54(5):530–540
Gotman J (2011) A few thoughts on what is a seizure? Epilepsy Behav 22:S2–S3
Guerrero MC, Parada JS, Espitia HE (2021) EEG signal analysis using classification techniques: logistic regression, artificial neural networks, support vector machines, and convolutional neural networks. Heliyon 7:e07258
Guo Y, Jiang X, Tao L, Meng L, Dai C, Long X, Wan F, Zhang Y, Van Dijk J, Aarts RM et al (2022) Epileptic seizure detection by cascading isolation forest-based anomaly screening and EasyEnsemble. IEEE Trans Neural Syst Rehabil Eng 30:915–924
Gupta V, Pachori RB (2019a) Epileptic seizure identification using entropy of FBSE based EEG rhythms. Biomed Signal Process Control 53:101569
Gupta V, Pachori RB (2019b) A new method for classification of focal and non-focal EEG signals. In: Machine intelligence and signal analysis. Springer, pp 235–246
Gupta V, Bhattacharyya A, Pachori RB (2017) Classification of seizure and non-seizure EEG signals based on EMD-TQWT method. In: 2017 22nd international conference on digital signal processing (DSP). IEEE, pp 1–5
Gupta V, Nishad A, Pachori RB (2018a) Focal EEG signal detection based on constant-bandwidth TQWT filter-banks. In: 2018 IEEE international conference on bioinformatics and biomedicine (BIBM). IEEE, pp 2597–2604
Gupta S, Krishna KH, Pachori RB, Tanveer M (2018b) Fourier-Bessel series expansion based technique for automated classification of focal and non-focal EEG signals. In: 2018 international joint conference on neural networks (IJCNN). IEEE, pp 1–6
Gupta A, Singh P, Karlekar M (2018c) A novel signal modeling approach for classification of seizure and seizure-free EEG signals. IEEE Trans Neural Syst Rehabil Eng 26(5):925–935
Gupta E, Gupta M, Sachdeva RA, Handa P, Goel N (2022a) Meta-EEG of CHB-MIT scalp EEG database v1.0.0.0. https://doi.org/10.5281/zenodo.6062372
Gupta M, Sachdeva RA, Gupta E, Handa P, Goel N (2022b) Meta-EEG of Siena scalp EEG database v1.0.0. https://doi.org/10.5281/zenodo.6061290
Hadiyoso S, Wijayanto I, Humairani A (2021) Signal dynamics analysis for epileptic seizure classification on EEG signals. Traitement du Signal 38(1):73–78
Handa P, Goel N (2021a) Peri-ictal and non-seizure EEG event detection using generated metadata. Expert Syst 39:e12929
Handa P, Goel N (2021b) Epileptic seizure detection using rhythmicity spectrogram and cross-patient test set. In: 2021 8th international conference on signal processing and integrated networks (SPIN). IEEE, pp 898–902
Handa P, Mathur M, Goel N (2021) Open and free EEG datasets for epilepsy diagnosis. arXiv Preprint http://arxiv.org/abs/2108.01030
Handa P, Goel N, Dev A (2023) A system and a method to detect peri-ictal and non-seizure EEG events using generated metadata, January 17. IN Patent 418365
Harati A, Lopez S, Obeid I, Picone J, Jacobson MP, Tobochnik S (2014) The TUH EEG CORPUS: a big data resource for automated EEG interpretation. In: 2014 IEEE signal processing in medicine and biology symposium (SPMB). IEEE, pp 1–5
Hassan KM, Islam MR, Nguyen TT, Molla MK (2022) Epileptic seizure detection in EEG using mutual information-based best individual feature selection. Expert Syst Appl 193:116414
HECOX, Kurt E (2021) Systems and methods for seizure detection based on changes in electroencephalogram (EEG) non-linearities, February 25
Hilal AM, Albraikan AA, Dhahbi S, Nour MK, Mohamed A, Motwakel A, Zamani AS, Rizwanullah M (2022) Intelligent epileptic seizure detection and classification model using optimal deep canonical sparse autoencoder. Biology 11(8):1220
Hossin M, Sulaiman MN (2015) A review on evaluation metrics for data classification evaluations. Int J Data Min Knowl Manag Process 5(2):1
Houssein EH, Hamad A, Hassanien AE, Fahmy AA (2019) Epileptic detection based on whale optimization enhanced support vector machine. J Inf Optim Sci 40(3):699–723
Howbert JJ, Patterson EE, Stead SM, Brinkmann B, Vasoli V, Crepeau D, Vite CH, Sturges B, Ruedebusch V, Mavoori J et al (2014) Forecasting seizures in dogs with naturally occurring epilepsy. PLoS ONE 9(1):e81920
Huang Z, Duan J (2023) GFBLS: graph-regularized fuzzy broad learning system for detection of interictal epileptic discharges. Eng Appl Artif Intell 125:106763
Huang S-A, Chang K-C, Liou H-H, Yang C-H (2019) A 1.9-MW SVM processor with on-chip active learning for epileptic seizure control. IEEE J Solid State Circuits 55(2):452–464
Hunter MRLL, Smith RLL, Wendy H, Rosso OA, Gerlach R, Rostas JAP, Williams D, Frans H (2005) The Australian EEG database. Clin EEG Neurosci 36(2):76–81
Hussain SF, Qaisar SM (2022) Epileptic seizure classification using level-crossing EEG sampling and ensemble of sub-problems classifier. Expert Syst Appl 191:116356
Hussein R, Palangi H, Ward R, Wang ZJ (2018) Epileptic seizure detection: a deep learning approach. arXiv Preprint http://arxiv.org/abs/1803.09848
Ihle M, Feldwisch-Drentrup H, Teixeira CA, Witon A, Schelter B, Timmer J, Schulze-Bonhage A (2012) EPILEPSIAE–a European epilepsy database. Comput Methods Progr Biomed 106(3):127–138
Ilakiyaselvan N, Khan AN, Shahina A (2022) Reconstructed phase space portraits for detecting brain diseases using deep learning. Biomed Signal Process Control 71:103278
Iqbal S, Khan YU, Farooq O (2015) Nonlinear analysis of EEG for seizure prediction. In: 2015 annual IEEE India conference (INDICON). IEEE, pp 1–5
Islam MK, Rastegarnia A, Yang Z (2015) A wavelet-based artifact reduction from scalp EEG for epileptic seizure detection. IEEE J Biomed Health Inform 20(5):1321–1332
Islam MS, Thapa K, Yang SH (2022) Epileptic-Net: an improved epileptic seizure detection system using dense convolutional block with attention network from EEG. Sensors 22(3):728
Jaiswal AK, Banka H (2017) Local pattern transformation based feature extraction techniques for classification of epileptic EEG signals. Biomed Signal Process Control 34:81–92
Jana GC, Sharma R, Agrawal A (2020) A 1D-CNN-spectrogram based approach for seizure detection from EEG signal. Procedia Comput Sci 167:403–412
Jia M, Liu W, Duan J, Chen L, Chen CP, Wang Q, Zhou Z (2022) Efficient graph convolutional networks for seizure prediction using scalp EEG. Front Neurosci 16:967116
Jiang Y, Chen W, Li M, Zhang T, You Y (2021) Synchroextracting chirplet transform-based epileptic seizures detection using EEG. Biomed Signal Process Control 68:102699
Kamousi B, Hajinoroozi M, Karunakaran S, Grant A, Yi J, Woo R, Parvizi J, Chao X (2020) Systems and methods for seizure prediction and detection, August 18. https://patents.google.com/patent/US10743809B1. US Patent 10,743,809
Kaur T, Diwakar A, Mirpuri P, Tripathi M, Chandra PS, Gandhi TK (2021) Artificial intelligence in epilepsy. Neurol India 69(3):560
Kavitha KVN, Ashok S, Imoize AL, Ojo S, Senthamil SK, Ahanger TA, Alhassan M (2022) On the use of wavelet domain and machine learning for the analysis of epileptic seizure detection from EEG signals. J Healthc Eng 2022:8928021
Khan P, Khan Y, Kumar S, Khan MS, Gandomi AH (2021) HVD-LSTM based recognition of epileptic seizures and normal human activity. Comput Biol Med 136:104684
Krishnan S, Athavale Y (2018) Trends in biomedical signal feature extraction. Biomed Signal Process Control 43:41–63
Kuhlmann L, Karoly P, Freestone DR, Brinkmann BH, Temko A, Barachant A, Li F, Titericz G Jr, Lang BW, Lavery D et al (2018) Epilepsyecosystem.org: crowd-sourcing reproducible seizure prediction with long-term human intracranial EEG. Brain 141(9):2619–2630
Kumar G, Chander S, Almadhor A (2022) An intelligent epilepsy seizure detection system using adaptive mode decomposition of EEG signals. Phys Eng Sci Med 45(1):261–272
Kusmakar S, Karmakar CK, Yan B, O’Brien TJ, Muthuganapathy R, Palaniswami M (2018) Automated detection of convulsive seizures using a wearable accelerometer device. IEEE Trans Biomed Eng 66(2):421–432
Li M, Chen W (2021) FFT-based deep feature learning method for EEG classification. Biomed Signal Process Control 66:102492
Li M, Chen W, Zhang T (2016) Automatic epilepsy detection using wavelet-based nonlinear analysis and optimized SVM. Biocybern Biomed Eng 36(4):708–718
Li Y, Cui WG, Huang H, Guo YZ, Li K, Tan T (2019a) Epileptic seizure detection in EEG signals using sparse multiscale radial basis function networks and the fisher vector approach. Knowl Based Syst 164:96–106
Li JW, Barma S, Mak PU, Pun SH, Vai MI (2019b) Brain rhythm sequencing using EEG signals: a case study on seizure detection. IEEE Access 7:160112–160124
Li C, Lammie C, Amirsoleimani A, Azghadi MR, Genov R (2023) Simulation of memristive crossbar arrays for seizure detection and prediction using parallel convolutional neural networks. Softw Impacts 15:100473
Lin S-k, Qomah I, Lin Y-s, Chiueh H, Lin C-Y (2018) Design and implementation of a smart headband for epileptic seizure detection and its verification using clinical database. In: 2018 IEEE international symposium on circuits and systems (ISCAS). pp 1–5
Ling G, Zheng Y, Guo H, Zhang K, Zhao Y, Wang H, Zheng J, Yang X (2019) Epilepsy detection method based on generative adversarial network. https://patentscope.wipo.int/search/ja/detail.jsf;jsessionid=C8E3E2D62DCF2672E5DEEC8DA6B28BB7.wapp2nA?docId=CN279819497 &_cid=P20-KE37ZO-52538-28. CN110575141
Liu Y, Zhou W, Yuan Q, Chen S (2012) Automatic seizure detection using wavelet transform and SVM in long-term intracranial EEG. IEEE Trans Neural Syst Rehabil Eng 20(6):749–755
Liu G, Xiao R, Xu L, Cai J (2021) Minireview of epilepsy detection techniques based on electroencephalogram signals. Front Syst Neurosci 15:44
Liu X, Wang J, Shang J, Liu J, Dai L, Yuan S (2022) Epileptic seizure detection based on variational mode decomposition and deep forest using EEG signals. Brain Sci 12(10):1275
Lopez S, Suarez G, Jungreis D, Obeid I, Picone J (2015) Automated identification of abnormal adult EEGs. In: 2015 IEEE signal processing in medicine and biology symposium (SPMB). IEEE, pp 1–5
Lopez S, Gross A, Yang S, Golmohammadi M, Obeid I, Picone J (2016) An analysis of two common reference points for EEGs. In: 2016 IEEE signal processing in medicine and biology symposium (SPMB). IEEE, pp 1–5
Lu D, Triesch J (2019) Residual deep convolutional neural network for EEG signal classification in epilepsy. arXiv Preprint http://arxiv.org/abs/1903.08100
Ma Y, Liu C, Ma MS, Yang Y, Truong ND, Kothur K, Nikpour A, Kavehei O (2023) TSD: transformers for seizure detection. bioRxiv. pp 2023–01
Madhavan S, Tripathy RK, Pachori RB (2019) Time-frequency domain deep convolutional neural network for the classification of focal and non-focal EEG signals. IEEE Sens J 20(6):3078–3086
Marni L, Hosseini M, Hopp J, Mohseni P, Mohsenin T (2018) A real-time wearable FPGA-based seizure detection processor using MCMC. In: 2018 IEEE international symposium on circuits and systems (ISCAS). pp 1–4
Mehla VK, Singhal A, Singh P, Pachori RB (2021) An efficient method for identification of epileptic seizures from EEG signals using Fourier analysis. Phys Eng Sci Med 44:443–456
Mishra S, Panda S, Mohanty MN, Shaik AB (2022) Epileptic seizure detection and classification using hog feature based MSCA-ELM model and embedded prototype development, 7. https://patents.google.com/patent/AU2021103884A4. AU Patent App. 2021103884
Mishra S, Kumar Satapathy S, Mohanty SN, Pattnaik CR (2023) A DM-ELM based classifier for EEG brain signal classification for epileptic seizure detection. Commun Integr Biol 16(1):2153648
Mitha M, Shiju SS, Viswanadhan M (2014) Automated epileptic seizure detection using relevant features in support vector machines. In: 2014 international conference on control, instrumentation, communication and computational technologies (ICCICCT). IEEE, pp 1000–1004
Moctezuma LA, Molinas M (2020) EEG channel-selection method for epileptic-seizure classification based on multi-objective optimization. Front Neurosci 14:593
Muhammed Shanir PP, Khan YU, Farooq O (2015) Time domain analysis of eeg for automatic seizure detection, 2015. Conference: emerging trends in electrical and electronics engineering (ETEEE-2015). Jamia Millia Islamia, New Delhi
Munirathinam R, Ponnan S, Chakraborty C, Umathurai S (2022) Improved performance on seizure detection in an automated electroencephalogram signal under evolution by extracting entropy feature. Multimed Tools Appl 81(10):13355–13370
Mursalin M, Zhang Y, Chen Y, Chawla NV (2017) Automated epileptic seizure detection using improved correlation-based feature selection with random forest classifier. Neurocomputing 241:204–214
Namazi H, Jafari S (2018) Age-based variations of fractal structure of EEG signal in patients with epilepsy. Fractals 26(04):1850051
Nandor L, Geza M, Ruben K, Gabor I, Orrin D (2011) System and device for seizure detection. https://patents.google.com/patent/US7885706B2/en
Narayan SM (2021) System and method for guiding direction to and treating targets for abnormal biological rhythms, August 26. US Patent App. 17/180,783
Naro D, Rummel C, Schindler K, Andrzejak RG (2014) Detecting determinism with improved sensitivity in time series: rank-based nonlinear predictability score. Phys Rev E 90(3):032913
Nasreddine W (2021) Epileptic EEG dataset. https://doi.org/10.17632/5pc2j46cbc.1
Nejedly P, Kremen V, Sladky V, Cimbalnik J, Klimes P, Plesinger F, Mivalt F, Travnicek V, Viscor I, Pail M et al (2020) Multicenter intracranial EEG dataset for classification of graphoelements and artifactual signals. Sci Data 7(1):1–7
Nishad A, Pachori RB (2020) Classification of epileptic electroencephalogram signals using tunable-Q wavelet transform based filter-bank. J Ambient Intell Humaniz Comput 15(1):877–891
Obeid I, Picone J (2016) The temple university hospital EEG data corpus. Front Neurosci 10:196
Orosco L, Correa GA, Laciar E (2013) A survey of performance and techniques for automatic epilepsy detection. J Med Biol Eng 33(6):526–537
Osorio I, Frei MG (2017) Algorithm for detecting a seizure from cardiac data, July 11. US Patent 9,700,256
Pachori RB, Bajaj V (2011) Analysis of normal and epileptic seizure EEG signals using empirical mode decomposition. Comput Methods Progr Biomed 104(3):373–381
Page A, Shea C, Mohsenin T (2016) Wearable seizure detection using convolutional neural networks with transfer learning. In: 2016 IEEE international symposium on circuits and systems (ISCAS). pp 1086–1089
Palak, Mangotra H, Goel N (2023) Effect of selection bias on automatic colonoscopy polyp detection. Biomed Signal Process Control 85:104915
Pan Y, Zhou X, Dong F, Wu J, Xu Y, Zheng S (2022) Epileptic seizure detection with hybrid time-frequency EEG input: a deep learning approach. Comput Math Methods Med 2022:8724536
Pandey A, Sequeria R, Kumar P, Kumar S (2019) A multistage deep residual network for biomedical cyber-physical systems. IEEE Syst J 14(2):1953–1962
Panwar S (2020) Single electrode EEG data of healthy and epileptic patients. https://doi.org/10.5281/zenodo.3684992
Panwar S, Joshi SD, Gupta A, Agarwal P (2019) Automated epilepsy diagnosis using EEG with test set evaluation. IEEE Trans Neural Syst Rehabil Eng 27(6):1106–1116
Parija S, Bisoi R, Dash PK, Sahani M (2021) Deep long short term memory based minimum variance kernel random vector functional link network for epileptic EEG signal classification. Eng Appl Artif Intell 105:104426
Patidar S, Panigrahi T (2017) Detection of epileptic seizure using Kraskov entropy applied on tunable-Q wavelet transform of EEG signals. Biomed Signal Process Control 34:74–80
Peachap AB, Tchiotsop D (2019) Epileptic seizures detection based on some new Laguerre polynomial wavelets, artificial neural networks and support vector machines. Inform Med Unlocked 16:100209
Peh WY, Thangavel P, Yao Y, Thomas J, Tan YL, Dauwels J (2023) Six-center assessment of CNN-transformer with belief matching loss for patient-independent seizure detection in EEG. Int J Neural Syst 33:2350012
Peng H, Lei C, Zheng S, Zhao C, Wu C, Sun J, Hu B (2021) Automatic epileptic seizure detection via stein kernel-based sparse representation. Comput Biol Med 132:104338
Prasanna J, Sairamya NJ, Thomas George S, Ruth Vinutha C, Subathra MSP (2019) Classification of non-focal and focal EEG signals using local binary pattern. In: 2019 international conference on computer communication and informatics (ICCCI). IEEE, pp 1–4
Prathaban BP, Balasubramanian R (2021) Dynamic learning framework for epileptic seizure prediction using sparsity based EEG reconstruction with optimized CNN classifier. Expert Syst Appl 170:114533
Praveena HD, Subhas C, Naidu KR (2021) Automatic epileptic seizure recognition using ReliefF feature selection and long short term memory classifier. J Ambient Intell Humaniz Comput 12(6):6151–6167
Pressler RM, Cilio MR, Mizrahi EM, Moshé SL, Nunes ML, Plouin P, Vanhatalo S, Yozawitz E, De Vries LS, Vinayan KP et al (2021) The ILAE classification of seizures and the epilepsies: modification for seizures in the neonate. Position paper by the ILAE task force on neonatal seizures. Epilepsia 62(3):615–628
Priyanka S, Dema D, Jayanthi T (2017) Feature selection and classification of epilepsy from EEG signal. In: 2017 international conference on energy, communication, data analytics and soft computing (ICECDS). IEEE, pp 2404–2406
Priyasad D, Fernando T, Denman S, Sridharan S, Fookes C (2021) Interpretable seizure classification using unprocessed EEG with multi-channel attentive feature fusion. IEEE Sens J 21:19186–19197
Qidwai U, Malik AS, Shakir M, Kamel N (2013) Hardware simulator for seizure, preseizure and normal mode signal generation in labview environment for research. Int J Biosci Biochem Bioinform. https://doi.org/10.7763/IJBBB.2013.V3.282
Qin H, Deng B, Wang J, Yi G, Wang R, Zhang Z (2020) Deep multi-scale feature fusion convolutional neural network for automatic epilepsy detection using EEG signals. In: 2020 39th Chinese control conference (CCC). IEEE, pp 7061–7066
Qiu S, Wang W, Jiao H (2022) LightSeizureNet: a lightweight deep learning model for real-time epileptic seizure detection. IEEE J Biomed Health Inform 27:1845–1856
Qiu X, Yan F, Liu H (2023) A difference attention ResNet-LSTM network for epileptic seizure detection using EEG signal. Biomed Signal Process Control 83:104652
Raghu S, Sriraam N, Temel Y, Rao SV, Hegde AS, Kubben PL (2019) Performance evaluation of DWT based sigmoid entropy in time and frequency domains for automated detection of epileptic seizures using SVM classifier. Comput Biol Med 110:127–143
Raghu S, Sriraam N, Temel Y, Rao SV, Kubben PL (2020) EEG based multi-class seizure type classification using convolutional neural network and transfer learning. Neural Netw 124:202–212
Rajendran T, Sridhar KP, Deepa S (2019) Performance analysis of fuzzy multilayer support vector machine for epileptic seizure disorder classification using auto regression features. Open Biomed Eng J 13(1):103–113
Rajinikanth V, Kadry S, Taniar D, Kamalanand K, Elaziz MA, Thanaraj KP (2022) Detecting epilepsy in EEG signals using synchro-extracting-transform (set) supported classification technique. J Ambient Intell Humaniz Comput 14:10123–10141
Rasheed K, Qayyum A, Qadir J, Sivathamboo S, Kwan P, Kuhlmann L, O’Brien T, Razi A (2020) Machine learning for predicting epileptic seizures using EEG signals: a review. IEEE Rev Biomed Eng 14:139–155
Rasheed K, Qadir J, O’Brien TJ, Kuhlmann L, Razi A (2021) A generative model to synthesize EEG data for epileptic seizure prediction. IEEE Trans Neural Syst Rehabil Eng 29:2322–2332
Reddy GRS, Rao R (2017) Automated identification system for seizure EEG signals using tunable-Q wavelet transform. Eng Sci Technol Int J 20(5):1486–1493
Reddy VYS, Sai AP, Suman D, Mudigonda M (2015) Discrete wavelet transform based statistical features for the diagnosis of epilepsy. In: 2015 annual IEEE India conference (INDICON). IEEE, pp 1–6
Romney A, Manian V (2021) Optimizing seizure prediction from reduced scalp EEG channels based on spectral features and MAML. IEEE Access 9:164348–164357
Rout SK, Biswal PK (2020) An efficient error-minimized random vector functional link network for epileptic seizure classification using VMD. Biomed Signal Process Control 57:101787
Rout SK, Sahani M, Dora C, Biswal PK, Biswal B (2022) An efficient epileptic seizure classification system using empirical wavelet transform and multi-fuse reduced deep convolutional neural network with digital implementation. Biomed Signal Process Control 72:103281
Sachdeva RA, Gupta E, Gupta M, Handa P, Goel N (2022) Dataset of rhythmicity spectrogram based images of seizure and non-seizure EEG signals. https://doi.org/10.1109/SPIN52536.2021.9566078
Sadiq MT, Akbari H, Rehman AU, Nishtar Z, Masood B, Ghazvini M, Too J, Hamedi N, Kaabar MKA (2021) Exploiting feature selection and neural network techniques for identification of focal and nonfocal EEG signals in TQWT domain. J Healthc Eng 2021:6283900
Sahani M, Rout SK, Dash PK (2021) FPGA implementation of epileptic seizure detection using semisupervised reduced deep convolutional neural network. Appl Soft Comput 110:107639
Saini J, Dutta M (2017) An extensive review on development of EEG-based computer-aided diagnosis systems for epilepsy detection. Netw Comput Neural Syst 28(1):1–27
Sairamya NJ, Susmitha L, Thomas George S, Subathra MSP (2019) Hybrid approach for classification of electroencephalographic signals using time–frequency images with wavelets and texture features. In: Intelligent data analysis for biomedical applications. Elsevier, pp 253–273
Sairamya NJ, Joel Premkumar M, Thomas George S, Subathra MSP (2021) Performance evaluation of discrete wavelet transform, and wavelet packet decomposition for automated focal and generalized epileptic seizure detection. IETE J Res 67(6):778–798
Sameer M, Gupta B (2020) Detection of epileptical seizures based on alpha band statistical features. Wirel Pers Commun 115(2):909–925
Sameer M, Gupta B (2022) CNN based framework for detection of epileptic seizures. Multimed Tools Appl 81(12):17057–17070
Samiee K, Kovács P, Gabbouj M (2017) Epileptic seizure detection in long-term EEG records using sparse rational decomposition and local Gabor binary patterns feature extraction. Knowl Based Syst 118:228–240
Saminu S, Xu G, Zhang S, Abd El Kader I, Zakariyya RS, Jabire AH (2019) Epilepsy detection and classification for smart IoT devices using hybrid technique. In: 2019 15th international conference on electronics, computer and computation (ICECCO). pp 1–6
Saminu S, Xu G, Shuai Z, Abd El Kader I, Jabire AH, Ahmed YK, Karaye IA, Ahmad IS (2021) A recent investigation on detection and classification of epileptic seizure techniques using EEG signal. Brain Sci 11(5):668
Saneesh Cleatus T, Thungamani M (2022) Epileptic seizure detection using spectral transformation and convolutional neural networks. J Inst Eng (India) Ser B 103(4):1115–1125
Santhosh NS, Sinha S, Satishchandra P (2014) Epilepsy: Indian perspective. Ann Indian Acad Neurol 17(Suppl 1):S3
Saputro IRD, Maryati ND, Solihati SR, Wijayanto I, Hadiyoso S, Patmasari R (2019) Seizure type classification on EEG signal using support vector machine. J Phys Conf Ser 1201:012065
Sarić R, Jokić D, Beganović N, Pokvić LG, Badnjević A (2020) FPGA-based real-time epileptic seizure classification using artificial neural network. Biomed Signal Process Control 62:102106
Sayeed MA, Mohanty SP, Kougianos E, Zaveri HP (2019) eSeiz: an edge-device for accurate seizure detection for smart healthcare. IEEE Trans Consum Electron 65(3):379–387
Schmidhuber J (2015) Deep learning in neural networks: an overview. Neural Netw 61:85–117
Şeker D, Özerdem MS (2021) A classification approach for focal/non-focal EEG detection using cepstral analysis. Dicle Üniversitesi Mühendislik Fakültesi Mühendislik Dergisi 12(4):603–613
Selvaraj TG, Ramasamy B, Jeyaraj SJ, Suviseshamuthu ES (2014) EEG database of seizure disorders for experts and application developers. Clin EEG Neurosci 45(4):304–309
Senthilpari C, Yong WH et al (2021) Epileptic EEG signal classifications based on DT-CWT and SVM classifier. J Eng Res 10(2A):95–104. https://doi.org/10.36909/jer.10523
Sethy PK, Panigrahi M, Vijayakumar K, Behera SK (2021) Machine learning based classification of EEG signal for detection of child epileptic seizure without snipping. Int J Speech Technol 26:559–570
Shaikh MHN, Farooq O, Chandel G (2019) EMD analysis of EEG signals for seizure detection. In: Advances in system optimization and control. Springer, pp 189–196
Shakir M, Malik AS, Kamel N, Qidwai U (2014) Rule-base wearable embedded platform for seizure detection from real EEG data in ambulatory state. In: 2014 IEEE region 10 symposium. IEEE, pp 45–49
Shankar A, Khaing HK, Dandapat S, Barma S (2021) Analysis of epileptic seizures based on EEG using recurrence plot images and deep learning. Biomed Signal Process Control 69:102854
Shariat A, Zarei A, Karvigh SA, Asl BM (2021) Automatic detection of epileptic seizures using Riemannian geometry from scalp EEG recordings. Med Biol Eng Comput 59:1431–1445
Sharma M, Bhurane AA, Acharya UR (2018) MMSFL-OWFB: a novel class of orthogonal wavelet filters for epileptic seizure detection. Knowl Based Syst 160:265–277
Sharma R, Sircar P, Pachori RB (2020) Automated focal EEG signal detection based on third order cumulant function. Biomed Signal Process Control 58:101856
Sharmila A, Geethanjali P (2019) A review on the pattern detection methods for epilepsy seizure detection from EEG signals. Biomed Eng Biomed Tech 64(5):507–517
Shekokar KS, Dour S (2021) Automatic epileptic seizure detection using LSTM networks. World J Eng 19:224–229
Shen M, Wen P, Song B, Li Y (2022) An EEG based real-time epilepsy seizure detection approach using discrete wavelet transform and machine learning methods. Biomed Signal Process Control 77:103820
Shoaran M, Farivar M, Emami A (2016) Hardware-friendly seizure detection with a boosted ensemble of shallow decision trees. In: 2016 38th annual international conference of the IEEE engineering in medicine and biology society (EMBC). pp 1826–1829
Shoaran M, Haghi BA, Taghavi M, Farivar M, Emami-Neyestanak A (2018) Energy-efficient classification for resource-constrained biomedical applications. IEEE J Emerg Sel Top Circuits Syst 8(4):693–707
Shoeibi A, Khodatars M, Ghassemi N, Jafari M, Moridian P, Alizadehsani R, Panahiazar M, Khozeimeh F, Zare A, Hosseini-Nejad H et al (2021) Epileptic seizures detection using deep learning techniques: a review. Int J Environ Res Public Health 18(11):5780
Shriram R, Vijaya BV, Martin B, Sundhararajan M, Daimiwal N (2019) Energy distribution and coherence-based changes in normal and epileptic electroencephalogram. In: Smart intelligent computing and applications. Springer, pp 625–635
Siddharth T, Tripathy RK, Pachori RB (2019) Discrimination of focal and non-focal seizures from EEG signals using sliding mode singular spectrum analysis. IEEE Sens J 19(24):12286–12296
Simozo FH, Destro-Filho J-B, Junior LOM (2014) Detrended-fluctuation-analysis (DFA) and high-frequency-oscillation (HFO) coefficients and their relationship to epileptic seizures. In: NEUROTECHNIX. pp 99–105
Singh A, Amalin PA (2015) FPGA implementation of second-order difference plot for epileptic seizure detection in EEG signals. In: 2015 annual IEEE India conference (INDICON). pp 1–5
Sivasaravanababu S, Prabhu V, Parthasarathy V, Mahendran RK (2022) An efficient epileptic seizure detection based on tunable Q-wavelet transform and DCVAE-stacked Bi-LSTM model using electroencephalogram. Eur Phys J Spec Top 231(11):2425–2437
Smith JR (1974) Automatic analysis and detection of EEG spikes. IEEE Trans Biomed Eng 1:1–7
Smith SJM (2005) EEG in the diagnosis, classification, and management of patients with epilepsy. J Neurol Neurosurg Psychiatry 76(suppl 2):ii2–ii7
Sopic D, Teijeiro T, Atienza D, Aminifar A, Ryvlin P (2022) Personalized seizure signature: an interpretable approach to false alarm reduction for long-term epileptic seizure detection. Epilepsia 64:S23–3S3
Sriraam N, Temel Y, Rao SV, Kubben PL et al (2019) A convolutional neural network based framework for classification of seizure types. In: 2019 41st annual international conference of the IEEE engineering in medicine and biology society (EMBC). IEEE, pp 2547–2550
Staba RJ, Matt S, Worrell GA (2014) Electrophysiological biomarkers of epilepsy. Neurotherapeutics 11(2):334–346
Steele AG, Parekh S, Azgomi HF, Ahmadi MB, Craik A, Pati S, Francis JT, Contreras-Vidal JL, Faghih RT (2021) A mixed filtering approach for real-time seizure state tracking using multi-channel electroencephalography data. IEEE Trans Neural Syst Rehabil Eng 29:2037–2045
Stevenson NJ, Karoliina T, Leena L, Sampsa V (2019) A dataset of neonatal EEG recordings with seizure annotations. Sci Data 6(1):1–8
Subasi A (2007) EEG signal classification using wavelet feature extraction and a mixture of expert model. Expert Syst Appl 32(4):1084–1093
Sui L, Zhao X, Zhao Q, Tanaka T, Cao J (2021) Hybrid convolutional neural network for localization of epileptic focus based on iEEG. Neural Plast 2021:6644365
Sun B, Lv JJ, Rui LG, Yang YX, Chen YG, Ma C, Gao ZK (2021) Seizure prediction in scalp EEG based channel attention dual-input convolutional neural network. Physica A 584:126376
Sunaryono D, Sarno R, Siswantoro J (2022) Gradient boosting machines fusion for automatic epilepsy detection from EEG signals based on wavelet features. J King Saud Univ Comput Inf Sci 34(10):9591–9607
Sunaryono D, Siswantoro J, Raharjo AB, Ridho R, Sarno R, Sabilla SI, Susilo RI (2023) Optimized one-dimension convolutional neural network for seizure classification from EEG signal based on whale optimization algorithm. Int J Intell Eng Syst 16(3):310–322
Supriya S, Siuly S, Hua W, Yanchun Z (2020) Automated epilepsy detection techniques from electroencephalogram signals: a review study. Health Inf Sci Syst 8(1):1–15
Supriya S, Siuly S, Wang H, Zhang Y (2021) New feature extraction for automated detection of epileptic seizure using complex network framework. Appl Acoust 180:108098
Svantesson M, Olausson H, Eklund A, Thordstein M (2021) Virtual EEG-electrodes: convolutional neural networks as a method for upsampling or restoring channels. J Neurosci Methods 355:109126
Svensson T, Chung UI, Tokuno S, Nakamura M, Svensson AK (2019) A validation study of a consumer wearable sleep tracker compared to a portable EEG system in naturalistic conditions. J Psychosom Res 126:109822
Swami P, Panigrahi B, Nara S, Bhatia M, Gandhi T (2016a) EEG epilepsy datasets. https://doi.org/10.13140/RG.2.2.14280.32006
Swami P, Gandhi T, Panigrahi BK, Bhatia M, Anand S (2016b) Locating ictal activities over human scalp with automated detection using EEG signals. In: 2016 3rd international conference on signal processing and integrated networks (SPIN). IEEE, pp 600–604
Swami P, Gandhi TK, Panigrahi BK, Bhatia M, Santhosh J, Anand S (2017) A comparative account of modelling seizure detection system using wavelet techniques. Int J Syst Sci Oper Logist 4(1):41–52
Syed Rafiammal S, Najumnissa D, Anuradha G, Mohideen SK, Jawahar PK, Mutalib SA (2019) A low power and high performance hardware design for automatic epilepsy seizure detection. Int J Electron Telecommun 65:707–712
Syed Rafiammal S, Najumnissa JD, Kaja MS (2020) Reconfigurable hardware design for automatic epilepsy seizure detection using EEG signals. Eng Technol Appl Sci Res 10(3):5803–5807
Syed Rafiammal S, Najumnissa Jamal D, Kaja Mohideen S (2021) Detection of epilepsy seizure in adults using discrete wavelet transform and cluster nearest neighborhood classifier. Iran J Sci Technol Trans Electr Eng 45:1103–1115
Taghavi M, Shoaran M (2019) Hardware complexity analysis of deep neural networks and decision tree ensembles for real-time neural data classification. In: 2019 9th international IEEE/EMBS conference on neural engineering (NER). IEEE, pp 407–410
Teijeiro AE, Shokrekhodaei M, Nazeran H (2019) The conceptual design of a novel workstation for seizure prediction using machine learning with potential eHealth applications. IEEE J Transl Eng Health Med 7:1–10
Tohidi M, Madsen JK, Moradi F (2019a) Low-power high-input-impedance EEG signal acquisition soc with fully integrated IA and signal-specific ADC for wearable applications. IEEE Trans Biomed Circuits Syst 13(6):1437–1450
Tohidi M, Madsen JK, Moradi F (2019b) A low-power, low-noise, high-accurate epileptic-seizure detection system for wearable applications. Microelectron J 92:104600
Toraman S (2021) Automatic recognition of preictal and interictal EEG signals using 1D-capsule networks. Comput Electr Eng 91:107033
Tuncer T (2021) A new stable nonlinear textural feature extraction method based EEG signal classification method using substitution Box of the Hamsi hash function: Hamsi pattern. Appl Acoust 172:107607
Tuncer E, Bolat ED (2022) Classification of epileptic seizures from electroencephalogram (EEG) data using bidirectional short-term memory (Bi-LSTM) network architecture. Biomed Signal Process Control 73:103462
Tuncer T, Dogan S, Ertam F, Subasi A (2020) A novel ensemble local graph structure based feature extraction network for EEG signal analysis. Biomed Signal Process Control 61:102006
Tuncer T, Dogan S, Acharya UR (2021) Automated EEG signal classification using chaotic local binary pattern. Expert Syst Appl 182:115175
Türk Ö, Özerdem MS (2019) Epilepsy detection by using scalogram based convolutional neural network from EEG signals. Brain Sci 9(5):115
Tzimourta KD, Tzallas AT, Giannakeas N, Astrakas LG, Tsalikakis DG, Angelidis P, Tsipouras MG (2019) A robust methodology for classification of epileptic seizures in EEG signals. Health Technol 9(2):135–142
Ullah I, Hussain M, ul Haq Qazi E, Aboalsamh H (2018) An automated system for epilepsy detection using EEG brain signals based on deep learning approach. Expert Syst Appl 107:61–71
Vijay Anand S, Shantha Selvakumari R (2019) Noninvasive method of epileptic detection using DWT and generalized regression neural network. Soft Comput 23(8):2645–2653
Wang F, Meng Q, Chen Y, Zhao Y (2014) Feature extraction method for epileptic seizure detection based on cluster coefficient distribution of complex network. WSEAS Trans Comput 13:351–60
Wang H, Shi W, Choy CS (2018) Hardware design of real time epileptic seizure detection based on STFT and SVM. IEEE Access 6:67277–67290
Wang X, Wang X, Liu W, Chang Z, Kärkkäinen T, Cong F (2021a) One dimensional convolutional neural networks for seizure onset detection using long-term scalp and intracranial EEG. Neurocomputing 459:212–222
Wang X, Ristaniemi T, Cong F (2021b) One and two dimensional convolutional neural networks for seizure detection using EEG signals. In: 2020 28th European signal processing conference (EUSIPCO). IEEE, pp 1387–1391
Wang Y, Dai Y, Liu Z, Guo J, Cao G, Ouyang M, Liu D, Shan Y, Kang G, Zhao G (2021c) Computer-aided intracranial EEG signal identification method based on a multi-branch deep learning fusion model and clinical validation. Brain Sci 11(5):615
Wu J, Zhou T, Li T (2020) Detecting epileptic seizures in EEG signals with complementary ensemble empirical mode decomposition and extreme gradient boosting. Entropy 22(2):140
Wu D, Shi Y, Wang Z, Yang J, Sawan M (2021) C\(^2\) SP-Net: joint compression and classification network for epilepsy seizure prediction. arXiv Preprint http://arxiv.org/abs/2110.13674
Xiang J, Li C, Li H, Cao R, Wang B, Han X, Chen J (2015) The detection of epileptic seizure signals based on fuzzy entropy. J Neurosci Methods 243:18–25
Xianji J (2021) Wearable epilepsy detection system based on multi-physiological information fusion and detection method thereof, January 15. https://patentscope.wipo.int/search/en/detail.jsf?docId=CN317065991 &_cid=P22-KY30AX-41930-1. CN112220454. Accessed 30 Jan 2022
Xiaofeng G, Quing T, Zhiguo Y (2019) Low-power epilepsy prediction circuit based on support vector machine, November 15. https://patentscope.wipo.int/search/en/detail.jsf?docId=CN277623276 &_cid=P22-KME9TQ-25069-32. CN110448273
Xin Q, Hu S, Liu S, Zhao L, Wang S (2021) WTRPNet: an explainable graph feature convolutional neural network for epileptic EEG classification. ACM Trans Multimed Comput Commun Appl (TOMM) 17(3s):1–18
Xinghua T, Lin L, Qinyi F, Yarong W, Zheng P, Zhenguo L (2020) The clinical value of long-term electroencephalogram (EEG) in seizure-free populations: implications from a cross-sectional study. BMC Neurol 20(1):1–7
Xu Y, Yang J, Ming W, Wang S, Sawan M (2023) Deep learning for short-latency epileptic seizure detection with probabilistic classification. arXiv Preprint http://arxiv.org/abs/2301.03465
Yan PZ, Fei W, Nathaniel K, Allen BB, Sotirios K, Zachary G (2019) Automated spectrographic seizure detection using convolutional neural networks. Seizure 71:124–131
Yao X, Cheng Q, Zhang G-Q (2019) A novel independent RNN approach to classification of seizures against non-seizures. arXiv Preprint http://arxiv.org/abs/1903.09326
Yedurkar DP, Metkar SP (2020) Multiresolution approach for artifacts removal and localization of seizure onset zone in epileptic EEG signal. Biomed Signal Process Control 57:101794
Yildiz A, Zan H, Said S (2021) Classification and analysis of epileptic EEG recordings using convolutional neural network and class activation mapping. Biomed Signal Process Control 68:102720
Yu J, Wang L, Chen X (2019) Epileptic seizure classification based on the combined features. In: Proceedings of the 2019 4th international conference on biomedical signal and image processing (ICBIP 2019). pp 7–12
Yuan Q, Zhou W, Li S, Cai D (2011) Epileptic EEG classification based on extreme learning machine and nonlinear features. Epilepsy Res 96(1–2):29–38
Yuan Q, Zhou W, Liu Y, Wang J (2012) Epileptic seizure detection with linear and nonlinear features. Epilepsy Behav 24(4):415–421
Yun F, Yapeng L, Jingzhao H, Yang L (2019) Portable adjustable head-mounted epilepsy monitor, July 16. https://patentscope.wipo.int/search/en/detail.jsf?docId=CN249762632 &_cid=P10-KY5E2T-64785-1. Cn110013249
Zaghloul ZS, Bayoumi M (2019) Early prediction of epilepsy seizures VLSI BCI system. arXiv Preprint http://arxiv.org/abs/1906.02894
Zarei R, He J, Siuly S, Huang G, Zhang Y (2019) Exploring Douglas-Peucker algorithm in the detection of epileptic seizure from multicategory EEG signals. BioMed Res Int 2019:5173589
Zhan T, Fatmi SZ, Guraya S, Kassiri H (2019) A resource-optimized VLSI implementation of a patient-specific seizure detection algorithm on a custom-made 2.2 cm2 wireless device for ambulatory epilepsy diagnostics. IEEE Trans Biomed Circuits Syst 13(6):1175–1185
Zhang Z, Parhi KK (2015) Seizure detection using regression tree based feature selection and polynomial SVM classification. In: 2015 37th annual international conference of the IEEE engineering in medicine and biology society (EMBC). pp 6578–6581
Zhang J, Wu H, Su W, Wang X, Yang M, Wu J (2018) A new approach for classification of epilepsy EEG signals based on temporal convolutional neural networks. In: 2018 11th international symposium on computational intelligence and design (ISCID), vol 02. pp 80–84
Zhang Z, Ren Y, Sabor N, Pan J, Luo X, Li Y, Chen Y, Wang G (2020a) DWT-NET: seizure detection system with structured EEG montage and multiple feature extractor in convolution neural network. J Sens 2020:3083910
Zhang B, Wang W, Xiao Y, Xiao S, Chen S, Chen S, Xu G, Che W (2020b) Cross-subject seizure detection in EEGs using deep transfer learning. Comput Math Methods Med 2020:7902072
Zhang T, Han Z, Chen X, Chen W (2021a) Quantifying randomness and complexity of a signal via maximum fuzzy membership difference entropy. Measurement 174:109053
Zhang T, Han Z, Chen X, Chen W (2021b) Subbands and cumulative sum of subbands based nonlinear features enhance the performance of epileptic seizure detection. Biomed Signal Process Control 69:102827
Zhang S, Chen D, Ranjan R, Ke H, Tang Y, Zomaya AY (2021c) A lightweight solution to epileptic seizure prediction based on EEG synchronization measurement. J Supercomput 77(4):3914–3932
Zhang S, Liu G, Xiao R, Cui W, Cai J, Hu X, Sun Y, Qiu J, Qi Y (2022a) A combination of statistical parameters for epileptic seizure detection and classification using VMD and NLTWSVM. Biocybern Biomed Eng 42(1):258–272
Zhang Y, Yao S, Yang R, Liu X, Qiu W, Han L, Zhou W, Shang W (2022b) Epileptic seizure detection based on bidirectional gated recurrent unit network. IEEE Trans Neural Syst Rehabil Eng 30:135–145
Zhao W, Zhao W, Wang W, Jiang X, Zhang X, Peng Y, Zhang B, Zhang G (2020) A novel deep neural network for robust detection of seizures using EEG signals. Comput Math Methods Med 2020:9689821
Zhao Y, Dong C, Zhang G, Wang Y, Chen X, Jia W, Yuan Q, Xu F, Zheng Y (2021) EEG-based seizure detection using linear graph convolution network with focal loss. Comput Methods Progr Biomed 208:106277
Zhao Y, Chu D, He J, Xue M, Jia W, Xu F, Zheng Y (2023a) Interactive local and global feature coupling for EEG-based epileptic seizure detection. Biomed Signal Process Control 81:104441
Zhao X, Yoshida N, Ueda T, Sugano H, Tanaka T (2023b) Epileptic seizure detection by using interpretable machine learning models. J Neural Eng 20:015002
Zhong J, Shuren Q, Chenglin P (2008) Study on separation for the frequency bands of EEG signal and frequency band relative intensity analysis based upon EMD. In: Proceedings of the 7th WSEAS international conference on signal processing, robotics and automation. Citeseer, pp 151–155
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no personal, academic, or financial conflict of interest associated with this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Handa, P., Lavanya, Goel, N. et al. Software advancements in automatic epilepsy diagnosis and seizure detection: 10-year review. Artif Intell Rev 57, 181 (2024). https://doi.org/10.1007/s10462-024-10799-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s10462-024-10799-y