Abstract
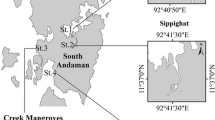
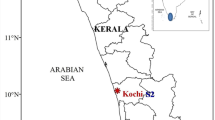
Temporal variation in the taxonomic structure of microphytobenthos (MPB) in a riverine mangrove ecosystem was studied along the southwest coast of India. About 122 species of microphytobenthos comprising diatoms (113 species), cyanobacteria (seven species), dinoflagellate (one species), and euglenophyte (one species) were observed, in which pennate diatoms dominated by 92%. Major subclasses of diatoms identified were Bacillariophycidae, Fragilariophycidae, Thalassiosirophycidae, Melosirophycidae, and Coscinodiscophycidae. Average levels of fluvial nutrients estimated in the porewater were NO3–N (28 ± 19.7 µmol L−1), PO4–P (3.77 ± 4.6 µmol L−1), and SiO4–Si (33.12 ± 27.2 µmol L−1). The colonization and persistence of dense cyanobacterial mats during monsoon resulted in less abundance and diversity of diatoms than in other seasons. The numerical abundance of MPB was at its maximum during July (monsoon season) due to the dense cyanobacterial mat formed by Oscillatoria princeps. MPB diversity was at its maximum during MON (H´- 3.2), followed by POM (H´- 3.08) and lowest during PRM (H´- 2.23). The statistically significant seasonal variations in the diversity of MPB were noticed during the study period (ANOVA F value 8.120; df 2; p value < 0.05). The present study identifies sediment temperature and porewater salinity (freshwater preference) along with rainfall, sediment pH and C:N ratio as the major governing factors in the benthic microalgal mat formation of the study area.










Similar content being viewed by others
Data availability
The manuscript has no associated data.
Code availability
Not applicable.
References
Al-Yamani FY, Saburova MA (2011) Illustrated guide on the benthic diatoms of Kuwait’s marine environment. In: Kuwait Institute for Scientific Research, Kuwait, pp 11–352
Anderson DM, Glibert PM, Burkholder JM (2002) Harmful algal blooms and eutrophication: nutrient sources, composition, and consequences. Estuaries 25(4):704–726
Basha CS (1991) Distribution of mangroves in Kerala. Indian for 117:439–449
Billerbeck M, Røy H, Bosselmann K, Huettel M (2007) Benthic photosynthesis in submerged Wadden Sea intertidal flats. Estuar Coast Shelf 71(3–4):704–716. https://doi.org/10.1016/j.ecss.2006.09.019
Blaber SJ, Cyrus DP, Albaret JJ, Ching CV, Day JW, Elliott M, Fonseca MS, Hoss DE, Orensanz J, Potter IC, Silvert W (2000) Effects of fishing on the structure and functioning of estuarine and nearshore ecosystems. ICES J Mar Sci 57(3):590–602. https://doi.org/10.1006/jmsc.2000.0723
Borrego J, Lopez M, Pendon JG, Morales JA (1998) C/S ratios in estuarine sediments of the Odiel River-mouth. SW Spain J Coast Res 14(4):1276–1283
Bunting P, Rosenqvist A, Lucas R, Rebelo LM, Hilarides L, Thomas N, Hardy A, Itoh T, Shimada M, Finlayson C (2018) The global mangrove watch—a new 2010 global baseline of mangrove extent. Remote Sens 10(10):1669. https://doi.org/10.3390/rs10101669
Cahoon LB (2014) The role of benthic microalgae in neritic ecosystems. Oceanogr Mar Biol 37:55–94
Castro-Rodríguez E, León-Luna I, Pinedo-Hernández J (2018) Biogeochemistry of mangrove sediments in the Swamp of Mallorquin, Colombia. Reg Stud Mar Sci 17:38–46. https://doi.org/10.1016/j.rsma.2017.11.005
Cook PL, Røy H (2006) Advective relief of CO2 limitation in microphytobenthos in highly productive sandy sediments. Limnol Oceanogr 51(4):1594–1601. https://doi.org/10.4319/lo.2006.51.4.1594
Costanza R, d’Arge R, De Groot R, Farber S, Grasso M, Hannon B, Limburg K, Naeem S, O’Neill RV, Paruelo J, Raskin RG, Sutton P, Van Den Belt M (1997) The value of the world’s ecosystem services and natural capital. Nature 387(6630):253–260
Crémière A, Strauss H, Sebilo M, Hong WL, Gros O, Schmidt S, Tocny J, Henry F, Gontharet S, Laverman AM (2017) Sulfur diagenesis under rapid accumulation of organic-rich sediments in a marine mangrove from Guadeloupe (French West Indies). Chem Geol 454:67–79. https://doi.org/10.1016/j.chemgeo.2017.02.017
Cunha-Lignon M, Coelho C Jr, Almeida R, Menghini RP, Schaeffer-Novelli Y, Cintrón G, Dahdouh-Guebas F (2011) Characterisation of mangrove forest types in view of conservation and management: a review of mangals at the Cananéia region, São Paulo State. Brazil J Coast Res SI 64:349–353
Dalu T, Wasserman RJ (2018) Cyanobacteria dynamics in a small tropical reservoir: understanding spatio-temporal variability and influence of environmental variables. Sci Total Environ 643:835–841. https://doi.org/10.1016/j.scitotenv.2018.06.256
Demaison GJ, Moore GT (1980) Anoxic environments and oil source bed genesis. Org Geochem 2:9–31. https://doi.org/10.1306/2F91945E-16CE-11D7-8645000102C1865D
Dubuc A, Baker R, Marchand C, Waltham NJ, Sheaves M (2019) Hypoxia in mangroves: occurrence and impact on valuable tropical fish habitat. Biogeosciences 16(20):3959–3976. https://doi.org/10.5194/bg-16-3959-2019
Eshleman KN, Hemond HF (1985) The role of soluble organics in acid-base status of waters at Bickford Watershed Massachussetts. Water Resour Res 21:1503–1510
Esteve I, Martínez-Alonso M, Mir J, Guerrero R (1992) Distribution, typology and structure of microbial mat communities in Spain: a preliminary study. Limnetica 8:185–195
Folk RL (1974) Petrology of sedimentary rocks: Austin. p 182
Fontana LF, Crapez MA, de Figueiredo Junior AG, Santos ES, da Silva FS, Ribeiro AM, Martins da Rocha CC, Netto AD (2012) Characterization and distribution of polycyclic aromatic hydrocarbons in sediments from Suruí Mangrove. Guanabara Bay, Rio De Janeiro, Brazil J Coast Res 28(1):156–162. https://doi.org/10.2112/JCOASTRES-D-10-00105.1
Gadeken K, Clemo WC, Ballentine W, Dykstra SL, Fung M, Hagemeyer A, Dorgan KM, Dzwonkowski B (2021) Transport of biodeposits and benthic footprint around an oyster farm, Damariscotta Estuary. Maine Peerj 9:e11862. https://doi.org/10.7717/peerj.11862
Gladstone-Gallagher RV, Mangan S, Thrush SF, Pilditch CA (2020) Porewater nutrient enrichment alters benthic-pelagic coupling on intertidal sandflats. J Sea Res 159:101876. https://doi.org/10.1016/j.seares.2020.101876
Grasshoff K, Ehrhardt M, Kremling K, Almgren T (1983) Methods of seawater analysis. Verlag Chemie, Weinheim, p 419
Ha HJ, Kim H, Noh J, Ha HK, Khim JS (2018) Rainfall effects on the erodibility of sediment and microphytobenthos in the intertidal flat. Environ Pollut 242:2051–2058
Halfen LN, Castenholz RW (1971) Gliding motility in the blue-green alga Oscillatoria princeps. J Phycol 7(2):133–145. https://doi.org/10.1111/j.1529-8817.1971.tb01492.x
Haziqah A, Hanipah A, Zhenren G (2020) Study of pollutant degradation coefficient in natural wetland of mangrove forests. Mater Sci Forum 997:121–125. https://doi.org/10.4028/www.scientific.net/MSF.997.121
Jennerjahn TC, Ittekkot V (1997) Organic matter in sediments in the mangrove areas and adjacent continental margins of Brazil. 1. Amino acids and hexosamines. Oceanolica Acta 20(2):359–369
Jesus B, Brotas V, Ribeiro L, Mendes CR, Cartaxana P, Paterson DM (2009) Adaptations of microphytobenthos assemblages to sediment type and tidal position. Cont Shelf Res 29(13):1624–1634. https://doi.org/10.1016/j.csr.2009.05.006
Kerekes J, Beauchamp S, Tordon R, Tremblay C, Pollock T (1986) Organic versus anthropogenic acidity in tributaries of the kejimkujik watersheds in western Nova Scotia. Wat Air Soil Pollut 30:165–174
Kesari V, Kumar S, Yadav I, Chatterjee A, Rai S, Pandey S (2022) Ganga river water quality assessment using combined approaches: physico-chemical parameters and cyanobacterial toxicity detection with special reference to microcystins and molecular characterization of microcystin synthetase (mcy) genes carrying cyanobacteria. Environ Sci Pollut Res 29(9):13122–13140. https://doi.org/10.1007/s11356-021-16589-1
Komárek J, Kaštovský J, Mare J, Johansen JR (2014) Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) 2014, using a polyphasic approach. Preslia 86(4):295–335
Komárek J (2003) Planktic oscillatorialean cyanoprokaryotes (short review according to combined phenotype and molecular aspects). In: Phytoplankton and Equilibrium Concept: The Ecology of Steady-State Assemblages. pp 367–382
Lorenzen CJ (1967) Determination of chlorophyll and pheo-pigments: spectrophotometric equations. Limnol Oceanogr 12(2):343–346. https://doi.org/10.4319/lo.1967.12.2.0343
Lu X, Tian C, Pei H, Hu W, Xie J (2013) Environmental factors influencing cyanobacteria community structure in Dongping Lake, China. J Environ Sci 25:2196–2206. https://doi.org/10.1016/S1001-0742(12)60297-6
Lugo AE, Snedaker SC (1974) The ecology of mangroves. Annu Rev Ecol Evol Syst 5:39–64
McGlathery KJ, Sundback K, Fong P (2012) Estuarine benthic algae. 2nd ed. In: Day JW et al, editors. Estuarine Ecology. Wiley-Blackwell; pp 203–234
Meyer-Reil LA, Köster M (2000) Eutrophication of marine waters: effects on benthic microbial communities. Mar Pollut Bull 41(1–6):255–263. https://doi.org/10.1016/S0025-326X(00)00114-4
Meyers PA (1994) Preservation of elemental and isotopic source identification of sedimentary organic matter. Chem Geol 144:289–302. https://doi.org/10.1016/0009-2541(94)90059-0
Mez K, Hanselmann K, Preisig HR (1998) Environmental conditions in high mountain lakes containing toxic benthic cyanobacteria. Hydrobiologia 368(1):1–15
Miller DC, Geider RJ, MacIntyre HL (1996) Microphytobenthos: the ecological role of the “secret garden” of unvegetated, shallow-water marine habitats II. Role in sediment stability and shallow-water food webs. Estuaries 19:202–212
Mitra A, Banerjee K, Bhattacharyya DP (2004) The Other Face of Mangroves. Department of Environment, Govt. of West Bengal, India
Moore TR, Jackson RJ (1989) Dynamics of dissolved organic carbon in forested and catchments, wetland, New Zealand, Larry River. Water Resour Res 5:1331–1339
Müller PJ (1977) C/N ratios in Pacific deep-sea sediments: effect of inorganic ammonium and organic nitrogen compounds sorbed by clays. Geochim Cosmochim Acta 41:765–776. https://doi.org/10.1016/0016-7037(77)90047-3
Odum EP (1968) A research challenge: evaluating the productivity of coastal and estuarine water. In Proceedings of the second sea grant Conference University of Rhode Island, pp 63–64
Ramachandran KK, Balasubramanion G, Kurien J, Thomas J (1985) The mangrove ecosystem of Kerala: its mapping, inventory and some environmental aspects. Centre Earth Sci Stud, Trivandrum, Kerala, p 51
Rani VU, Perumal UE, Palanivel S (2016) Morphology and taxonomy of Oscillatoria princeps vaucher ex gomont (oscillatoriales, oscillatoriaceae). Indian J Edu Inf Manage 5(1):1–5
Redzuan NS, Underwood GJC (2020) Movement of microphytobenthos and sediment between mudflats and salt marsh during spring tides. Front Mar Sci. https://doi.org/10.3389/fmars.2020.00496
Ridd PV, Stieglitz T (2002) Dry season salinity changes in arid estuaries fringed by mangroves and salt flats. Estuar Coast Shelf Sci 54(6):1039–1049. https://doi.org/10.1006/ecss.2001.0876
Robertson AI, Alongi DM (1995) Role of riverine mangrove forests in organic carbon export to the tropical coastal ocean: a preliminary mass balance for the fly delta (Papua New Guinea). Geo-Mar Lett 15:134–139
Robertson AI, Blaber SJM (1992) Plankton, Epibenthos and Fish Communities. Coastal and Estuarine Studies In: Robertson AI, Alongi DM eds, editor/s. Tropical mangrove ecosystems pp 173–224 https://doi.org/10.1029/CE041p0173
Schaeffer P, Adam P, Philippe E, Trendel JM, Schmid JC, Behrens A (2006) The wide diversity of hopanoid sulfides evidenced by the structural identification of several novel hopanoid series. Org Geochem 37:1590–1616. https://doi.org/10.1016/j.orggeochem.2006.04.005
Shedage S, Shrivastava PK, Behara LK (2019) Carbon rich mangrove forests: an overview for strategic management and climate change mitigation. Adv Res 18(2):1–9
Shukovsky ES, Halfen LN (1976) Cellular differentiation of terminal regions of trichomes of Oscillatoria princeps (Cyanophyceae). J Phycol 12:336–342. https://doi.org/10.1111/j.1529-8817.1976.tb02853.x
Strickland JDH, Parsons TR (1968) Determination of dissolved oxygen. A practical handbook of seawater analysis. J Fish Res Board Can 167:71–75
van der Wal D, Wielemaker-van den Dool A, Herman PM (2010) Spatial synchrony in intertidal benthic algal biomass in temperate coastal and estuarine ecosystems. Ecosystems 13:338–351. https://doi.org/10.1007/s10021-010-9322-9
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci 37(1):29–38
Whiting MC, Mclntire CD (1985) An investigation of distributional patterns in the diatom flora of Netarts Bay, Oregon, by correspondence analysis. J Phycol 21:655–661
Wood SA, Kelly L, Bouma-Gregson K, Humbert JF, Laughinghous HD, Lazorchak J, McAllister T, McQueen A, Pokrzywinski K, Puddick J, Quiblier C, Reitz LA, Ryan K, Vadeboncoeur Y, Zastepa A, Davis TW (2020) Toxic benthic freshwater cyanobacterial proliferations: challenges and solutions for enhancing knowledge and improving monitoring and mitigation. Freshw Biol 65(10):1824–1842. https://doi.org/10.1111/fwb.13532
Zedler JB (1982) Salt marsh algal mat composition: spatial and temporal comparisons. Bull South Calif Acad Sci 81(1):41–50
Acknowledgements
The first author is thankful to the Cochin University of Science and Technology, Kochi, Kerala, India, for the award of the Junior Research Fellowship (U-JRF).
Funding
This study was funded by the Cochin University of Science and Technology.
Author information
Authors and Affiliations
Contributions
NB Wrote the manuscript with input from LCT. NB performed the field study. NB and LCT contributed to the taxonomic identification. KBP supervised this study and provided research materials. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Ethics approval
No animal testing was performed during this study.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by Man Xiao.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Benny, N., Thomas, L.C. & Padmakumar, K.B. Analysis of climatic and edaphic variabilities on the microphytobenthic mat characteristics of a riverine mangrove ecosystem along the southwest coast of India. Aquat Ecol (2024). https://doi.org/10.1007/s10452-023-10084-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10452-023-10084-0