Abstract
Stormwater retention ponds receive a variety of urban and highway pollutants that may have adverse effects on water-dwelling organisms. In this exploratory study, the benthic diatom community composition at genus level of nine such ponds servicing highway, residential, industrial, and mixed industrial/residential catchments was examined. Thirteen biocides were measured in the pond water as one of the possible explanatory factors for diatom taxonomic variability. The uppermost 1 cm of sediment was sampled, and a total of 50 diatom genera were identified. Moderate to high similarities were found among the diatom communities of the ponds. Two genera, namely Navicula and Nitzschia, were the most abundant and accounted for 19–47% of the relative abundance in the ponds. Estimated relative abundances of diatom genera and measured biocide concentrations in the ponds were grouped according to land use. Highway ponds were found to be significantly different from ponds servicing residential and industrial catchments, while no significant differences were found between residential and industrial ponds. The presence of biocides alone could not explain diatom taxonomic variability, although some evidence was found that communities differed depending on the catchment type of the ponds. The results of this exploratory study are an important contribution to future works investigating stormwater diatom communities, where combined effects of biocides and other stormwater contaminants and community stressors, e.g., metals, PAHs, road salt, should be explicitly looked at.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Increasing urbanization and human activities create pollution pressure on the natural environment. Thus, a number of regulations, directives, and guidelines have been established to examine and maintain a good ecological status of various terrestrial and aquatic ecosystems (WFD 2000; DEQS 2008; GCERA 2008). Surface water bodies are habitats for a diversity of fauna and flora. Therefore, they receive particular attention with respect to contamination. Investigations of rivers, streams, and lakes over the years have resulted in a number of data sets, providing an insight into water chemistry parameters and their relationship with the living organism community structure (Benetti et al. 2012; Martín and Fernandez 2012; Pereira et al. 2012).
In urban areas, artificial reservoirs, basins, and wetlands are commonly constructed to serve as stormwater detention and treatment facilities, or as a recreational element of an urban environment. One common type is the stormwater retention pond constructed to detain stormwater and retain its pollutants (Hvitved-Jacobsen et al. 2010). Surface runoff contains a substantial amount of particulate and dissolved pollutants, originating from different sources such as vehicles, pavements, installations, and buildings (Bannerman et al. 1993; McLeod et al. 2006). One such pollutant group that has received increasing attention—and which has been found in significant concentrations in urban stormwater runoff—is biocides from building materials (Bollmann et al. 2014).
Biocides are added to a range of building materials to prevent algal and fungal growth, which otherwise would cause discoloration or even degradation of surfaces (Paulus 2004). During rain events, these biocides are released from the materials and transported in the stormwater runoff to ponds or recipients (Burkhardt et al. 2007). For instance, Bollmann et al. (2014) document biocide concentrations in stormwater runoff at a high temporal and spatial resolution and found a wide range of these substances. Highest median concentrations were reported for carbendazim and terbutryn, namely 45 and 52 ng L−1, respectively. During peak events, they occurred in concentrations as high as 306 and 1840 ng L−1, respectively. Other biocides occurred in concentrations between 1 and 10 ng L−1, but with occasional peaks of approx. 100 ng L−1. Generally, the concentration of pollutants in the incoming water varies orders of magnitude between events, with the degree of variation depending on the pollutant type (Leisenring et al. 2014).
The degree of contamination in stormwater ponds also depends on the fate of pollutants. Suspended solids and pollutants associated with particles are partially removed from the water column by sedimentation (Stanley 1996; Karlsson et al. 2010b). Received loads of nutrients can be, for instance, reduced by pond plant and algae uptake (Hurley and Forman 2011; Chang et al. 2012; Gold et al. 2019). Some pollutants, such as dissolved organic matter (McEnroe et al. 2013a), certain herbicides (Bois et al. 2013), and pharmaceuticals (Liu et al. 2019), are also to some extent susceptible to biological and chemical degradation. Numerous studies report significant pollutant removal, for instance of 80% for metals, 70% for phosphorus, and 30% for nitrogen, depending on the period of the year (Starzec et al. 2005). Thus, stormwater ponds are widely recognized as pollutant retention and accumulation basins (Karlsson et al. 2010a; Weinstein et al. 2010).
Being an integral part of the urban environment, stormwater retention ponds in time also turn into habitats for a variety of aquatic flora and fauna (Scher and Thiery 2005; Le Viol et al. 2009; Sun et al. 2019). However, factors like high loads of anthropogenic pollutants, high nutrient loads, short hydraulic residence time, and high water column mixing rates make these habitats quite different from natural lakes (Hvitved-Jacobsen et al. 2010). Only a few studies have addressed how these differences affect ponds in terms of habitats for aquatic flora and fauna. Among these, Stephansen et al. (2016) studied and compared stormwater ponds and small shallow lakes in Denmark with respect to their invertebrate diversity and bioaccumulation of heavy metals. The authors found similar invertebrate populations in both types of water bodies despite a higher heavy metal bioaccumulation in pond invertebrates. In a study by Hassal and Anderson (2015), stormwater pond macroinvertebrate communities were compared to those of natural wetlands. Although the water quality parameters of the two types of water bodies were quite different, the macroinvertebrate biodiversity was found to be similar in the richest ponds and the natural water bodies. Le Viol et al. (2009) studied macroinvertebrate biodiversity in highway ponds and compared it with that of the surrounding natural ponds. The authors reported similar macroinvertebrate communities in all investigated ponds, despite the measured differences in their abiotic conditions. These and other studies indicate that ponds constructed for stormwater management have the potential to positively contribute to local and regional biodiversity and not only be pollutant retention facilities.
The few studies that have been published on this topic mainly cover invertebrates (e.g., Le Viol et al. 2009; Stephansen et al. 2016), vertebrates (e.g., Ackley and Meylan 2010; Brand et al. 2010), and macrophytes (e.g., Istenič et al. 2012). However, aquatic ecosystems are complex, with a range of different biotic and abiotic parameters governing their behavior. Analysis of a plant and animal community composition of a water body can provide valuable knowledge about ecosystem health or indicate changes in environmental conditions (Parmar et al. 2016). For instance, increased nutrient concentrations are well reflected by shifts in phytoplankton or macroinvertebrate population taxonomic composition (Smith et al. 2007; Parmar et al. 2016). Heavy metal contamination can be indicated by certain macrophyte species (Ladislas et al. 2012; Phillips et al. 2015). While invertebrates, vertebrates, and macrophytes of stormwater ponds have been studied in some detail, algae have received rather limited attention, even though they are of a particular importance to the aquatic ecosystems as they form the basis of the aquatic food webs (John et al. 2011). Especially, those benthic algae associated with bottom sediments, such as diatoms, can be exposed to elevated levels of pollutants and have to either adapt to the environment or be eliminated (Gallagher et al. 2011).
Diatoms are well known for their fast response to changing environmental conditions, which is usually well reflected by changes in diatom species composition (Kelly et al. 1998; Martín and Fernandez 2012). They are good indicators of different water quality parameters, such as nutrient levels, acidity, and turbidity and are widely used for the assessment of the ecological status of water bodies, particularly for routine monitoring of streams and rivers (Kelly et al. 1998; Martín and Fernandez 2012). A number of authors investigating wetland and lake benthic diatom communities also report a close correspondence of certain taxa presence and distribution in the sediment to the measured elevated levels of water column nutrients and salinity (Gell et al. 2002; Zalat and Vildary 2005; Della Bella et al. 2007). A variety of diatom taxa found in the upper centimeter of the sediments of the ponds might hence be related to a range of experienced environmental conditions of the near past.
As described above, effects of physicochemical parameters on benthic diatom assemblages in freshwater bodies are widely studied and well defined in a number of scientific works and reports. With respect to the growing importance of biocide contamination, to our knowledge, there is a lack of studies focusing on benthic diatom community sensitivity to these contaminants in lakes and stormwater retention ponds. On the other hand, a number of laboratory assessments of pesticide toxicity to diatoms on a single species level or to laboratory-grown biofilm diatoms have been reported. For example, Moisset et al. (2015) showed that the biocide diuron at concentrations of 1 and 10 µg L−1 impacted the photosynthetic activity and altered the growth pattern of Eolimna minima, Nitzschia palea, and Planothidium lanceolatum. Proia et al. (2011) found increased biofilm diatom mortality and inhibited photosynthesis by diuron at concentrations up to 15 µg L−1. Debenest et al. (2009) studied periphyton exposure to herbicides isoproturon and s-metolachlor in controlled microcosm experiments and reported biomass growth inhibition at herbicide concentrations of 5 and 30 µg L−1. Regarding taxonomic identification, the authors also found that several diatom species, such as Melosira varians, Nitzschia dissipata, and Cocconeis placentula, were tolerant to the tested herbicide concentrations.
In the present exploratory study, we investigate the taxonomic composition of benthic diatom communities at genus level in sediments from stormwater retention ponds from catchments of different land use. We chose to focus on one of the emerging contaminants, namely, biocides from building materials, some of which are algaecides and hence very likely could influence the survival or otherwise cause changes in diatom communities. We aimed to investigate whether the presence of these contaminants could explain the examined diatom taxonomic variability. With this work, we intended to establish a solid basis for future studies to investigate stormwater diatom communities on a larger scale.
Materials and methods
Sampling sites, sediment extraction, and biocide measurements
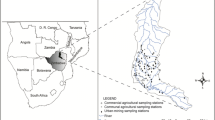
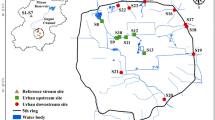
For benthic diatom analysis, nine stormwater retention ponds were sampled in August 2014 in Northern and Central Jutland, Denmark (Fig. 1). Samples were collected when heavy rain disturbance was not present and the water column was back to non-turbulent conditions. In order to cover a range of environmental conditions, ponds were selected in catchments encompassing four different types of land use—highway, residential, industrial, and mixed residential/industrial. Highway ponds are located further away from the built environment and have lower impervious cover in their catchment areas. The separation between residential and industrial areas is based on that the former mainly comprise single-family houses, while the latter contain impervious surfaces of more variable use and buildings of different economic activities. We speculated that highway ponds receive lower concentrations and variety of biocides compared to the industrial and residential ones as there are no obvious biocide-source in these catchments. Pond characteristics are given in Table 1, and the geographical distances among the ponds are provided in the supplementary material Table S1. Sediments were sampled from five locations distributed along the shore line of each pond, at a water depth of approx. 70 cm. This was done in order to evaluate diatom taxa presence and distribution throughout the pond and whether there were large differences among the identified communities depending on the sampled location in the pond. The stormwater pond water column is generally considered to be rather well mixed, although its homogeneity has been questioned in some works (McEnroe et al. 2013b). Sampling sites are shown in Fig. 1a, and pond sampling locations are exemplified for pond R2 in Fig. 1b.
Sediment samples were collected using a corer consisting of a 5 cm inner diameter plastic cylinder attached to a coring stick. The uppermost 1 cm of sediment was taken from each sample, preserved in ethanol (99.5% VWR Chemicals, 20% final concentration) and stored in cool and dark for further laboratory treatment (Taylor et al. 2007). The final analysis was done within a year of the sampling. Sediment accumulation rates in stormwater retention ponds are much higher than in natural lakes and depend on different pond and catchment characteristics, such as its size, percentage of impervious surface. Analysis of the uppermost 1 cm of sediment was chosen as an approximation of the more recent history of the stormwater pond diatom population (Schroer et al. 2018).
Water quality parameters, such as pH, dissolved oxygen, conductivity, temperature, nitrite and nitrate nitrogen, total phosphorus, and chlorophyll-a, were determined once based on grab sampling of the pond water, simultaneously with the sediment sampling. However, one-time measurements do not represent long-term water column conditions, as ponds are subject to frequent water exchange related with precipitation events and new loads of runoff pollutants and were hence only used for chemical characterization of the ponds and not further discussed nor correlated with the diatom data. All water quality measurements are provided in the supplementary material.
Water samples for biocide concentration measurements were collected three times as grab samples with 3–4 weeks intervals starting from mid-August. A total of 13 biocides were analyzed: terbutryn (TB), diuron (DR), irgarol (IRG), carbendazim (CD), tebuconazole (TBU), isoproturon (IP), propiconazole (PPZ), methylisothiazolinone (MI), benzylisothiazolinone (BIT), noctylisothiazolinone (OIT), mecoprop (MCPP), iodo-propynyl butylcarbamate (IPBC), dichloro-n-octylisothiazolinone (DCOIT). Measurements were carried out by means of HPLC–MS/MS, as described in Bollmann et al. (2014).
Diatom slide preparation and analysis
Each sediment sample was well mixed, and a subsample of 3 mL was extracted for further treatment according to the hot 30% H2O2 method (Kelly 2000; DARES 2004; Taylor et al. 2007). After treatment of at least 5 h, any H2O2 remaining was washed from the sample by centrifugation and resuspension of the pellet in demineralized water (Kelly 2000; Taylor et al. 2007). A 1.5 mL treated and washed subsample was distributed over a cover slip, previously soaked in nitric acid in order to avoid diatom valve clumping. Then the cover slip was dried and fixed with a mounting agent Naphrax (Brunel Microscopes Ltd) on a glass slide. Diatoms were identified to genus level using identification keys, either as books or online data bases (Kelly 2000; Spaulding et al. 2010). At least 300 valves per slide were counted as random fields of view using an upright microscope (Meiji Techno MT6520) with a 1000 × magnification objective and oil immersion (DARES 2004).
Statistical analysis
Measured biocide concentrations in the ponds were grouped according to the catchment type of the pond and analyzed using one-way ANOVA in SigmaPlot 12.3 (Snedecor and Cochran 1989; Systat Software 2016). The mixed industrial and residential land use was excluded from the ANOVA test as there was only one pond with this land use (Table 1). Where significant differences were found, pairwise post hoc comparisons between the groups were performed using either Tukey’s or Dunn’s methods, depending on whether the data were normal or not-normal distributed (Conover 1980; Snedecor and Cochran 1989). Pond diatom communities were compared among the three catchment types and tested for significant differences by an analysis of similarities (ANOSIM) test in Primer (Primer E-Ldt 2016). Similarities in species composition among the five sampling locations of each pond were analyzed using Bray–Curtis similarity indices (0 indicates no similarity and 1 indicates perfect similarity) in Past v 3.15 (Hammer et al. 2001). Possible grouping of the nine ponds was tested by Classical Bray–Curtis Cluster analysis and Detrended Correspondence Analysis (DCA) in Past v. 3.15 as well (Hammer et al. 2001; Legendre and Legendre 2012). Correlations among different pond parameters: pond surface area, mean depth, mean water residence time, as well as measured biocide concentrations, and taxonomic diatom data, were analyzed by Spearman’s rank correlations (Conover 1980).
Results
Biocide concentrations in the ponds
All measured water column biocide concentrations in the nine ponds are shown in Fig. 2. The numbers are also provided in the supplementary data Table S3.
Biocide concentrations (ng L−1) of the 9 investigated ponds, measured with 3–4 weeks intervals starting from mid-August, 2014. The figure shows all measured data, i.e., 3 values for each substance and pond. Substances below detection limits are not shown. MCPP stands for mecoprop, IP—isoproturon, DR—diuron, TB—terbutryn, TBU—tebuconazole, PPZ—propiconazole, CD—carbendazim, OIT—n-octylisothiazolinone, DCOIT—dichloro-n-octylisothiazolinone
Four herbicides—mecoprop (MCPP), isoproturon (IP), diuron (DR), and terbutryn (TB), four fungicides—tebuconazole (TBU), propiconazole (PPZ), carbendazim (CD), and n-octylisothiazolinone (OIT), and one broad spectrum antifouling agent—dichloro-n-octylisothiazolinone (DCOIT), were detected. In all ponds, methylisothiazolinone (MI), benzylisothiazolinone (BIT), iodo-propynyl butylcarbamate (IPBC), and irgarol (IRG) were below the detection limit. Concentrations varied from “not detected” to 113 ng L−1 depending on sampling time, compound, and pond.
Two biocides—the fungicide TBU and the broad spectrum biocidal agent DCOIT—were detected in all ponds throughout the sampling period. TBU concentrations ranged from 2 to 56 ng L−1, while DCOIT ranged from 3 to 40 ng L−1. Several of the compounds detected at the higher concentrations were herbicides: TB in ponds R1 (28–59 ng L−1) and M1 (36–38 ng L−1), MCPP in M1 (37–57 ng L−1), DR in M1 (58–113 ng L−1), and once in H1 (53 ng L−1), or fungicides: PPZ in pond I1 (38–44 ng L−1), CD in R1 (44–78 ng L−1), I3 (44–49 ng L−1), and M1 (37–47 ng L−1). The herbicide IP was detected only in pond R1 and at concentrations of 5 to 12 ng L−1. The concentrations of the antifungal biocide OIT, when detected, ranged from 3 to 12 ng L−1.
Very low or no concentrations of biocides were detected in highway ponds, except in one of the samples from pond H1 where there were 53 ng L−1 of DR and 34 ng L−1 of DCOIT, and one of the sample from H3 with 40 ng L−1 of DCOIT. The highest concentrations of most of the biocides were measured in pond M1, which is located in a mixed industrial and residential area.
Using one-way ANOVA analyses on biocide concentrations, no significant differences were detected between residential and industrial ponds (p > 0.05). On the other hand, highway ponds were significantly different both from residential and industrial ponds (p < 0.05), except for OIT and DCOIT, where no significant differences were detected among any of the 3 groups. Overall, measured biocide concentrations clearly separated highway ponds from residential and industrial ponds.
Diatom diversity and abundance in the ponds
The five sampled locations were analyzed separately for each pond and subsequently pooled for further analysis. A total of 50 diatom genera were identified in the nine ponds. An overview of the genera, sorted according to the frequency that they were found in each of the water bodies, is given in Table 2. The genera found in 3–5 locations of a pond were considered as common and are marked in grey, while the genera found in 0–2 locations of a pond were considered as absent or rare and are marked in white. The total number of genera identified in each pond is given as a range, e.g., 24–37. The first number indicates how many genera were found in all five sampling locations, while the last number tells how many genera were found in at least one of the locations in a pond.
Similar numbers of genera were found in all nine ponds, ranging from 25 identified at one or more of the five sampled locations of I1 to 40 identified in H2. Only pond I1 could be distinguished from the other ponds to some degree, as it had the lowest number of genera: 15–25. With respect to abundant and dominant genera (found in 4 or 5 locations of a pond), similar numbers were observed in all ponds: 23–27 in highway ponds, 23–24 in residential ponds, 15–23 in industrial ponds and 28 in the mixed industrial and residential pond. Eight genera, namely, Fragilaria, Gomphonema, Navicula, Nitzschia, Planothidium, Sellaphora, Surirella, and Tabularia, were frequently observed (3–5 sampled locations) in all nine ponds. The rest of the genera occurrence differed depending on a pond (Table 2), which is highly likely a consequence of habitat diversity, physical and chemical pond parameters, etc.
As the frequency at which a genus was observed within a pond only partly reflects its distribution, the relative abundance of each identified genus was estimated as well. Based on a study of Thayer et al. (1983), all genera were categorized as: absent/very rare (0–1%), rare (1–5%), few (5–10%), common (10–20%), and abundant (> 20%). In order to analyze the genera abundance within a pond, the data were plotted for the 5 sampled locations of each pond (supplementary material, Fig. S2). Only the common and abundant genera were selected in order to have a clearer overview of genera distribution among the locations.
In total 15 genera were found as common (10–20%) or abundant (> 20%) at least in one location of a pond. The list of those genera and their environmental preferences is described in supplementary material (Table S4). These genera accounted for 45–80% of the relative abundance in the ponds. Two genera, namely Navicula and Nitzschia, were found in high abundances in all locations of the nine ponds. Together they constituted 19–47% of the relative abundance of benthic diatoms in the ponds.
Analyzing common and abundant genera (at least in one location of a pond), a few differences emerged among the ponds (supplementary material, Fig. S2). For instance, several genera were found only in a certain pond: Diatoma in H2 (5–12%), Gyrosigma in H3 (4–12%), Asterionella in R1 (7–11%), Neidium, Pinnularia, and Synedra in I1 (1–11%, 6–15% and 6–15%, respectively), Planothidium and Surirella in I2 (5–11% and 4–14%, respectively). The five sampling locations of each pond were rather similar in terms of diatom composition, with an exception of Fragilaria which was noted as a dominant genus only at one of the five sampled locations of pond H1 (28.9% versus 1.7–4.4% in the other locations) (see discussion below).
Bray–Curtis similarity test performed for the different locations of a pond has shown that all locations of a pond were highly similar, with Bray–Curtis values ranging from 0.57 to 0.92. The lowest similarity was found for the one location in pond H1, which was mainly due to the somewhat higher abundance of Fragilaria.
Because of the high similarity of the sampling locations, the five locations of each pond were grouped, and the mean relative abundance of each genus was calculated for each pond. The genera were categorized according to their abundance, as described above. In order to have a clearer overview of genera dominance, only the genera commonly found (10–20%) or abundant (> 20%) at least in one of the 9 ponds were selected and plotted (Fig. 3). Only two genera, namely, Navicula and Nitzschia, were found common or abundant in all nine ponds: 12–22% and 11–20%, respectively. Several other genera found in high abundance (> 10%) were Amphora in R2 (12%), Cocconeis in all ponds (11–26%) except H1, R1 and I1, Synedra in I1 (12%), and Cyclotella in R1 and I3 (12% and 11%, respectively).
Statistical Bray–Curtis similarity was compared among the 9 ponds. It ranged from 0.44 to 0.87, indicating moderate to high diatom community similarities among the ponds. Most of the lowest similarities were found when comparing ponds R1 and I1 with highway ponds (0.46–0.55). Other moderate dissimilar ponds were R1 and R2 (0.5), R2 and I1 (0.46), and I1 and I2 (0.44). Bray–Curtis similarity indices were also plotted against the geographical distances between the ponds to test whether pond diatom communities had higher similarity for the ponds located closer to one another. No similarity–distance correlation was found (supplementary material, Fig. S1).
A pond comparison was also carried out by an ANOSIM test, where ponds were grouped according to their catchment type (highway, residential, industrial). Dissimilarities in benthic diatom communities among ponds were not large (ANOSIM, global R = 0.242). However, some significant differences were found. Highway ponds were found to be different from residential ponds (ANOSIM, R = 0.359, p = 0.002), as well as industrial ones (ANOSIM, R = 0.258, p = 0.001), while residential and industrial ponds were not significantly different from each other (p = 0.087).
The taxonomic diatom data and estimated genera relative abundances were submitted to a DCA analysis (Fig. 4) to test any possible grouping of ponds and whether it could be related to any of the measured chemical or physical pond parameters. No grouping with respect to catchment characteristics could be observed. The majority of identified genera and the dominant genera were found to be similar for all ponds. Several physical and chemical pond parameters, such as pond surface area, mean water depth, water residence time, and biocide concentration, were correlated with pond DCA axis 1 coordinates. Axis 1 was found to significantly (p < 0.05) correlate with the average concentrations of mecoprop (MCPP) (rs = 0.73), terbutryn (TB) (rs = 0.80), and propiconazole (PPZ) (rs = 0.77).
Discussion
Twenty-five (pond I1) to 40 (pond H2) diatom genera were identified in the nine ponds of this study. Numbers were similar in all ponds, except pond I1, which had the lowest number of genera (15–25). This pond is located in an industrial area, and it is probable that it receives a wider range of pollutants compared to residential or highway ponds. Another explanation could be that this pond has the lowest water residence time and the largest catchment area compared to the other ponds. This could result in a more frequent pollutant load and correspondingly higher exposure of diatoms hereto. As a consequence, only the most tolerant diatom communities would remain in the pond (Cattaneo et al. 2011).
Diatom composition at the five sampling locations of each pond was comparatively similar. Only in pond H1, Fragilaria stood out as a dominant genus at just one of the five sampled locations. Whether the presence of certain genera only in some locations of a pond could have been caused by location-specific parameters, such as light availability or the presence/absence of macrophytes, or was simply due to random variability, is not known. Some studies also report higher Fragilaria species abundance as a consequence of increased agricultural activities and other human caused disturbances (Watchorn et al. 2008; Della Bella and Marcini 2009). Nevertheless, it is rather difficult to apply such findings to the case of the present study, as Fragilaria genus was found as dominant only in one of the studied five pond locations.
Additionally, several of the common genera (10–20% at least in one location of a pond), namely, Diatoma, Gyrosigma, Asterionella, Neidium, Pinnularia, Synedra, Planothidium, and Surirella, were found only in one of the nine ponds. Despite that, the differences among dominant genera did not seem to depend on the land use type of the pond—highway, residential, industrial or mixed residential and industrial. Additionally, regardless of a few differences, the mentioned eight taxa are tolerant to either a range of water pH, salinity, trophy, saprobity or are motile, which makes them adaptive to various environmental conditions (supplementary material, Table S4).
Although diatom communities in the nine ponds were found to be composed of a variety of genera, only a few of them, specifically, Amphora, Cocconeis, Synedra, and Cyclotella, were found at a relative abundance of more than 10% in at least one of the nine ponds. Two genera—Navicula and Nitzschia—constituted the largest part of the diatom communities in all nine ponds. All six genera are alkaliphilous or tolerate a range of pH values, as well as prefer nutrient rich, eutrophic waters, which is often the case of stormwater ponds. Ponds of the present study fall into categories of eutrophic or hypertrophic waters (Bellinger and Sigee 2010), according to the measured total phosphorus and chlorophyll-a concentrations (supplementary material, Table S2). Navicula and Nitzschia are also motile genera, able to adapt to nutrient rich and turbulent environments (Rimet 2012), and some of Navicula species are also listed as pollution tolerant (Kelly 2000). According to Ludikova (2016), higher Cyclotella abundance might be related to higher phosphorus concentrations. The same author has investigated 53 urban ponds and lakes of St. Petersburg and found the genera Achnanthidium, Cocconeis, Cyclostephanos, Cyclotella, Gomphonema, Lemnicola, Navicula, Nitzschia, and Stephanodiscus as the most common.
Regarding the measured biocide concentrations in the nine ponds of the present study, they varied from “not detected” to 113 ng L−1 depending on sampling time, compound, and pond (catchment). None of the measurements exceeded the recommended annual average values of the European Water Framework Directive for priority substances in inland waters (WFD 2013). The directive suggests not to exceed concentrations of 64 ng L−1 for TB, 200 ng L−1 for DR, and 300 ng L−1 for IP. The concentrations measured in the present study are several orders of magnitude lower than some of those used in controlled investigations by other authors testing the effect of biocides on diatom communities. For instance, Rimet and Bouchez (2011) carried out a river mesocosm study where diuron and terbutryn were two of the three pesticides used. The total pesticide concentrations ranged from 1.11 to 3.01 µg L−1 for long-term exposure and from 20.25 to 29.50 µg L−1 for acute exposure. These levels caused diatom community shifts toward higher abundances of pollution tolerant taxa such as Navicula, Nitzschia, and Cocconeis, which exhibit motility or forming of mucous tubules. Despite the lower biocide concentrations in the stormwater retention ponds of the present study, the higher abundances of these diatom genera in the nine ponds of this study might have been influenced by their higher tolerance to contamination. Following Bester et al. (1995), on the other hand, effects of triazine herbicides could be determined down to 100 ng L−1 in a marine ecosystem. Another study performed by Marcel et al. (2013) analyzed a large monitoring data set of river diatoms and correlated their ecological guilds—groups of diatom taxa that prefer certain environmental conditions—with herbicide contamination. The authors found that “high profile” guild diatom abundance, “genera such as Diatoma, Eunotia, Fragilaria, Gomphoneis, Gomphonema and Ulnaria,” decreased significantly with an increase in herbicide atrazine concentration. They observed a similar tendency for herbicides diuron and isoproturon as well.
The taxonomic differences of the benthic diatom communities of the present study could not be connected to the measured concentrations of biocides alone, although the statistical analysis indicated that diatom community composition was to some degree influenced by the pond catchment characteristics. One-way ANOVA tests for the measured biocide concentrations showed a clear separation between highway ponds compared to residential and industrial ones, while no significant differences were found between residential and industrial ponds. The same result was obtained by an ANOSIM test, carried out to compare diatom communities of the three types of ponds. The DCA study also provided some evidence that diatom community composition in ponds might be related to biocide concentrations. However, the evidence was not strong enough to discard the possibility that biocide concentration could be merely a proxy of another structuring parameter or set of parameters.
This was probably due to differences in runoff volumes, and runoff patterns of pollutants and nutrients, as well as the potential colonization from adjacent ponds and lakes (Naselli-Flores et al. 2016). The majority of the ponds investigated in this study were separated by only a few kilometers (3–9 km) or tens of kilometers (32–57 km), except for two highway ponds (H1 and H2), which were located further away (81–123 km) from the rest of the sampling sites (see Table S1). Moreover, sediment type is an important factor influencing diatom species presence and distribution in a water body as well. Linnan et al. (2017) found for instance that fine sediments favor diatom abundance. Similarly, Babeesh et al. (2016) showed a close association of sediment texture and diatom distribution, with species such as Eunotia pectinales and Encyonema latum negatively correlated with sandy, but positively correlated with muddy, sediments. Although sediment properties were not analyzed in the present study, this aspect could be potentially interesting to consider in the future works as well.
Overall, the results of this study provide evidence that benthic diatom communities were somewhat affected by the catchment land use type of the ponds, especially when comparing highway ponds to residential and industrial ones. The overall diatom taxonomic composition depends not only on the effect of specific pollutants and their concentrations, but also on a combination of different factors such as species forming the initial diatom community, physical and chemical water parameters, morphology of the water body, biological links to other aquatic ecosystems, and etcetera. The rather weak correlation between biocides and ponds, and land use and ponds (as a proxy for pollutant type and concentration) indicates that those other factors play a major role for the overall diatom taxonomic composition of a stormwater retention pond. The results found in this study on the response of benthic diatom communities of stormwater ponds toward contaminants such as biocides indicate that further attention should be devoted to elucidating the combined effects of biocides and other contaminants, such as heavy metals (especially copper), poly aromatic hydrocarbons, road salt.
Conclusions
The benthic diatom community composition of nine stormwater retention ponds located in catchments of different land use and receiving varying levels of biocide contamination was examined. In total, 50 diatom genera were identified with the pollutant tolerant taxa Navicula and Nitzschia making up a large part of diatom communities in all nine ponds and constituting 19–47% of the relative abundance. According to the measured biocide concentrations, as well as the estimated diatom relative abundance, highway ponds were found to be significantly different from the residential and industrial ones. However, the taxonomic variations of diatom communities in ponds of different land use type could not be connected to the presence and concentrations of the measured biocides alone.
The results clearly demonstrate that artificial water bodies constructed to treat stormwater prior to discharge to the environment had benthic sediment diatom communities composed of a variety of genera. This suggests that artificial stormwater ponds serve as ecosystems of ecological value. Diatoms are an important part of the ecosystem, forming the base of the food web, and their dynamics should be clearly understood when engineering stormwater ponds not only as treatment facilities but also as assets to enhance regional biodiversity. While the present study is still far from explaining all the observed community patterns, it demonstrates that stormwater pollutants found in our stormwater ponds certainly do not render these water bodies uninhabitable. Nevertheless, biocides might have an impact on the ecosystem, which should be better understood for optimal management of stormwater runoff. Accordingly, further works are needed to examine combined effects of biocides and other stormwater contaminants on diatom communities, such as metals, PAHs, road salt.
References
Ackley JW, Meylan PA (2010) Watersnake eden: use of stormwater retention ponds by mangrove salt marsh snakes (Nerodia Clarkii Compressicuada) in urban Florida. Herpetological conservation and biology 5(1):17–22
Bannerman RT, Owens DW, Dodds RB, Hornewer NJ (1993) Sources of pollutants in Wisconsin stormwater. Wat Sci Technol 28(3–5):241–259
Bellinger EG, Sigee DC (2010) Freshwater algae: identification and use as bioindicators. Wiley, New York, p 271p
Benetti CJ, Perez-Bilbao A, Garrido J (2012) Macroinvertebrates as indicators of water quality in running waters: 10 years of research in rivers with different degrees of anthropogenic impacts. Ecological water quality - water treatment and reuse, Dr. Voudouris (Ed.), ISBN: 978–953–51–0508- 4, InTech
Bester K, Huhnerfuss H, Brockmann U, Rick HJ (1995) Biological effects of triazine herbicide contamination on marine-phytoplankton. Arch Environ Contam Toxicol 29:277–283
Bois P, Huguenot D, Jézéquel K, Lollier M, Cornu JY, Lebeau T (2013) Herbicide mitigation in microcosms simulating stormwater basins subject to polluted water inputs. Water Res 47(3):1123–1135
Bollmann UE, Vollertsen J, Carmeliet J, Bester K (2014) Dynamics of biocide emissions from buildings in a suburban stormwater catchment–concentrations, mass loads and emission processes. Water Res 56:66–76
Brand AB, Snodgrass JW (2010) Value of artificial habitats for amphibian reproduction in altered landscapes. Conserv Biol 24(1):295–301
Burkhardt M, Kupper T, Hean S, Haag R, Schmid P, Kohler M, Boller M (2007) Biocides used in building materials and their leaching behavior to sewer systems. Water Sci Technol 56(12):63–67
Cattaneo A, Couillard Y, Wunsam S, Fortin C (2011) Littoral diatoms as indicators of recent water and sediment contamination by metals in lakes. J Environ Monit 13:572–582
Chang N-B, Islam K, Marimon Z, Wanielista MP (2012) Assessing biological and chemical signatures related to nutrient removal by floating islands in stormwater mesocosms. Chemosphere 88:736–743
Conover WJ (1980) Practical nonparametric statistics, 2nd edn. Wiley, New York
DARES (2004) Enumeration protocol, Version 1.0. https://craticula.ncl.ac.uk/DARES/methods/DARES_Protocol_Diatom_Counting.pdf
Debenest T, Pinelli E, Coste M, Silvestre J, Mazzella N, Madigou C, Delmas F (2009) Sensitivity of freshwater periphytic diatoms to agricultural herbicides. Aquat Toxicol 93:11–17
Della Bella V, Puccinelli C, Marcheggiani S, Mancini L (2007) Benthic diatom communities and their relationship to water chemistry in wetlands of central Italy. Ann Limnol 43(2):89–99
Della Bella V, Mancini L (2009) Freshwater diatom and macroinvertebrate diversity of coastal permanent ponds along a gradient of human impact in a Mediterranean eco-region. Hydrobiologia. https://doi.org/10.1007/s10750-009-9890-x
DEQS (Directive on Environmental Quality Standards) (2008) Directive 2008/105/EC of European Parliament and of the Council on environmental quality standards in the field of water policy, amending and subsequently repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and amending Directive 2000/60/EC”, OJ L348, p.84–97, 24.12.2008. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32008L0105
Gallagher MT, Snodgrass JW, Ownby DR, Brand AB, Casey RE, Lev S (2011) Watershed-scale analysis of pollutant distributions in stormwater management ponds. Urban Ecosyst 14:469–484
GCERA (Guidance for Conducting Ecological Risk Assessments) (2008) Ohio environmental protection agency division of environmental response and revitalization, DERR - 00 - RR - 031, October 2006, revised April 2008. https://www.epa.state.oh.us/portals/30/rules/RR-031.pdf
Gell PA, Sluiter IR, Fluin J (2002) Seasonal and interannual variations in diatom assemblages in Murray River connected wetlands in north-west Victoria, Australia. Mar Freshw Res 53:981–992
Gold AC, Thompson SP, Piehler MF (2019) Nitrogen cycling processes within stormwater control measures: a review and call for research. Water Res 149:578–587
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4(1): 9pp. https://palaeo-electronica.org/2001_1/past/issue1_01.htm
Hurley SE, Forman RTT (2011) Stormwater ponds and biofilters for large urban sites: modeled arrangements that achieve the phosphorus reduction for Boston’s Charles River, USA. Ecol Eng 37(6):850–863
Hvitved-Jacobsen T, Vollertsen J, Nielsen AH (2010) Urban and highway stormwater pollution—concepts and engineering. CRC Press, Boca Raton
Istenič D, Arias CA, Vollertsen J, Nielsen AH, Wium-Andersen T, Hvitved-Jacobsen T, Brix H (2012) Improved urban stormwater treatment and pollutant removal pathways in amended wet detention ponds. J Environ Sci Health Part A 47:1466–1477
John DM, Whitton BA, Brook AJ (2011) The freshwater algal flora of the british isles, an identification guide to freshwater and terrestrial algae, 2nd edn. Cambridge University Press, Cambridge
Karlsson K, German J, Viklander M (2010) Stormwater pond sediments: temporal trends in heavy metal concentrations and sediment removal. Soil Sedim Contamin 19:217–230
Karlsson K, Viklander M, Scholes L, Revitt M (2010) Heavy metal concentrations and toxicity in water and sediment from stormwater ponds and sedimentation tanks. J Hazard Mater 178(1–3):612–618
Kelly MG, Cazaubon A, Coring E, Dell’Uomo A, Ector L, Goldsmith B, Guasch H, Hürlimann J, Jarlman A, Kawecka B, Kwandrans J, Laugaste R, Lindstrøm E-A, Leitao M, Marvan P, Padisak J, Pipp E, Prygiel J, Rott E, Sabater S, Van Dam H, Vizinet J (1998) Recommendations for the routine sampling of diatoms for water quality assessments in Europe. J Appl Phycol 10:215–224
Kelly MG (2000) Identification of common benthic diatoms in rivers. Field Stud 9(4):583–700
Ladislas S, El-Mufleh A, Gérente C, Chazarenc F, Andrés Y, Béchet B (2012) Potential of aquatic macrophytes as bioindicators of heavy metal pollution in urban stormwater runoff. Water Air Soil Pollut 223:877–888
Legendre P, Legendre L (2012) Numerical ecology, vol 24, Elsevier, Oxford
Leisenring M, Clary J, Hobson P (2014) International stormwater best management practices (BMP) database pollutant category statistical summary report—solids, bacteria, nutrients, and metals. International Stormwater BMP Database
Le Viol I, Mocq J, Julliard R, Kerbiriou C (2009) The contribution of motorway stormwater retention ponds to the biodiversity of aquatic macroinvertebrates. Biol Cons 142:3163–3171
Liu F, Nielsen AH, Vollertsen J (2019) Sorption and degradation potential of pharmaceuticals in sediments from a stormwater retention pond. Water 11(3):526. https://doi.org/10.3390/w11030526
Ludikova AV (2016) Surface-sediment diatom assemblages of the urban ponds of St. Petersburg Russia. Inland Water Biol 9(3):274–282
Martín G, Fernandez M, de Los R (2012) Diatoms as indicators of water quality and ecological status: sampling, analysis and some ecological remarks, ecological water quality—water treatment and reuse, Dr. Voudouris (Ed.), ISBN: 978–953–51–0508–4, InTech, 508p. https://www.intechopen.com/books/ecological-water-quality-water-treatment-and-reuse/diatoms-as-indicators-of-water-quality-and-ecological-status-sampling-analysis-and-some-ecological-r
McEnroe NA, Williams CJ, Xenopoulos MA, Porcal P, Frost PC (2013) Distinct optical chemistry of dissolved organic matter in urban pond ecosystems. PLoS ONE 8(11):e80334. https://doi.org/10.1371/journal.pone.0080334
McEnroe NA, Buttle JM, Marsalek J, Pick FR, Xenopoulos MA, Frost PC (2013) Thermal and chemical stratification of ponds: are they ‘completely mixed reactors’? Urban Ecosyst 16:327–339. https://doi.org/10.1007/s11252-012-0258-z
McLeod S, Kells J, Putz G (2006) Urban runoff quality characterization and load estimation in Saskatoon Canada. J Environ Eng 132(11):1470–1481
Moisset S, Tiam SK, Feurtet-Mazel A, Morin S, Delmas F, Mazzella N, Gonzalez P (2015) Genetic and physiological responses of three freshwater diatoms to realistic diuron exposures. Environ Sci Pollut Res 22:4046–4055
Naselli-Flores L, Termine R, Barone R (2016) Phytoplankton colonization patterns. Is species richness depending on distance among freshwaters and on their connectivity? Hydrobiologia 764:103–113
Parmar TK, Rawtani D, Agrawal YK (2016) Bioindicators: the natural indicator of environmental pollution. Front Life Sci 9(2):110–118. https://doi.org/10.1080/21553769.2016.1162753
Paulus W (2004) Directory of microbicides for the protection of materials—a handbook. Kluwer Academic Publishers, Dordrecht
Pereira AS, Trindade CRT, Albertoni EF, Palma-Silva C (2012) Aquatic macrophytes as indicators of water quality in subtropical shallow lakes Southern Brazil. Acta Limnol Brasil 24(1):52–63
Phillips DP, Human LRD, Adams JB (2015) Wetland plants as indicators of heavy metal contamination. Marine Pollution Bulletin 92(1–2):227–232. https://doi.org/10.1016/j.marpolbul.2014.12.038
Primer E-Ltd (2016) Primer software package, version 7. Primer-E ltd, Plymouth
Proia L, Morin S, Peipoch M, Romani AM, Sabater S (2011) Resistance and recovery of ricer biofilms receiving short pulses of Triclosan and Diuron. Sci Total Environ 409:3129–3137
Rimet F, Bouchez A (2011) Use of diatom life-forms and ecological guilds to assess pesticide contamination in rivers: Lotic mesocosm approaches. Ecol Ind 11:489–499
Rimet F (2012) Diatoms: an ecoregional indicator of nutrients, organic matter and micropollutants pollution. Agricultural Sciences, Universite de Grenoble, 2012. Doctoral Thesis
Scher O, Thiery A (2005) Odonata, amphibia and environmental characteristics in motorway stormwater retention ponds (Southern France). Hydrobiologia 551:237–251
Schroer WF, Benitez-Nelson CR, Smith EM, Ziolkowski LA (2018) Drivers of sediment accumulation and nutrient burial in coastal stormwater detention ponds, South Carolina USA. Ecosystems 21:1118–1138. https://doi.org/10.1007/s10021-017-0207-z
Smith AJ, Bode RW, Kleppel GS (2007) A nutrient biotic index (NBI) for use with benthic macroinvertebrate communities. Ecol Ind 7(2):371–386. https://doi.org/10.1016/j.ecolind.2006.03.001
Snedecor GW, Cochran WG (1989) Statistical Methods. Iowa State College Press, Ames
Spaulding SA, Lubinski DJ, Potapova M (2010) Diatoms of the United States. https://westerndiatoms.colorado.edu
Stanley WD (1996) Pollutant removal by a stormwater dry detention pond. Water Environ Res 68(6):1076–1083
Stephansen DA, Nielsen AH, Hvitved-Jacobsen T, Pedersen ML, Vollertsen J (2016) Invertebrates in stormwater wet detention ponds—sediment accumulation and bioaccumulation of heavy metals has no effect on biodiversity and community structure. Sci Total Environ 566–567:1579–1587
Sun Z, Sokolova E, Brittain JE, Saltveit SJ, Rauch S, Meland S (2019) Impact of environmental factors on aquatic biodiversity in roadside stormwater ponds. Sci Rep 9:5994
Systat Software Inc (2016) Sigmaplot v 132. Systat Inc., San Jose
Taylor JC, Harding WR, Archibald CGM (2007) a methods manual for the collection, preparation and analysis of diatom samples, Version 1.0. Report to the Water Research Commission. WRC Report TT 281/07. ISBN 1–77005–483–9
Thayer VL, Johnson TC, Schrader HJ (1983) Distribution of diatoms in lake superior sediments. J Great Lakes Res 9(4):497–507
Watchorn MA, Hamilton PB, Anderson TW, Roe HM, Patterson RT (2008) Diatoms and pollen as indicators of water quality and land-use change: a case study from the Oak Ridges Moraine, Southern Ontario, Canada. J Paleolimnol 39:491–509. https://doi.org/10.1007/s10933-007-9126-x
WFD (Water Framework Directive) (2000) Directive 2000/60/EC of the European parliament and of the council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off J Eur Union L 327(43):1–73
WFD (Water Framework Directive) (2013) Directive 2013/39/EU of the European Parliament and of the countil of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Off J Eur Union L 226:1–17
Weinstein JE, Crawford KD, Garner TR, Flemming AJ (2010) Screening-level ecological and human health risk assessment of polycyclic aromatic hydrocarbons in stormwater detention pond sediments of Coastal South Carolina, USA. J Hazard Mater 178:906–916
Zalat A, Vildary SS (2005) Distribution of diatom assemblages and their relationship to environmental variables in the surface sediments of three northern Egyptian lakes. J Paleolimnol 34:159–174
Acknowledgements
The authors acknowledge funding obtained from the Danish Environmental Protection Agency (Project MST- 667–00151).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Télesphore Sime-Ngando
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Minelgaite, G., van Alst, N., Stephansen, D.A. et al. An exploratory study of benthic diatom communities in stormwater ponds of different land uses and varying biocide contamination. Aquat Ecol 54, 761–774 (2020). https://doi.org/10.1007/s10452-020-09773-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-020-09773-x