Abstract
Metacommunity models describe species occupancy frequency distribution (hereinafter ‘SOFD’). Our goal is to present how the differences in eight macroinvertebrate orders dispersal ability affect SOFD patterns. A total of 293 species from eight macroinvertebrate orders were observed in 14 eutrophic lakes in southern Finland. Species occupancy ranged from 1 to 14. About 30% (89 out of 293) of the species were found in only one lake, yielding a surprisingly high number of rare species. So, there were few widely distributed common species and numerous rare species with a restricted distribution. Combined data from eight macroinvertebrate orders supported the bimodal truncated SOFD pattern. Similarly, the low dispersal ability orders, watermites and mayflies, fitted the bimodal truncated SOFD pattern. However, bimodal symmetric SOFD pattern also fitted relatively well to the dragonflies and damselflies with high dispersal ability. It seems that differences in dispersal ability among different macroinvertebrate orders may partly explain observed differences. Moreover, our results supported slightly more a niche-based model rather than a metapopulation dynamics model in eutrophic lakes littoral macroinvertebrate metacommunities. Our results highlight that the dispersal ability is important trait for species conservation in patchily distributed habitat.
Similar content being viewed by others
Introduction
Species which occurred isolated in patches, like waterbodies, form local communities. At the landscape level, most species either appear at a small number of suitable patches (satellite species; often rare) or at many patches (core species; often common and abundant). Species occupancy frequency distribution (hereinafter SOFD) is a proper method for characterizing this kind of local communities at regional scales. A SOFD approach offers information on the number or proportion of local communities in which each species occurred. The shape of the SOFD is one of the most studied patterns within community ecology (see reviews by Tokeshi 1992; McGeoch and Gaston 2002; Jenkins 2011; Hui 2012). Reviews (McGeoch and Gaston 2002; Jenkins 2011; Hui 2012) in this topic found that the two most common models which explain variations in the SOFD pattern are: (1), the metapopulation dynamic model (hereafter MPDM; Hanski 1982, 1999) and (2) the niche-based model (hereafter NBM; Brown 1984). These two models are not mutually exclusive. Local communities that share several (core) species and simultaneously have several species, which are occupying only one or few locations (satellite) conform to a bimodal core-satellite pattern, resulting from a MPDM, which is based on local extinction and colonization dynamics (Hanski 1982, 1999). Alternatively, the so-called unimodal-satellite-dominant or niche-based model relies on the idea that local communities have many rare narrow-niche specialist species and few common broad-niche generalist species (Brown 1984).
Previous studies have found that SOFDs generally have a bimodal core-satellite pattern in terrestrial communities (Hanski 1982, 1998, 1999; McGeoch and Gaston 2002; Jenkins 2011; Autio et al. 2013). However, in aquatic communities, the support for bimodal core-satellite pattern is relatively weak and the unimodal-satellite-dominant pattern seems to be more common (Tokeshi 1992; Heino 2008, 2015; Verberk et al. 2010; Korkeamäki et al. 2018; Renner et al. 2020). For example, Jenkins (2011) re-analysed two freshwater macroinvertebrate datasets (Wiberg-Larsen et al. 2000; Oertli et al. 2008) and he found unimodal-satellite SOFD pattern in both cases.
Most previous SOFD studies have, however, focused on single taxonomic group in aquatic macroinvertebrate assemblages, such as chironomids (Diptera: Chironomidae) (Tokeshi 1992), caddisflies (Trichoptera) (Wiberg-Larsen et al. 2000) and damselflies and dragonflies (Odonata) (Korkeamäki et al. 2018; Renner et al. 2020), or combined data several macroinvertebrate taxonomic groups together (Heino 2008, 2015; Verberk et al. 2010; see also Jenkins 2011). Therefore, it was not clear whether several species groups in aquatic invertebrate communities would produce unimodal-satellite SOFD pattern or whether there is variation between taxonomic groups in the SOFD patterns in a regional scale. The general purpose of our study was to test how dispersal ability modifies SOFD distribution in eight macroinvertebrate orders from 14 eutrophic lakes in southern Finland. Previous study has found that different aquatic insect orders differed in dispersal ability (Sarremejane et al. 2020).
We explored four main research questions. First, are there differences in macroinvertebrate watermite dispersal ability in relation to other aquatic insect orders? We assumed that non-flying water mite had lower dispersal ability than flying aquatic insect orders. Second, are there differences in species richness in relation to regional species’ richness between macroinvertebrate orders? We predicted that a proportion of observed species from regional species pool increases with dispersal ability. Third, is unimodal-satellite SOFD pattern fitted the best with combined macroinvertebrate data? We expected that a unimodal-satellite SOFD pattern would occur in eutrophic lakes as this is frequently observed in other aquatic macroinvertebrates orders (Tokeshi 1992; Heino 2008, 2015; Verberk et al. 2010; Jenkins 2011; Korkeamäki et al. 2018; Renner et al. 2020). Fourth, how dispersal ability affects SOFD pattern (organismal scaling hypothesis) (e.g. Collins and Glenn 1997)? Local patchily distributed and geographically isolated communities are linked to each other with species dispersal. Only a species with a high dispersal ability can colonize distant patches. Therefore, species with high dispersal ability will occur in most communities. Alternatively, species with a poor dispersal ability occur only at a few local communities in a region. Thus, we predicted that macroinvertebrate orders with a poor dispersal ability will occur only at a few sites in a region, whereas species with good dispersal ability will be found at most sites, as suggested by Collins and Glenn (1997), and are predicted to exhibit bimodal SOFDs with both equal number of cores (common) and satellite (rare) species. A satellite-dominant unimodal SOFD pattern is predicted to occur in poor dispersal ability macroinvertebrate orders (Collins and Glenn 1997).
Materials and methods
Dispersal ability of macroinvertebrates
There are already published data from aquatic insect dispersal ability (Sarremejane et al. 2020), but not for watermites, so here, to estimate the dispersal ability of local Finnish species belonging to eight different macroinvertebrate orders, we sent a questionnaire to experienced entomologists in Finland. In this study, we used eight macroinvertebrate orders: watermites (Hydracarina), mayflies (Ephemeroptera), dragonflies and damselflies (Odonata), caddisflies (Trichoptera), water bugs (Heteroptera), aquatic dipterans (Diptera, excluded chironomids), chironomids (Chironomidae) and aquatic beetles (Coleoptera). Lepidoptera and Megaloptera (Sialidae) and spiders (Arachne) were excluded from our data.
In the questionnaire, we asked each of them to rank eight macroinvertebrate orders in their dispersal ability. To ensure that all taxonomic groups followed the same methodology and to ensure comparability, we followed the same procedure for all taxonomic groups. In the questionnaire, value 1 indicates that a given taxon has the lowest dispersal ability, while value 8 means that a given taxon has the highest dispersal ability. To obtain the dispersal ability index for each macroinvertebrate order, we calculated the average dispersal ability index from the returned questionnaires (n = 10) (Supplementary Table 2).
Study area of field data
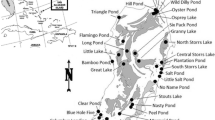
Our study was performed in Kanta-Häme and Pirkanmaa regions in southern Finland (61 30′ N, 24 20′ E) within an area of 50 × 80 km. We sampled 14 lakes (see more details in Suutari et al. 2009). The minimum distance between lakes was 0.8 km. All study sites are eutrophic with rich aquatic macrophyte communities with emergent, floating-leaved and submerged macrophytes, which cover more than half of the studied lakes or bays of large lakes. The surface area of lakes and bays varied greatly from few hectares to more than hundreds of hectares. All studied waterbodies were shallow and the coverage of the bottom was mud.
Macroarthropod samples
We collected macroinvertebrate samples in late May–early June 2000 (sweep samples) and September (traps). We took eight 2-min-sweep samples with a pond net (D-shape, width 35 cm, height 25 cm and mesh size 0.4 mm) from each lake. The samples were taken from the lower littoral zone vegetation (depth 0.5–1.0 m). To obtain a more extensive picture of the species richness in our study locations, we placed three cylinder-like traps covered with nets (mesh size 0.5 mm) (description of traps in Bagge 1999) in each study lake. Traps were left overnight (ca. 17 h) on the bottom vegetation in lower littoral zone. Two traps were provided with fish baits and one with a yellow light stick. Arthropods captured by pond net and traps were conserved in 70% ethanol. L. Paasivirta identified the Diptera and watermite species and J. Salmela identified all specimens from other insect orders to the species level with a microscope. From the collected samples, we were able to identify most of the macroinvertebrate specimens to the species level. Nevertheless, in few cases when species-level identification was impossible, individuals were identified to the higher systematic level such as genus or family level (Supplementary table 1).
Statistical methods
To make sure that our measure of dispersal ability index is reliable, we tested the level of agreement between ten judges agreed on their dispersal rankings of eight different macroinvertebrate species groups we used Kendall’s coefficient of concordance W. Kendall’s W varied from perfect agreement, W = 1, and perfect disagreement W = 0. For test differences between macroinvertebrate orders in their dispersal ability, we used a related-samples Friedman’s two-way ANOVA because our rankings are ordinal variables. We used Bonferroni corrected P values in comparison between the macroinvertebrates species groups.
In the figures, we used classes of 10% occupancy and the number of species in each 10% class, as suggested by McGeoch and Gaston (2002), to demonstrate the variation in SOFD between eight macroinvertebrate orders and combined data from all macroinvertebrate orders in eutrophic lakes.
We used the recently developed methods to identify different SOFD patterns (Jenkins 2011; Hui 2012) where the multi-model inference approach was applied to regressions of ranked species occupancy curves (RSOCs). For statistical test, we constructed nine binary (presence/absence) species-by-site matrixes (one combined all species data and eight macroinvertebrate orders). In each matrix, species are in rows and 14 eutrophic lakes in columns. Then, we calculated the occupancy frequency of each species as the sum of all the studied lakes where it was found. Each macroinvertebrate species’ occupancy frequency was divided by the total number of studied lakes (n = 14), and then, these relative occupancy values (Oi) were sorted in decreasing order. Each macroinvertebrate species had its own rank value, Ri, which was inversely correlated with the relative occupancy value. For each dataset, we then performed five regression analyses in which the relative occupancy of a species (Oi) is the dependent variable and Ri is the independent variable. Finally, we evaluated the five most common SOFD patterns: (1) a unimodal-satellite dominant, (2) a bimodal symmetrical, (3) a bimodal asymmetrical, (4) bimodal truncated or (5) a uniform (random) pattern best fitted our macroinvertebrate assemblage in eutrophic lakes in Finland (cf., Jenkins 2011; Hui 2012). As in Jenkins (2011) and Hui (2012), the Levenberg–Marquardt algorithm (with 999 iterations) was used for the nonlinear regressions, estimating the parameters (y0, a, b and c) of the following four equations (by means of ordinary least squares (OLS)) to find the best fitting SOFD pattern. The equations are:
-
(1)
Oi = y0 + a*exp(− bRi) with initial parameters y0 = 0.01, a = 1.0, b = 0.01: unimodal-satellite mode (exponential concave) pattern.
-
(2)
Oi = a/(1 + exp(− bRi + c) with initial parameters a = 1.0, b = − 0.1, c = − 1.0: bimodal symmetrical (sigmoidal symmetric) pattern.
-
(3)
Oi = a[1 − exp(− bRic)] with initial parameters a = 1.0, b = − 1.0, c = − 1.0: bimodal asymmetric (sigmoidal asymmetric) pattern.
-
(4)
Oi = aRibexp(− cRi) with initial parameters, a = 100, b = 0.05, c = − 0.04: bimodal truncated (power exponential) pattern.
-
(5)
Oi = aRi + b with initial parameters a = 0.01, b = 0.01: uniform (random) pattern.
We also examined the homogeneity of variance, normality of residuals and independent error terms, as well as the tails and shoulders from graphs of regression.
We used the Akaike information criterion for small sample sizes (AICc) to compare the five alternative models where the one with the smallest AICc value would fit the best with data. Alternative models with ΔAICc (= AICcmin − AICci) < 7 would be considered as equally supported (Anderson et al. 2000; Burnham and Anderson 2000; Burnham et al. 2011; Jenkins 2011). We used the IBM SPSS statistical package version 26 for all statistical calculations.
Results
Dispersal ability of macroinvertebrate orders
The experts (n = 10) agreed with each other in dispersal ability between eight macroinvertebrate orders (Kendall’s W = 0.51, χ2 = 35.87, df = 7, P < 0.001). There were differences between dispersal abilities between macroinvertebrate orders (related-samples Friedman’s two-way ANOVA, χ2 = 35.87, df = 7, P < 0.001; Table 2). The experts evaluated that dragonflies and damselflies had the highest and watermites the lowest dispersal ability (Bonferroni corrected P < 0.001). Odonates also have a higher dispersal ability than mayflies (P < 0.001) and chironomids (P = 0.020). All other macroinvertebrate species groups did not differ from each other (P > 0.073 in every case) (Table 1).
Species richness
We found 293 macroinvertebrate species or higher systematic in the 14 eutrophic lakes (Table 1). Chironomids and aquatic beetles were the most species rich macroinvertebrate orders (Table 1). Both taxonomic groups had more than 60 species. All other macroinvertebrate groups had less than 40 species (Table 1). The lowest dispersal ability insect orders covered only small proportion of regional species pool: caddisflies (14%) and chironomids flies (27%) (Table 2). A high fraction of macroinvertebrate orders, which had high dispersal ability, was observed from national species pool (Table 2). We found that almost half of the waterbug species observed in this study have been also recorded in Finland (24 out of 50 species). Furthermore, two-thirds of aquatic beetles observed in Finland were found in this study. Moreover, a high proportion of odonates and mayflies was found in this study from a national species pool (Table 2).
Species occupancy frequencies
The macroinvertebrate species occurrence varied from 1 to 14 lakes. Most species (30% or 89 out of 293 of species) were found only in one lake (Fig. 1). On average, each species occurred in less than one-third (mean = 4.4, SD = 3.7, n = 293) of the studied eutrophic lakes. However, each dragonfly and damselfly species occurred in almost half (mean 6.8, SD = 4.2, n = 18) of the studied lakes. For all other macroinvertebrate groups, one species occurred in about one-third of the studied lakes: waterbugs (Heteroptera, 4.9, SD = 3.5, n = 24), chironomids (Chironomidae, 4.8 SD = 3.9, n = 91), other aquatic dipterans (Diptera excluded chironomids, 3.5, SD = 3.5), watermites (Hydracarina) (4.3, SD = 3.3), mayflies (Ephemeroptera, 4.2, SD = 4.2, n = 14), caddisflies (Trichoptera, 3.9, SD = 4.2, n = 25) and water beetles (Coleoptera 3.4, SD = 3.1, n = 61).
SOFD patterns
The combined macroinvertebrate species data fitted best with the bimodal truncated SOFD pattern (Table 3; Fig. 1). All alternative SOFD pattern models did not conform to combined data (ΔAICc > 7; Table 3). Most of the studied macroinvertebrate orders had the unimodal-satellite or bimodal truncated SOFD pattern (Table 3; Fig. 2). According to ΔAICc values, both SOFD models fitted most of the macroinvertebrate orders very well (Table 3). The SOFD pattern varied considerably between the studied macroinvertebrate orders as expected by their differences in the dispersal abilities (Table 3; Fig. 2). The lowest dispersal ability macroinvertebrate order fitted with bimodal truncated distribution such as watermites (Fig. 2a) and mayflies (2b). On the other hand, dragonflies and damselflies, which had the highest dispersal ability, had a bimodal symmetrical SOFD pattern (Table 2; Fig. 2c). Also, in water bugs both bimodal symmetrical and bimodal asymmetrical SOFD patterns fitted equally well (Table 3, Fig. 2d).
Number of species in each eight macroinvertebrate groups in relation to the proportion of occupied eutrophic lakes (%) (n = 14 water bodies) in southern Finland. Studied macroinvertebrate groups are: a Hydracarina (36 species), b Ephemeroptera (14), c Odonata (18), d Heteroptera (24), e Trichoptera (25), f Diptera, excluded chironomids (24), g Chironomidae (91) and h Coleoptera (61)
Discussion
We found that aquatic insect orders varied in their dispersal ability. The lowest dispersal ability was in wingless watermites. The highest dispersal ability was in dragonflies and damselflies as in Sarremejane et al.’s (2020) study. Also, other aquatic insect orders were ranked similarly in the Sarremejane et al. (2020) study as our one. For example, in our study the lowest dispersal ability in aquatic insect order was found for mayflies (Ephemeroptera), Chironomidae and other aquatic flies (Diptera), as in Sarremejane et al. study (2020).
Our results emphasize the high macroinvertebrate diversity of eutrophic lakes in southern Finland. We found a total of 293 macroinvertebrate species in the 14 studied lakes. In our study, we observed about half of macroinvertebrate species recorded from regional species pool. The only exception was caddisfly (14%) and chironomids fly species (27%), which was observed less in our study than regional species pool. Moreover, a high fraction of several macroinvertebrate orders was observed from national species pool (Table 1). For example, we found about 35% of the 52 odonate species recorded from Finland (Karjalainen 2002). This means that our results contrast with the results of many previous studies where oligotrophic and dystrophic lakes usually harbour a lower number of odonate species in Finland (Korkeamäki 2003; Honkanen et al. 2011; Korkeamäki et al. 2018) and other studies in northern Europe (e.g. Oertli et al. 2002; Flenner and Sahlén 2008; Koch et al. 2014). In the current study, we observed 14 mayfly species which was about 25% from all species (55) recorded in Finland (Savolainen 2009) (Table 1).
We found both unimodal-satellite-dominant and bimodal symmetrical SOFD patterns in different macroinvertebrate orders in eutrophic lakes. Sampling artefacts may partly affect our observed SOFD patterns (see discussion in McGeoch and Gaston 2002). The patterns are affected by the accuracy of the sampling (McGeoch and Gaston 2002; Heatherly et al. 2007), but our data were gathered using two different methods known to detect the majority of the macroinvertebrate species present at an aquatic vegetation zone of lakes. Our sample size (the number of water bodies) was large enough, 14 lakes, and larger than the minimum, ten sites (McGeoch and Gaston 2002; Hui 2012). Hui (2012) also found that the number of species affects SOFD patterns. If the number of species was less than ten, then bimodal asymmetric pattern occurred more often. In our study, the number of species varied from 14 up to 293 (Table 1) So, it is unlikely that that all observed SOFD patterns are due to sampling artefacts or low number of study sites nor low number of species.
We found unimodal-satellite SOFD pattern in our combined macroinvertebrate data from 14 eutrophic lakes (Table 3; Fig. 1). This result is also in accordance with previously published results for aquatic habitats (e.g. Verberk et al. 2010; Heino 2015; Korkeamäki et al. 2018; Renner et al. 2020). The general theory of species community structure, suggested by e.g. Brown (1984) and discussed by Lennon et al. (2004), coincides well with the pattern observed by us: our studied lakes harbour merely a small number of common species and numerous rare ones. According to the NBM, common species with wider niches and a high tolerance of environmental variation are widely distributed and locally abundant. On the other hand, rare species with narrow niches and a greater sensitivity to environmental variation occur more locally and have a limited regional distribution (Brown 1984). This SOFD pattern may be dependent on both biotic and abiotic factors, as suggested by McGeoch and Gaston (2002) and Jenkins (2011). This will result in a unimodal-satellite SOFD pattern with many satellite species, in particular when data from numerous taxonomically dissimilar species with different niches requirement pooled together from communities (see Heino 2015; Jokimäki et al. 2016; Korkeamäki et al. 2018; Renner et al. 2020). According to our data, few common species occupied a larger proportion of the lakes and numerous rare species occupied only small proportion of lakes, which supports NBM.
According to Hui (2012), two most frequent models were unimodal-satellite-dominant and the bimodal truncated SOFD patterns, particularly in insects and arthropods. We found very similar frequencies in this study. It seems that in most of macroinvertebrates orders unimodal-satellite-dominant and the bimodal truncated SOFD patterns are the most common patterns (Table 3; Fig. 2). In their reviews, both McGeoch and Gaston (2002) and Hui (2012) estimated that one-fourth of SOFD patterns were bimodal symmetric. Our data support this frequency because we observed two out of nine models were bimodal symmetric.
We found that the SOFD patterns varied between macroinvertebrate orders (Table 3; Fig. 2). A dispersal ability seems to affect SOFD pattern as predicted by organismal scaling hypothesis. This hypothesis predicts a unimodal SOFD pattern dominated by satellite species with poor dispersal ability, such as wingless water mites and mayflies in our study (Collins and Glenn 1997). Macroinvertebrate orders with very good dispersal ability have bimodal symmetric SOFD pattern, as we found in dragonflies and damselflies in our study and in previous studies (Andersen et al. 2016; Suhling et al. 2017; Suhonen et al. 2010b). According to the MPDM (Hanski 1982, 1999), bimodality should result from random colonization and extinction events among the species in the local communities. The rare species with low dispersal ability occupied only part of suitable eutrophic lakes. Moreover, rare species seems to have smaller population size which increases local extinction risk (Korkeamäki and Suhonen 2002; Suhonen et al. 2010a, 2014).
The common species might also be widely distributed due to a low local extinction risk and a high colonization probability due to their high dispersal ability (Hanski and Gyllenberg 1997; Hanski 1998). This may partly explain bimodal symmetric SOFD pattern in odonates and water bugs in our study.
Conclusion
To conclude, our results demonstrate a general SOFD pattern of numerous rare and few common macroinvertebrate species, as in several previous studies in aquatic environments (Tokeshi 1992; Heino 2008, 2015; Verberk et al. 2010; Jenkins 2011; Korkeamäki et al. 2018; Renner et al. 2020). However, high dispersal ability of macroinvertebrate orders fitted relatively well with bimodal symmetric SODF pattern as predicted by organismal scaling hypothesis (Collins and Glenn 1997). The differences between our and most of the previous studies were that there was pooled species with different dispersal ability species groups together. Pooling different kinds of species together may shift the results towards unimodal-satellite-dominant SOFD pattern similarly as increasing extent of study area (e.g. McGeoch and Gaston 2002). To achieve a better understanding of the variation in SOFD for an aquatic macroinvertebrate assemblage, more field studies from several macroinvertebrate orders simultaneously collected from several waterbodies within same ecoregion are required. Different macroinvertebrate orders should be analysed separately to understand whether dispersal ability is modified SOFD patterns. Our results provide support for both the NBM (Brown 1984) and the MPDM (Hanski 1982) due to a high variation between macroinvertebrate orders in their dispersal ability (Sarremejane et al. 2020) and predicted by organismal scaling hypothesis (Collins and Glenn 1997). Moreover, we also need studies from different locations to understand geographical variation in SOFD patterns as was found in odonate assemblages in northern Europe (Korkeamäki et al. 2018). Our results highlight that the dispersal ability is important trait for species conservation in a scarce, but patchily distributed habitat.
References
Andersen E, Nilsson B, Sahlén G (2016) Survival possibilities of the dragonfly Aeshna viridis (Insecta, Odonata) in southern Sweden predicted from dispersal possibilities. J Insect Conserv 20:179–188. https://doi.org/10.1007/s10841-016-9850-5
Anderson D, Burnham K, Thompson W (2000) Null hypothesis testing: Problems, prevalence, and an alternative. J Wildl Manag 64:912–923. https://doi.org/10.2307/3803199
Autio O, Salmela J, Suhonen J (2013) Species richness and rarity of crane flies (Diptera, Tipuloidea) in a boreal mire. J Insect Conserv 17:1125–1136
Bagge P (1999) Water mites of small boreal forest lakes (Central Finland). In: Bruin J, van der Geest LPS, Sabelis MW (eds) Ecology and evolution of the acari. Kluwer Academic Publishers, Amsterdam, pp 483–489
Bagge AM, Bagge P (2009) Finnish water mites (Acari: Hydrachnidia, Halacaroidea), the list and distribution. Mem Soc Fauna Flora Fennica 85:69–78
Brown J (1984) On the relationship between abundance and distribution of species. Am Nat 124:255–279
Burnham P, Anderson DR (2000) Model selection and multimodel inference. A practical information-theoretic approach. Springer, New York
Burnham KP, Anderson DR, Huyvaert KP (2011) AIC model selection and multimodel inference in behavioural ecology: some background, observation, and comparisons. Behav Ecol Sociobiol 65:23–35
Collins S, Glenn S (1997) Effects of organismal and distance scaling on analysis of species distribution and abundance. Ecol Appl 7:543–551. https://doi.org/10.1890/1051-0761(1997)007[0543:EOOADS]2.0.CO;2
Flenner I, Sahlén G (2008) Dragonfly community re-organisation in boreal forest lakes: rapid species turnover driven by climate change? Insect Conserv Div 1:169–179. https://doi.org/10.1111/j.1752-4598.2008.00020.x
Hanski I (1982) Dynamics of regional distribution—the core and satellite species hypothesis. Oikos 38:210–221
Hanski I (1998) Metapopulation dynamics. Nature 396:41–49
Hanski I (1999) Metapopulation ecology. Oxford University Press, Oxford
Hanski I, Gyllenberg M (1997) Uniting two general patterns in the distribution of species. Science 275:397–400. https://doi.org/10.1126/science.275.5298.397
Heatherly T, Whiles MR, Gibson DJ, Collins SL, Huryn AD, Jackson JK, Palmer MA (2007) Stream insect occupancy-frequency patterns and metapopulation structure. Oecologia 151:313–321. https://doi.org/10.1007/s00442-006-0596-8
Heino J (2008) Temporally stable abundance–occupancy relationships and occupancy frequency patterns in stream insects. Oecologia 157:337–347
Heino J (2015) Deconstructing occupancy frequency distributions in stream insects: effects of body size and niche characteristics in different geographical regions. Ecol Entomol 40:491–499. https://doi.org/10.1111/een.12214
Honkanen M, Sorjanen A, Mönkkönen M (2011) Deconstructing responses of dragonfly species richness to area, nutrients, water plant diversity and forestry. Oecologia 166:457–467. https://doi.org/10.1007/s00442-010-1846-3
Hui C (2012) Scale effect and bimodality in the frequency distribution of species occupancy. Comm Ecol 13:30–35
Jenkins DG (2011) Ranked species occupancy curves reveal common patterns among diverse metacommunities. Global Ecol Biogeogr 20:486–497. https://doi.org/10.1111/j.1466-8238.2010.00617.x
Jokimäki J, Suhonen J, Kaisanlahti-Jokimäki M (2016) Urbanization and species occupancy frequency distribution pattern in core zone areas of European towns. Eur J Ecol 2:23–43. https://doi.org/10.1515/eje-2016-0014
Karjalainen S (2002) Suomen sudenkorennot (Odonata). Kustannusosakeyhtiö Tammi. Porvoo.
Koch K, Wagner C, Sahlén G (2014) Farmland versus forest: comparing changes in Odonata species composition in western and eastern Sweden. Insect Conserv Div 7:22–31. https://doi.org/10.1111/icad.12034
Korkeamäki E (2003) Isoukonkorennon (Aeshna crenata) elinympäristöt Salpausselällä. Kymijoen Vesi Ja Ympäristö Ry:n Tutkimusraportti 217:1–11
Korkeamäki E, Suhonen J (2002) Distribution and habitat specialization of species affect local extinction in dragonfly Odonata populations. Ecography 25:459–465. https://doi.org/10.1034/j.1600-0587.2002.250408.x
Korkeamäki E, Elo M, Sahlén G, Salmela J, Suhonen J (2018) Regional variations in occupancy frequency distribution patterns between odonate assemblages in Fennoscandia. Ecosphere 9:e02192. https://doi.org/10.1002/ecs2.2192
Lennon J, Koleff P, Greenwood J, Gaston K (2004) Contribution of rarity and commonness to patterns of species richness. Ecol Lett 7:81–87. https://doi.org/10.1046/j.1461-0248.2004.00548.x
McGeoch M, Gaston K (2002) Occupancy frequency distributions: patterns, artefacts and mechanisms. Biol Rev 77:311–331. https://doi.org/10.1017/S1464793101005887
Oertli B, Auderset Joye D, Castella E, Juge R, Cambin D, Lachavanne J (2002) Does size matter? The relationship between pond area and biodiversity. Biol Conserv 104:59–70. https://doi.org/10.1016/S0006-3207(01)00154-9
Oertli B, Indermuehle N, Angelibert S, Hinden H, Stoll A (2008) Macroinvertebrate assemblages in 25 high alpine ponds of the Swiss national park (Cirque of Macun) and relation to environmental variables. Hydrobiologia 597:29–41. https://doi.org/10.1007/s10750-007-9218-7
Rassi P (ed.) (1993) Frequency score of Coleoptera in Finland 1.1.1960–1.1.1990. Viestipaino, Helsinki
Renner S, Dalzochio MS, Périco E, Sahlén G, Suhonen J (2020) Odonate species occupancy frequency distribution and abundance—occupancy relationship patterns in temporal and permanent water bodies in a subtropical area. Ecol Evol. https://doi.org/10.1002/ece3.6478
Rintala T, Rinne V (2010) Suomen luteet. Hyönteistarvike Tibiale Oy, Helsinki.
Salokannel J, Mattila K (2018) Suomen vesiperhoset. Trichoptera of Finland. Tibiale Oy, Helsinki
Sarremejane R, Cid N, Stubbington R, Datry T, Alp M, Canedo-Arguelles M, Cordero-Rivera A, Csabai Z, Gutierrez-Canovas C, Heino J, Forcellini M, Millan A, Paillex A, Paril P, Polasek M, Tierno de Figueroa JM, Usseglio-Polatera P, Zamora-Munoz C, Bonada N (2020) DISPERSE, a trait database to assess the dispersal potential of European aquatic macroinvertebrates. Sci Data 7:386. https://doi.org/10.1038/s41597-020-00732-7
Savolainen E (2009) Distribution of mayflies (Ephemeroptera) in Finland. Kulumus 15:1–35
Suhling F, Martens A, Suhling I (2017) Long-distance dispersal in Odonata: examples from arid Namibia. Austral Ecol 42:544–552. https://doi.org/10.1111/aec.12472
Suhonen J, Hilli-Lukkarinen M, Korkeamäki E, Kuitunen M, Kullas J, Penttinen J, Salmela J (2010a) Local extinction of dragonfly and damselfly populations in low- and high-quality habitat patches. Conserv Biol 24:1148–1153. https://doi.org/10.1111/j.1523-1739.2010.01504.x
Suhonen J, Honkavaara J, Rantala MJ (2010b) Activation of the immune system promotes insect dispersal in the wild. Oecologia 162:541–547
Suhonen J, Korkeamäki E, Salmela J, Kuitunen M (2014) Risk of local extinction of Odonata freshwater habitat generalists and specialists. Conserv Biol 28:783–789. https://doi.org/10.1111/cobi.12231
Suutari E, Salmela J, Paasivirta L, Rantala MJ, Tynkkynen K, Suhonen LM, J, (2009) Macroarthropod species richness and conservation priorities in Stratiotes aloides (L.) lakes. J Insect Conserv 13:413–419
Tokeshi M (1992) Dynamics of distribution in animal communities: theory and analysis. Res Popul Ecol 34:249–273
Verberk WCEP, van der Velde G, Esselink H (2010) Explaining abundance-occupancy relationships in specialists and generalists: a case study on aquatic macroinvertebrates in standing waters. J Anim Ecol 79:589–601. https://doi.org/10.1111/j.1365-2656.2010.01660.x
Wiberg-Larsen P, Brodersen K, Birkholm S, Gron P, Skriver J (2000) Species richness and assemblage structure of Trichoptera in Danish streams. Freshwat Biol 43:633–647. https://doi.org/10.1046/j.1365-2427.2000.00546.x
Acknowledgements
We thank the associate editor Maria J Feio, Sylvia Suhonen and unknown referees for suitable comments and corrections to previous versions of the manuscript. We thank the Finnish Environment Institute and Regional Environment Centres of Häme and Pirkanmaa for the study permissions. We are grateful to the Academy of Finland (to J. Suhonen and M. Rantala), the Finnish Cultural Foundation (Häme) (to J. Suhonen), Jenny and Antti Wihuri Fund (to E. Suutari) and the Finnish Entomological Society (to J. Salmela) for financing our study. We are also thankful to M. Luojumäki and K. Tynkkynen to collect macroinvertebrate individuals from field samples. Jari Haimi, Jari Ilmonen, Jaakko Ilvonen, Esa Korkeamäki, Markku Kuitunen and Linda Laakso helped us to evaluate macroinvertebrate species groups dispersal ability.
Funding
Open access funding provided by University of Turku (UTU) including Turku University Central Hospital.
Author information
Authors and Affiliations
Contributions
MJR, JSa and JSu collected the field data. JSu developed both the study concept and analysed the data. LP identified Diptera and watermite samples, and JSa identified all other insect order specimens. ES entered and handled the raw data. All authors wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Additional information
Handling Editor: Maria J. Feio.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Suhonen, J., Paasivirta, L., Rantala, M.J. et al. Macroinvertebrate species occupancy frequency distribution patterns in eutrophic lakes. Aquat Ecol 56, 201–212 (2022). https://doi.org/10.1007/s10452-021-09909-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-021-09909-7