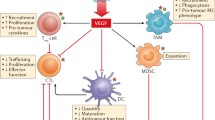
Abstract
Angiogenesis is critical for the initiation and progression of solid tumors, as well as hematological malignancies. While angiogenesis in solid tumors has been well characterized, a large body of investigation is devoted to clarify the impact of angiogenesis on lymphoma development. B-cell non-Hodgkin lymphoma (B-NHL) is the most common lymphoid malignancy with a highly heterogeneity. The malignancy remains incurable despite that the addition of rituximab to conventional chemotherapies provides substantial improvements. Several angiogenesis-related parameters, such as proangiogenic factors, circulating endothelial cells, microvessel density, and tumor microenvironment, have been identified as prognostic indicators in different types of B-NHL. A better understanding of how these factors work together to facilitate lymphoma-specific angiogenesis will help to design better antiangiogenic strategies. So far, VEGF-A monoclonal antibodies, receptor tyrosine kinase inhibitors targeting VEGF receptors, and immunomodulatory drugs with antiangiogenic activities are being tested in preclinical and clinical studies. This review summarizes recent advances in the understanding of the role of angiogenesis in B-NHL, and discusses the applications of antiangiogenic therapies.

Similar content being viewed by others
References
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285(21):1182–1186
Koster A, Raemaekers JM (2005) Angiogenesis in malignant lymphoma. Curr Opin Oncol 17(6):611–616
Ruan J, Hajjar K, Rafii S, Leonard JP (2009) Angiogenesis and antiangiogenic therapy in non-Hodgkin’s lymphoma. Ann Oncol 20(3):413–424
Angeli V, Ginhoux F, Llodra J, Quemeneur L, Frenette PS, Skobe M et al (2006) B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity 24(2):203–215
Gratzinger D, Zhao S, Marinelli RJ, Kapp AV, Tibshirani RJ, Hammer AS et al (2007) Microvessel density and expression of vascular endothelial growth factor and its receptors in diffuse large B-cell lymphoma subtypes. Am J Pathol 170(4):1362–1369
Chen H, Treweeke AT, West DC, Till KJ, Cawley JC, Zuzel M et al (2000) In vitro and in vivo production of vascular endothelial growth factor by chronic lymphocytic leukemia cells. Blood 96(9):3181–3187
Bairey O, Boycov O, Kaganovsky E, Zimra Y, Shaklai M, Rabizadeh E (2004) All three receptors for vascular endothelial growth factor (VEGF) are expressed on B-chronic lymphocytic leukemia (CLL) cells. Leuk Res 28(3):243–248
Ganjoo KN, Moore AM, Orazi A, Sen JA, Johnson CS, An CS (2008) The importance of angiogenesis markers in the outcome of patients with diffuse large B cell lymphoma: a retrospective study of 97 patients. J Cancer Res Clin Oncol 134(3):381–387
Gratzinger D, Zhao S, Tibshirani RJ, Hsi ED, Hans CP, Pohlman B et al (2008) Prognostic significance of VEGF, VEGF receptors, and microvessel density in diffuse large B cell lymphoma treated with anthracycline-based chemotherapy. Lab Invest 88(1):38–47
Gratzinger D, Advani R, Zhao S, Talreja N, Tibshirani RJ, Shyam R et al (2010) Lymphoma cell VEGFR2 expression detected by immunohistochemistry predicts poor overall survival in diffuse large B cell lymphoma treated with immunochemotherapy (R-CHOP). Br J Haematol 148(2):235–244
Jorgensen JM, Sorensen FB, Bendix K, Nielsen JL, Funder A, Karkkainen MJ et al (2009) Expression level, tissue distribution pattern, and prognostic impact of vascular endothelial growth factors VEGF and VEGF-C and their receptors Flt-1, KDR, and Flt-4 in different subtypes of non-Hodgkin lymphomas. Leuk Lymphoma 50(10):1647–1660
Ferrajoli A, Manshouri T, Estrov Z, Keating MJ, O’Brien S, Lerner S et al (2001) High levels of vascular endothelial growth factor receptor-2 correlate with shortened survival in chronic lymphocytic leukemia. Clin Cancer Res 7(4):795–799
Riihijarvi S, Nurmi H, Holte H, Bjorkholm M, Fluge O, Pedersen LM et al (2012) High serum vascular endothelial growth factor level is an adverse prognostic factor for high-risk diffuse large B-cell lymphoma patients treated with dose-dense chemoimmunotherapy. Eur J Haematol 89(5):395–402
Niitsu N, Okamato M, Nakamine H, Yoshino T, Tamaru J, Nakamura S et al (2002) Simultaneous elevation of the serum concentrations of vascular endothelial growth factor and interleukin-6 as independent predictors of prognosis in aggressive non-Hodgkin’s lymphoma. Eur J Haematol 68(2):91–100
Salven P, Orpana A, Teerenhovi L, Joensuu H (2000) Simultaneous elevation in the serum concentrations of the angiogenic growth factors VEGF and bFGF is an independent predictor of poor prognosis in non-Hodgkin lymphoma: a single-institution study of 200 patients. Blood 96(12):3712–3718
Rueda A, Rifa J, Quero C, Gomez-Codina J, Murias A, Garcia-Arroyo FR et al (2014) High serum levels of vascular endothelial growth factor-C have a positive impact on outcome of patients with advanced diffuse large B cell lymphoma. Leuk Lymphoma 55(6):1413–1416
Labidi SI, Menetrier-Caux C, Chabaud S, Chassagne C, Sebban C, Gargi T et al (2010) Serum cytokines in follicular lymphoma. Correlation of TGF-beta and VEGF with survival. Ann Hematol 89(1):25–33
Molica S, Vitelli G, Levato D, Ricciotti A, Digiesi G (2002) Clinicoprognostic implications of increased serum levels of vascular endothelial growth factor and basic fibroblastic growth factor in early B-cell chronic lymphocytic leukaemia. Br J Cancer 86(1):31–35
Monestiroli S, Mancuso P, Burlini A, Pruneri G, Dell’Agnola C, Gobbi A et al (2001) Kinetics and viability of circulating endothelial cells as surrogate angiogenesis marker in an animal model of human lymphoma. Cancer Res 61(11):4341–4344
Mancuso P, Burlini A, Pruneri G, Goldhirsch A, Martinelli G, Bertolini F (2001) Resting and activated endothelial cells are increased in the peripheral blood of cancer patients. Blood 97(11):3658–3661
Igreja C, Courinha M, Cachaco AS, Pereira T, Cabecadas J, da Silva MG et al (2007) Characterization and clinical relevance of circulating and biopsy-derived endothelial progenitor cells in lymphoma patients. Haematologica 92(4):469–477
Alshenawy HA (2010) Prognostic significance of vascular endothelial growth factor, basic fibroblastic growth factor, and microvessel density and their relation to cell proliferation in B-cell non-Hodgkin’s lymphoma. Ann Diagn Pathol 14(5):321–327
Cardesa-Salzmann TM, Colomo L, Gutierrez G, Chan WC, Weisenburger D, Climent F et al (2011) High microvessel density determines a poor outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus chemotherapy. Haematologica 96(7):996–1001
Taskinen M, Jantunen E, Kosma VM, Bono P, Karjalainen-Lindsberg ML, Leppa S (2010) Prognostic impact of CD31-positive microvessel density in follicular lymphoma patients treated with immunochemotherapy. Eur J Cancer 46(13):2506–2512
Vesela P, Tonar Z, Salek D, Vokurka S, Trneny M, Kodet R et al (2014) Microvessel density of mantle cell lymphoma. A retrospective study of its prognostic role and the correlation with the Ki-67 and the mantle cell lymphoma international prognostic index in 177 cases. Virchows Arch 465(5):587–597
Koster A, van Krieken JH, Mackenzie MA, Schraders M, Borm GF, van der Laak JA et al (2005) Increased vascularization predicts favorable outcome in follicular lymphoma. Clin Cancer Res 11(1):154–161
Crivellato E, Nico B, Vacca A, Dammacco F, Rebatti D (2002) Mast cell heterogeneity in B-cell non-Hodgkin’s lymphomas: an ultrastructural study. Leuk Lymphoma 43(11):2201–2205
Duse AO, Ceausu RA, Mezei T, Cimpean AM, Gaje P, Ionita H et al (2011) Mast cells contribute to the angiogenesis in non-Hodgkin lymphoma. An immunohistochemical study based on the relationship with microvessel density. Rom J Morphol Embryol 52(3 Suppl):1091–1096
Hedstrom G, Berglund M, Molin D, Fischer M, Nilsson G, Thunberg U et al (2007) Mast cell infiltration is a favourable prognostic factor in diffuse large B-cell lymphoma. Br J Haematol 138(1):68–71
Taskinen M, Karjalainen-Lindsberg ML, Leppa S (2008) Prognostic influence of tumor-infiltrating mast cells in patients with follicular lymphoma treated with rituximab and CHOP. Blood 111(9):4664–4667
Nam SJ, Go H, Paik JH, Kim TM, Heo DS, Kim CW et al (2014) An increase of M2 macrophages predicts poor prognosis in patients with diffuse large B-cell lymphoma treated with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone. Leuk Lymphoma 55(11):2466–2476
Riihijarvi S, Fiskvik I, Taskinen M, Vajavaara H, Tikkala M, Yri O et al (2015) Prognostic influence of macrophages in patients with diffuse large B-cell lymphoma: a correlative study from a Nordic phase II trial. Haematologica 100(2):238–245
Canioni D, Salles G, Mounier N, Brousse N, Keuppens M, Morchhauser F et al (2008) High numbers of tumor-associated macrophages have an adverse prognostic value that can be circumvented by rituximab in patients with follicular lymphoma enrolled onto the GELA-GOELAMS FL-2000 trial. J Clin Oncol 26(3):440–446
Carreras J, Lopez-Guillermo A, Fox BC, Colomo L, Martinez A, Roncador G et al (2006) High numbers of tumor-infiltrating FOXP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood 108(9):2957–2964
Lee NR, Song EK, Jang KY, Choi HN, Moon WS, Kwon K et al (2008) Prognostic impact of tumor infiltrating FOXP3 positive regulatory T cells in diffuse large B-cell lymphoma at diagnosis. Leuk Lymphoma 49(2):247–256
Farinha P, Al-Tourah A, Gill K, Klasa R, Connors JM, Gascoyne RD (2010) The architectural pattern of FOXP3-positive T cells in follicular lymphoma is an independent predictor of survival and histologic transformation. Blood 115(2):289–295
Wahlin BE, Sander B, Christensson B, Kimby E (2007) CD8 + T-cell content in diagnostic lymph nodes measured by flow cytometry is a predictor of survival in follicular lymphoma. Clin Cancer Res 13(2 Pt 1):388–397
Alvaro T, Lejeune M, Salvado MT, Lopez C, Jaen J, Bosch R et al (2006) Immunohistochemical patterns of reactive microenvironment are associated with clinicobiologic behavior in follicular lymphoma patients. J Clin Oncol 24(34):5350–5357
Carreras J, Lopez-Guillermo A, Roncador G, Villamor N, Colomo L, Martinez A et al (2009) High numbers of tumor-infiltrating programmed cell death 1-positive regulatory lymphocytes are associated with improved overall survival in follicular lymphoma. J Clin Oncol 27(9):1470–1476
Wang ES, Teruya-Feldstein J, Wu Y, Zhu Z, Hicklin DJ, Moore MA (2004) Targeting autocrine and paracrine VEGF receptor pathways inhibits human lymphoma xenografts in vivo. Blood 104(9):2893–2902
Goto H, Kudo E, Kariya R, Taura M, Katano H, Okada S (2015) Targeting VEGF and interleukin-6 for controlling malignant effusion of primary effusion lymphoma. J Cancer Res Clin Oncol 141(3):465–474
Mori F, Ishida T, Ito A, Sato F, Masaki A, Takino H et al (2012) Potent antitumor effects of bevacizumab in a microenvironment-dependent human lymphoma mouse model. Blood Cancer J 2(4):e67
Stopeck AT, Unger JM, Rimsza LM, Bellamy WT, Iannone M, Persky DO et al (2009) A phase II trial of single agent bevacizumab in patients with relapsed, aggressive non-Hodgkin lymphoma: Southwest oncology group study S0108. Leuk Lymphoma 50(5):728–735
Ruan J, Gregory SA, Christos P, Martin P, Furman RR, Coleman M et al (2014) Long-term follow-up of R-CHOP with bevacizumab as initial therapy for mantle cell lymphoma: clinical and correlative results. Clin Lymphoma Myeloma Leuk 14(2):107–113
Stopeck AT, Unger JM, Rimsza LM, LeBlanc M, Farnsworth B, Iannone M et al (2012) A phase 2 trial of standard-dose cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) and rituximab plus bevacizumab for patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: SWOG 0515. Blood 120(6):1210–1217
Fu Z, Zhu J, Zheng W, Liu W, Ying Z, Xie Y et al (2014) Safety and efficacy of bevacizumab combined with R-CHOP regimen in seven Chinese patients with untreated diffuse large B-cell lymphoma. Cancer Cell Int 14(1):5
Hainsworth JD, Greco FA, Raefsky EL, Thompson DS, Lunin S, Reeves J Jr et al (2014) Rituximab with or without bevacizumab for the treatment of patients with relapsed follicular lymphoma. Clin Lymphoma Myeloma Leuk 14(4):277–283
Lockhart AC, Rothenberg ML, Dupont J, Cooper W, Chevalier P, Sternas L et al (2010) Phase I study of intravenous vascular endothelial growth factor trap, aflibercept, in patients with advanced solid tumors. J Clin Oncol 28(2):207–214
Romero M, Briere J, de Bazelaire C, Leboeuf C, Wang L, Ratajczak P et al (2011) Aflibercept-mediated early angiogenic changes in aggressive B-cell lymphoma. Cancer Chemother Pharmacol 68(5):1135–1143
Carlo-Stella C, Locatelli SL, Giacomini A, Cleris L, Saba E, Righi M et al (2013) Sorafenib inhibits lymphoma xenografts by targeting MAPK/ERK and AKT pathways in tumor and vascular cells. PLoS One 8(4):e61603
Chapuy B, Schuelper N, Panse M, Dohm A, Hand E, Schroers R et al (2011) Multikinase inhibitor sorafenib exerts cytocidal efficacy against Non-Hodgkin lymphomas associated with inhibition of MAPK14 and AKT phosphorylation. Br J Haematol 152(4):401–412
Xargay-Torrent S, Lopez-Guerra M, Montraveta A, Saborit-Villarroya I, Rosich L, Navarro A et al (2013) Sorafenib inhibits cell migration and stroma-mediated bortezomib resistance by interfering B-cell receptor signaling and protein translation in mantle cell lymphoma. Clin Cancer Res 19(3):586–597
Guidetti A, Carlo-Stella C, Locatelli SL, Malorni W, Pierdominici M, Barbati C et al (2012) Phase II study of sorafenib in patients with relapsed or refractory lymphoma. Br J Haematol 158(1):108–119
Greenwald DR, Li H, Luger SM, Go RS, King D, Patel T et al (2013) A phase II study of sorafenib (BAY 43-9006) in recurrent diffuse large B cell lymphoma: an eastern cooperative oncology group study (E1404). J Hematol Oncol 6:46
Guidetti A, Carlo-Stella C, Locatelli SL, Malorni W, Mortarini R, Viviani S et al (2014) Phase II study of perifosine and sorafenib dual-targeted therapy in patients with relapsed or refractory lymphoproliferative diseases. Clin Cancer Res 20(22):5641–5651
Ikezoe T, Nishioka C, Tasaka T, Yang Y, Komatsu N, Togitani K et al (2006) The antitumor effects of sunitinib (formerly SU11248) against a variety of human hematologic malignancies: enhancement of growth inhibition via inhibition of mammalian target of rapamycin signaling. Mol Cancer Ther 5(10):2522–2530
Buckstein R, Kuruvilla J, Chua N, Lee C, Macdonald DA, Al-Tourah AJ et al (2011) Sunitinib in relapsed or refractory diffuse large B-cell lymphoma: a clinical and pharmacodynamic phase II multicenter study of the NCIC Clinical Trials Group. Leuk Lymphoma 52(5):833–841
Shanafelt T, Zent C, Byrd J, Erlichman C, Laplant B, Ghosh A et al (2010) Phase II trials of single-agent anti-VEGF therapy for patients with chronic lymphocytic leukemia. Leuk Lymphoma 51(12):2222–2229
Brander D, Rizzieri D, Gockerman J, Diehl L, Shea TC, Decastro C et al (2013) Phase II open label study of the oral vascular endothelial growth factor-receptor inhibitor PTK787/ZK222584 (vatalanib) in adult patients with refractory or relapsed diffuse large B-cell lymphoma. Leuk Lymphoma 54(12):2627–2630
Ruan J, Luo M, Wang C, Fan L, Yang SN, Cardenas M et al (2013) Imatinib disrupts lymphoma angiogenesis by targeting vascular pericytes. Blood 121(26):5192–5202
Dredge K, Dalgleish AG, Marriott JB (2003) Thalidomide analogs as emerging anti-cancer drugs. Anticancer Drugs 14(5):331–335
Corral LG, Haslett PA, Muller GW, Chen R, Wong LM, Ocampo CJ et al (1999) Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol 163(1):380–386
Davies FE, Raje N, Hideshima T, Lentzsch S, Young G, Tai YT et al (2001) Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood 98(1):210–216
Eleutherakis-Papaiakovou V, Bamias A, Dimopoulos MA (2004) Thalidomide in cancer medicine. Ann Oncol 15(8):1151–1160
D’Amato RJ, Loughnan MS, Flynn E, Folkman J (1994) Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci U S A 91(9):4082–4085
Kenyon BM, Browne F, D’Amato RJ (1997) Effects of thalidomide and related metabolites in a mouse corneal model of neovascularization. Exp Eye Res 64(6):971–978
Palumbo A, Facon T, Sonneveld P, Blade J, Offidani M, Gay F et al (2008) Thalidomide for treatment of multiple myeloma: 10 years later. Blood 111(8):3968–3977
Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P et al (1999) Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med 341(21):1565–1571
Pro B, Younes A, Albitar M, Dang NH, Samaniego F, Romaguera J et al (2004) Thalidomide for patients with recurrent lymphoma. Cancer 100(6):1186–1189
Damaj G, Lefrere F, Delarue R, Varet B, Furman R, Hermine O (2003) Thalidomide therapy induces response in relapsed mantle cell lymphoma. Leukemia 17(9):1914–1915
Ruan J, Martin P, Coleman M, Furman RR, Cheung K, Faye A et al (2010) Durable responses with the metronomic rituximab and thalidomide plus prednisone, etoposide, procarbazine, and cyclophosphamide regimen in elderly patients with recurrent mantle cell lymphoma. Cancer 116(11):2655–2664
Ji D, Li Q, Cao J, Guo Y, Lv F, Liu X et al (2016) Thalidomide enhanced the efficacy of CHOP chemotherapy in the treatment of diffuse large B cell lymphoma: A phase II study. Oncotarget 7(22):33331–33339
Song K, Herzog BH, Sheng M, Fu J, McDaniel JM, Chen H et al (2013) Lenalidomide inhibits lymphangiogenesis in preclinical models of mantle cell lymphoma. Cancer Res 73(24):7254–7264
Zhang L, Qian Z, Cai Z, Sun L, Wang H, Bartlett JB et al (2009) Synergistic antitumor effects of lenalidomide and rituximab on mantle cell lymphoma in vitro and in vivo. Am J Hematol 84(9):553–559
Witzig TE, Wiernik PH, Moore T, Reeder C, Cole C, Justice G et al (2009) Lenalidomide oral monotherapy produces durable responses in relapsed or refractory indolent non-Hodgkin’s Lymphoma. J Clin Oncol 27(32):5404–5409
Wiernik PH, Lossos IS, Tuscano JM, Justice G, Vose JM, Cole CE et al (2008) Lenalidomide monotherapy in relapsed or refractory aggressive non-Hodgkin’s lymphoma. J Clin Oncol 26(30):4952–4957
Witzig TE, Vose JM, Zinzani PL, Reeder CB, Buckstein R, Polikoff JA et al (2011) An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin’s lymphoma. Ann Oncol 22(7):1622–1627
Hernandez-Ilizaliturri FJ, Deeb G, Zinzani PL, Pileri SA, Malik F, Macon WR et al (2011) Higher response to lenalidomide in relapsed/refractory diffuse large B-cell lymphoma in nongerminal center B-cell-like than in germinal center B-cell-like phenotype. Cancer 117(22):5058–5066
Eve HE, Carey S, Richardson SJ, Heise CC, Mamidipudi V, Shi T et al (2012) Single-agent lenalidomide in relapsed/refractory mantle cell lymphoma: results from a UK phase II study suggest activity and possible gender differences. Br J Haematol 159(2):154–163
Goy A, Sinha R, Williams ME, Kalayoglu Besisik S, Drach J, Ramchandren R et al (2013) Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study. J Clin Oncol 31(29):3688–3695
Habermann TM, Lossos IS, Justice G, Vose JM, Wiernik PH, McBride K et al (2009) Lenalidomide oral monotherapy produces a high response rate in patients with relapsed or refractory mantle cell lymphoma. Br J Haematol 145(3):344–349
Trneny M, Lamy T, Walewski J, Belada D, Mayer J, Radford J et al (2016) Lenalidomide versus investigator’s choice in relapsed or refractory mantle cell lymphoma (MCL-002; SPRINT): a phase 2, randomised, multicentre trial. Lancet Oncol 17(3):319–331
Ruan J, Martin P, Shah B, Schuster SJ, Smith SM, Furman RR et al (2015) Lenalidomide plus Rituximab as Initial Treatment for Mantle-Cell Lymphoma. N Engl J Med 373(19):1835–1844
Wang M, Fayad L, Wagner-Bartak N, Zhang L, Hagemeister F, Neelapu SS et al (2012) Lenalidomide in combination with rituximab for patients with relapsed or refractory mantle-cell lymphoma: a phase 1/2 clinical trial. Lancet Oncol 13(7):716–723
Wang M, Fowler N, Wagner-Bartak N, Feng L, Romaguera J, Neelapu SS et al (2013) Oral lenalidomide with rituximab in relapsed or refractory diffuse large cell, follicular and transformed lymphoma: a phase II clinical trial. Leukemia 27(9):1902–1909
Leonard JP, Jung SH, Johnson J, Pitcher BN, Bartlett NL, Blum KA et al (2015) Randomized Trial of Lenalidomide Alone Versus Lenalidomide Plus Rituximab in Patients With Recurrent Follicular Lymphoma: CALGB 50401 (Alliance). J Clin Oncol 33(31):3635–3640
Tuscano JM, Dutia M, Chee K, Brunson A, Reed-Pease C, Abedi M et al (2014) Lenalidomide plus rituximab can produce durable clinical responses in patients with relapsed or refractory, indolent non-Hodgkin lymphoma. Br J Haematol 165(3):375–381
Kiesewetter B, Willenbacher E, Willenbacher W, Egle A, Neumeister P, Voskova D et al (2017) A phase 2 study of rituximab plus lenalidomide for mucosa-associated lymphoid tissue lymphoma. Blood 129(3):383–385
Nowakowski GS, LaPlant B, Habermann TM, Rivera CE, Macon WR, Inwards DJ et al (2011) Lenalidomide can be safely combined with R-CHOP (R2CHOP) in the initial chemotherapy for aggressive B-cell lymphomas: phase I study. Leukemia 25(12):1877–1881
Nowakowski GS, LaPlant B, Macon WR, Reeder CB, Foran JM, Nelson GD et al (2015) Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non-germinal center B-cell phenotype in newly diagnosed diffuse large B-Cell lymphoma: a phase II study. J Clin Oncol 33(3):251–257
Vitolo U, Chiappella A, Franceschetti S, Carella AM, Baldi I, Inghirami G et al (2014) Lenalidomide plus R-CHOP21 in elderly patients with untreated diffuse large B-cell lymphoma: results of the REAL07 open-label, multicentre, phase 2 trial. Lancet Oncol 15(7):730–737
Feldman T, Mato AR, Chow KF, Protomastro EA, Yannotti KM, Bhattacharyya P et al (2014) Addition of lenalidomide to rituximab, ifosfamide, carboplatin, etoposide (RICER) in first-relapse/primary refractory diffuse large B-cell lymphoma. Br J Haematol 166(1):77–83
Martin A, Redondo AM, Dlouhy I, Salar A, Gonzalez-Barca E, Canales M et al (2016) Lenalidomide in combination with R-ESHAP in patients with relapsed or refractory diffuse large B-cell lymphoma: a phase 1b study from GELTAMO group. Br J Haematol 173(2):245–252
Bates DO, Cui TG, Doughty JM, Winkler M, Sugiono M, Shields JD et al (2002) VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res 62(14):4123–4131
Eswarappa SM, Potdar AA, Koch WJ, Fan Y, Vasu K, Lindner D et al (2014) Programmed translational readthrough generates antiangiogenic VEGF-Ax. Cell 157(7):1605–1618
Kronke J, Udeshi ND, Narla A, Grauman P, Hurst SN, McConkey M et al (2014) Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 343(6168):301–305
Streubel B, Chott A, Huber D, Exner M, Jager U, Wagner O et al (2004) Lymphoma-specific genetic aberrations in microvascular endothelial cells in B-cell lymphomas. N Engl J Med 351(3):250–259
Jain RK (2001) Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med 7(9):987–989
Rivera LB, Bergers G (2015) CANCER. Tumor angiogenesis, from foe to friend. Science 349(6249):694–695
Wong PP, Demircioglu F, Ghazaly E, Alrawashdeh W, Stratford MR, Scudamore CL et al (2015) Dual-action combination therapy enhances angiogenesis while reducing tumor growth and spread. Cancer Cell 27(1):123–137
Acknowledgements
The work was supported by the Natural Science Foundation of Ningbo (2019A610270), the National Natural Science Foundation of China (81400098), Zhejiang Key Laboratory of Pathophysiology (201909), and the K.C. Wong Magna Fund in Ningbo University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiang, L., Li, N. B-cell non-Hodgkin lymphoma: importance of angiogenesis and antiangiogenic therapy. Angiogenesis 23, 515–529 (2020). https://doi.org/10.1007/s10456-020-09729-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-020-09729-7