Abstract
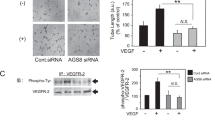
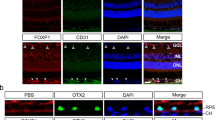
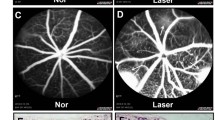
Loss of visual acuity in neovascular age-related macular degeneration (nAMD) occurs when factors activate choroidal endothelial cells (CECs) to transmigrate the retinal pigment epithelium into the sensory retina and develop into choroidal neovascularization (CNV). Active Rac1 (Rac1GTP) is required for CEC migration and is induced by different AMD-related stresses, including vascular endothelial growth factor (VEGF). Besides its role in pathologic events, Rac1 also plays a role in physiologic functions. Therefore, we were interested in a method to inhibit pathologic activation of Rac1. We addressed the hypothesis that IQGAP1, a scaffold protein with a Rac1 binding domain, regulates pathologic Rac1GTP in CEC migration and CNV. Compared to littermate Iqgap1+/+, Iqgap1−/− mice had reduced volumes of laser-induced CNV and decreased Rac1GTP and phosphorylated VEGFR2 (p-VEGFR2) within lectin-stained CNV. Knockdown of IQGAP1 in CECs significantly reduced VEGF-induced Rac1GTP, mediated through p-VEGFR2, which was necessary for CEC migration. Moreover, sustained activation of Rac1GTP induced by VEGF was eliminated when CECs were transfected with an IQGAP1 construct that is unable to bind Rac1. IQGAP1-mediated Src activation was involved in initiating Rac1 activation, CEC migration, and tube formation. Our findings indicate that CEC IQGAP1 interacts with VEGFR2 to mediate Src activation and subsequent Rac1 activation and CEC migration. In addition, IQGAP1 binding to Rac1GTP results in sustained activation of Rac1, leading to CEC migration toward VEGF. Our study supports a role of IQGAP1 and the interaction between IQGAP1 and Rac1GTP to restore CECs quiescence and, therefore, prevent vision-threatening CNV in nAMD.









Similar content being viewed by others
References
Zarbin MA (2004) Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol 122(4):598–614
Hartnett ME, Elsner AE (1996) Characteristics of exudative age-related macular degeneration determined in vivo with confocal and indirect infrared imaging. Ophthalmology 103(1):58–71
Spaide RF, Jaffe GJ, Sarraf D et al (2020) Consensus nomenclature for reporting neovascular age-related macular degeneration data. Ophthalmology 127:616–636
Hartnett ME, Elsner AE (1996) Characteristics of exudative age-related macular degeneration determined in vivo with confocal and indirect infrared imaging. Ophthalmology 103:58–71
Daniel E et al (2019) Five-year follow-up of nonfibrotic scars in the comparison of age-related macular degeneration treatments trials. Ophthalmology 126(5):743–751
Pershing S et al (2019) Use of bevacizumab and ranibizumab for wet age-related macular degeneration: influence of CATT results and introduction of aflibercept. Am J Ophthalmol 207:385–394
Jaffe GJ et al (2019) Macular morphology and visual acuity in year five of the comparison of age-related macular degeneration treatments trials. Ophthalmology 126(2):252–260
Engers R et al (2001) Rac affects invasion of human renal cell carcinomas by up-regulating tissue inhibitor of metalloproteinases (TIMP)-1 and TIMP-2 expression. J Biol Chem 276(45):41889–41897
Ridley AJ et al (2003) Cell migration: integrating signals from front to back. Science 302(5651):1704–1709
Burridge K (1999) Crosstalk between Rac and Rho. Science 283:2028–2029
Peterson LJ et al (2006) Heterotypic RPE-choroidal endothelial cell contact increases choroidal endothelial cell transmigration via PI 3-kinase and Rac1. Exp Eye Res 84:737
Peterson LJ et al (2006) Heterotypic RPE-choroidal endothelial cell contact increases choroidal endothelial cell transmigration via PI 3-kinase and Rac1. Exp Eye Res 84:737
Wang H et al (2016) TNF-alpha mediates choroidal neovascularization by upregulating VEGF expression in RPE through ROS-dependent beta-catenin activation. Mol Vis 22:116–128
Monaghan-Benson E, Burridge K (2009) The regulation of VEGF-induced microvascular permeability requires Rac and ROS. J Biol Chem 284:25604
Wittchen ES et al (2005) Rap1 GTPase inhibits leukocyte transmigration by promoting endothelial barrier function. J Biol Chem 280:11675–11682
Burridge K, Wennerberg K (2004) Rho and Rac take center stage. Cell 116(2):167–179
Panday A et al (2015) NADPH oxidases: an overview from structure to innate immunity-associated pathologies. Cell Mol Immunol 12(1):5–23
Acevedo A, Gonzalez-Billault C (2018) Crosstalk between Rac1-mediated actin regulation and ROS production. Free Radic Biol Med 116:101–113
Bid HK et al (2013) RAC1: an emerging therapeutic option for targeting cancer angiogenesis and metastasis. Mol Cancer Ther 12(10):1925–1934
Bergers G, Benjamin LE (2003) Tumorigenesis and the angiogenic switch. Nat Rev Cancer 3(6):401–410
Hedman AC, Smith JM, Sacks DB (2015) The biology of IQGAP proteins: beyond the cytoskeleton. EMBO Rep 16(4):427–446
White CD, Erdemir HH, Sacks DB (2012) IQGAP1 and its binding proteins control diverse biological functions. Cell Signal 24(4):826–834
Mataraza JM et al (2003) IQGAP1 promotes cell motility and invasion. J Biol Chem 278(42):41237–41245
Mataraza JM et al (2007) Multiple proteins mediate IQGAP1-stimulated cell migration. Cell Signal 19(9):1857–1865
Jadeski L et al (2008) IQGAP1 stimulates proliferation and enhances tumorigenesis of human breast epithelial cells. J Biol Chem 283(2):1008–1017
Balenci L et al (2007) IQGAP1 regulates adult neural progenitors in vivo and vascular endothelial growth factor-triggered neural progenitor migration in vitro. J Neurosci 27(17):4716–4724
Wang H et al (2015) Rap1 GTPase Inhibits Tumor Necrosis Factor-alpha-Induced Choroidal Endothelial Migration via NADPH Oxidase- and NF-kappaB-Dependent Activation of Rac1. Am J Pathol 185(12):3316–3325
Geisen P, McColm JR, Hartnett ME (2006) Choroidal endothelial cells transmigrate across the retinal pigment epithelium but do not proliferate in response to soluble vascular endothelial growth factor. Exp Eye Res 82:608–619
Mataraza JM et al (2003) Identification and characterization of the Cdc42-binding site of IQGAP1. Biochem Biophys Res Commun 305(2):315–321
Sullivan DP et al (2019) Endothelial IQGAP1 regulates leukocyte transmigration by directing the LBRC to the site of diapedesis. The Journal of experimental medicine 216(11):2582–2601
Yamaoka-Tojo M et al (2006) IQGAP1 mediates VE-cadherin-based cell-cell contacts and vegf signaling at adherence junctions linked to angiogenesis. Arterioscl Thromb Vasc Biol 26(9):1991–1997
Meyer RD, Sacks DB, Rahimi N (2008) IQGAP1-dependent signaling pathway regulates endothelial cell proliferation and angiogenesis. PLoS ONE 3(12):e3848
Huang H, Shen J, Vinores SA (2011) Blockade of VEGFR1 and 2 suppresses pathological angiogenesis and vascular leakage in the eye. PLoS ONE 6(6):e21411
Cruz-Gonzalez F et al (2014) Predictive value of VEGF A and VEGFR2 polymorphisms in the response to intravitreal ranibizumab treatment for wet AMD. Graefes Arch Clin Exp Ophthalmol 252(3):469–475
Askou AL et al (2019) Suppression of Choroidal Neovascularization by AAV-Based Dual-Acting Antiangiogenic Gene Therapy. Molecular therapy. Nucleic acids 16:38–50
Lukason M et al (2011) Inhibition of choroidal neovascularization in a nonhuman primate model by intravitreal administration of an AAV2 vector expressing a novel anti-VEGF molecule. Mol Ther 19(2):260–265
Martin DF (2018) Evolution of intravitreal therapy for retinal diseases: from CMV to CNV: the LXXIV Edward Jackson Memorial Lecture. Am J Ophthalmol. https://doi.org/10.1016/j.ajo.2017.12.019
Owen D et al (2008) The IQGAP1-Rac1 and IQGAP1-Cdc42 interactions: interfaces differ between the complexes. J Biol Chem 283(3):1692–1704
Wang H et al (2011) The Role of RPE Cell-Associated VEGF189 in Choroidal Endothelial Cell Transmigration across the RPE. Invest Ophthalmol Vis Sci 52(1):570–578
Mezquita B et al (2014) Unlocking Doors without Keys: Activation of Src by Truncated C-terminal Intracellular Receptor Tyrosine Kinases Lacking Tyrosine Kinase Activity. Cells 3(1):92–111
Li Z et al (2018) Mesenchymal stem cells promote endothelial progenitor cell migration, vascularization, and bone repair in tissue-engineered constructs via activating CXCR2-Src-PKL/Vav2-Rac1. Faseb j 32(4):2197–2211
Joussen AM, Bornfeld N (2009) The treatment of wet age-related macular degeneration. Dtsch Arztebl Int 106(18):312–317
Haller JA (2013) Current anti-vascular endothelial growth factor dosing regimens: benefits and burden. Ophthalmology 120(5 Suppl):S3–S7
Rofagha S et al (2013) Seven-Year Outcomes in Ranibizumab-Treated Patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology. https://doi.org/10.1016/j.ophtha.2013.03.046
Wang H, Hartnett ME (2016) Regulation of signaling events involved in the pathophysiology of neovascular AMD. Mol Vis 22:189–202
Handa JT et al (2019) A systems biology approach towards understanding and treating non-neovascular age-related macular degeneration. Nat Commun 10(1):3347
Farecki ML et al (2017) Characteristics of type 1 and 2 CNV in exudative AMD in OCT-angiography. Graefes Arch Clin Exp Ophthalmol 255(5):913–921
Grunwald JE et al (2012) Photographic assessment of baseline fundus morphologic features in the comparison of age-related macular degeneration treatments trials. Ophthalmology 119(8):1634–1641
Monaghan-Benson E et al (2010) The role of vascular endothelial growth factor-induced activation of NADPH oxidase in choroidal endothelial cells and choroidal neovascularization. Am J Pathol 177(4):2091–2102
Wang H et al (2016) Retinal inhibition of CCR3 induces retinal cell death in a murine model of choroidal neovascularization. PLoS ONE 11(6):e0157748
Takeda A et al (2009) CCR3 is a target for age-related macular degeneration diagnosis and therapy. Nature 460(7252):225–230
Wang H et al (2011) Upregulation of CCR3 by age-related stresses promotes choroidal endothelial cell migration via VEGF-dependent and -independent signaling. Investig Ophthalmol Vis Sci 52(11):8271–8277
Churchill AJ et al (2006) VEGF polymorphisms are associated with neovascular age-related macular degeneration. Hum Mol Genet 15(19):2955–2961
Urao N et al (2008) Role of nox2-based NADPH oxidase in bone marrow and progenitor cell function involved in neovascularization induced by hindlimb ischemia. Circ Res 103(2):212–220
Becker S et al (2008) Targeted Knockdown of Overexpressed VEGFA or VEGF164 in Muller cells maintains retinal function by triggering different signaling mechanisms. Sci Rep 8(1):2003
Saint-Geniez M et al (2008) Endogenous VEGF is required for visual function: evidence for a survival role on muller cells and photoreceptors. PLoS ONE 3(11):e3554
D’Amore PA, Smith SR (1993) Growth factor effects on cells of the vascular wall: a survey. Growth Factors 8(1):61–75
Abe K et al (2000) Vav2 Is an activator of Cdc42, Rac1, and RhoA. J Biol Chem 275(14):10141–10149
Acknowledgements
This work was supported by the National Institutes of Health EY014800 and an Unrestricted Grant from Research to Prevent Blindness, Inc., New York, NY, to the Department of Ophthalmology & Visual Sciences, University of Utah; and the National Institutes of Health R01EY015130 and R01EY017011 to M.E.H and by T32 EY024234 to A. R. D.B.S. is supported by the Intramural Research Program of the National Institutes of Health. We thank Dr. William A. Muller for providing adenovirus-IQGAP1shRNA constructs.
Author information
Authors and Affiliations
Contributions
HW designed and performed the experiments and wrote the manuscript; AR and EK performed experiments and housed the animals; DBS provided the animals, reagents, and wrote the manuscript; MEH designed the experiments, wrote the manuscript, and provided funding supports.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared there were no conflicts of interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, H., Ramshekar, A., Kunz, E. et al. IQGAP1 causes choroidal neovascularization by sustaining VEGFR2-mediated Rac1 activation. Angiogenesis 23, 685–698 (2020). https://doi.org/10.1007/s10456-020-09740-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-020-09740-y