Abstract
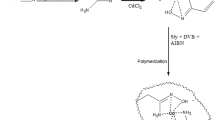
In this study, a cesium ion imprinted polymer (Cs(I)-IIP) was prepared by free radical thermal polymerization using 18-crown-6-ether (18C6) as ligand, methacrylic acid (MAA) as functional monomer, and ethylene glycol dimethacrylate (EGDMA) as crosslinking agent, which can be used for adsorption and separation of cesium ions from low-concentration solutions. The adsorption kinetic and isotherm results showed that the adsorption of Cs+ fitted to the pseudo-second-order kinetic model and Langmuir model, indicating that the adsorption of Cs+ on Cs(I)-IIP was the monolayer chemical adsorption. The maximum adsorption capacity was 84.21 mg·g− 1. The selective adsorption properties are performed in Cs+, Li+, Na+, and K+ multicomponent systems. The results showed that the Cs(I)-IIP has a high selectivity in the presence of coexisting Li+, Na+, and K+, and the selectivity coefficients (K’) of Cs(I)-IIP for Cs+/Li+, Cs+/Na+, Cs+/K+ are 2.1, 1.56, and 1.33, respectively. The high adsorption capacity and selectivity are attributed to the introduction of imprinting technology to form specific Cs+ recognition adsorption sites, and the 18C6 cavity was easier to recognize Cs+ in the competitive adsorption process. Finally, the Cs(I)-IIP can be regenerated and reused for 10 times with the adsorption capacity only decreased by 8.1%, indicating that the polymer has good reuse performance.









Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Zhang, X.F., et al.: Efficient co-extraction of lithium, rubidium, cesium and potassium from lepidolite by process intensification of chlorination roasting. Chem. Eng. Processing-Process Intensif. 147, 8 (2020). https://doi.org/10.1016/j.cep.2019.107777
Guo, H., et al.: Enhanced acid treatment to extract lithium from lepidolite with a fluorine-based chemical method. Hydrometallurgy. 183, 9–19 (2019). https://doi.org/10.1016/j.hydromet.2018.10.020
Hill, T.G., Ensor, D.D., Delmau, L.H., Moyer, B.A.: Thermal stability study of a new guanidine suppressor for the next-generation caustic-side solvent extraction process. Sep. Sci. Technol. 51, 1133–1140 (2016). https://doi.org/10.1080/01496395.2016.1143509
Chen, W.S., et al.: Recovery of Rubidium and Cesium resources from Brine of Desalination through t-BAMBP extraction. Metals. 10, 15 (2020). https://doi.org/10.3390/met10050607
Jagasia, P., et al.: Recovery of radio-cesium from actual high level liquid waste using solvents containing calix 4 arene-crown-6 ligands. J. Environ. Chem. Eng. 5, 4134–4140 (2017). https://doi.org/10.1016/j.jece.2017.07.055
Lee, C.H., Chen, W.S.: Extraction of cesium from aqueous solution through t-SAMBP/C(2)mimNTf(2) and recovery of cesium from waste desalination brine. Desalination Water Treat. 235, 193–199 (2021). https://doi.org/10.5004/dwt.2021.27540
Ye, Y., Li, K., Zhang, W.C., Liu, C.: Precipitation of cesium lead halide perovskite nanocrystals in glasses based on liquid phase separation. J. Am. Ceram. Soc. 105, 6105–6115 (2022). https://doi.org/10.1111/jace.18547
Lv, L.M., Chen, C., Hou, H.W., Zhang, X.H., Lan, P.: Structure analysis and cesium adsorption mechanism evaluation of sodium copper ferrocyanide. J. Radioanal. Nucl. Chem. 331, 5835–5842 (2022). https://doi.org/10.1007/s10967-022-08633-2
Jang, S.C., et al.: Magnetic composites as an effective technology for removal of radioactive cesium. Int. J. Environ. Sci. Technol. 12, 3695–3700 (2015). https://doi.org/10.1007/s13762-015-0853-7
Dumat, C., Quiquampoix, H., Staunton, S.: Adsorption of cesium by synthetic clay-organic matter complexes: Effect of the nature of organic polymers. Environ. Sci. Technol. 34, 2985–2989 (2000). https://doi.org/10.1021/es990657o
Pangeni, B., Paudyal, H., Inoue, K., Ohto, K., Kawakita, H.: Preparation of Natural Bio-adsorbent from Green Tea Extract Powder and its application for selective removal of cs(I) from Water. J. Chem. Eng. Jpn. 54, 638–647 (2021). https://doi.org/10.1252/jcej.20we172
Zhang, A.Y., Xiao, C.L., Xue, W.J., Chai, Z.F.: Chromatographic separation of cesium by a macroporous silica-based supramolecular recognition agent impregnated material. Sep. Purif. Technol. 66, 541–548 (2009). https://doi.org/10.1021/je400735z
Zhang, J.F., et al.: Kinetics-controlled separation intensification for Cesium and Rubidium isolation from Salt Lake Brine. Ind. Eng. Chem. Res. 57, 4399–4406 (2018). https://doi.org/10.1021/acs.iecr.7b04820
Patra, K., et al.: Achieving highly efficient and selective cesium extraction using 1,3-di-octyloxycalix 4 arene-crown-6 in n-octanol based solvent system: Experimental and DFT investigation. RSC Adv. 11, 21323–21331 (2021). https://doi.org/10.1039/d1ra02661e
Wang, J.C.: Co-extraction of strontium and cesium from simulated high-level liquid waste (HLLW) by calixcrown and crown ether. J. Nucl. Sci. Technol. 52, 171–177 (2015). https://doi.org/10.1080/00223131.2014.938136
Zhang, A.Y., Dai, Y., Xu, L., Chai, Z.F.: Solvent extraction of Cesium with a New Compound Calix 4 arene-bis (4-methyl-1,2-phenylene)-crown-6. J. Chem. Eng. Data. 58, 3275–3281 (2013). https://doi.org/10.1016/j.seppur.2009.02.002
Zhang, K., et al.: Adsorption behavior of cs(I) on natural soils: Batch experiments and model-based quantification of different adsorption sites. Chemosphere. 290, 9 (2022). https://doi.org/10.1016/j.chemosphere.2021.132636
Chen, R.Z., et al.: Preparation of a film of copper hexacyanoferrate nanoparticles for electrochemical removal of cesium from radioactive wastewater. Electrochem. Commun. 25, 23–25 (2012). https://doi.org/10.1016/j.elecom.2012.09.012
Nisola, G.M., et al.: Covalently decorated crown ethers on magnetic graphene oxides as bi-functional adsorbents with tailorable ion recognition properties for selective metal ion capture in water. Chem. Eng. J. 389, 12 (2020). https://doi.org/10.1016/j.cej.2019.123421
Bereczki, R., Agai, B., Bitter, I., Toke, L., Toth, K.: Bis(benzo-18-crown-6) derivatives: Synthesis and ion-sensing properties in plasticized PVC membranes. J. Incl. Phenom. Macrocyclic Chem. 45, 45–50 (2003). https://doi.org/10.1023/a:1023095617641
Chaudhury, S., Bhattacharyya, A., Goswami, A.: Electrodriven Ion Transport through Crown Ether-Nafion Composite membrane: Enhanced selectivity of cs + over na + by Ion Gating at the Surface. Ind. Eng. Chem. Res. 53, 8804–8809 (2014). https://doi.org/10.1021/ie500934v
Fan, Q.H., Tanaka, M., Tanaka, K., Sakaguchi, A., Takahashi, Y.: An EXAFS study on the effects of natural organic matter and the expandability of clay minerals on cesium adsorption and mobility. Geochim. Cosmochim. Acta. 135, 49–65 (2014). https://doi.org/10.1016/j.gca.2014.02.049
Awual, M.R., et al.: Radioactive cesium removal from nuclear wastewater by novel inorganic and conjugate adsorbents. Chem. Eng. J. 242, 127–135 (2014). https://doi.org/10.1016/j.cej.2013.12.072
Liu, Z., et al.: Experimental and theoretical investigations of cs + adsorption on crown ethers modified magnetic adsorbent. J. Hazard. Mater. 371, 712–720 (2019). https://doi.org/10.1016/j.jhazmat.2019.03.022
Yang, L., Li, S.F., Sun, C.Y.: Selective adsorption and separation of cs(I) from salt lake brine by a novel surface magnetic ion-imprinted polymer. J. Dispers. Sci. Technol. 38, 1547–1555 (2017). https://doi.org/10.1080/01932691.2016.1261361
Li, X.Z., Sun, Y.P.: Evaluation of ionic imprinted polymers by electrochemical recognition of rare earth ions. Hydrometallurgy. 87, 63–71 (2007). https://doi.org/10.1016/j.hydromet.2007.02.003
Wang, W.S., et al.: Effective removal of Fe(II) impurity from rare earth solution using surface imprinted polymer. Chem. Eng. Res. Des. 91, 2759–2764 (2013). https://doi.org/10.1016/j.cherd.2013.05.006
Jing, Z.F., et al.: Selectivity of 18-crown-6 ether to alkali ions by density functional theory and molecular dynamics simulation. J. Mol. Liq. 311, 9 (2020). https://doi.org/10.1016/j.molliq.2020.113305
Handy, N.C.: The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors - comment. Mol. Phys. 100, 63–63 (2002). https://doi.org/10.1080/00268970110088893
Zhang, J., Lu, T.: Efficient evaluation of electrostatic potential with computerized optimized code. Phys. Chem. Chem. Phys. 23, 20323–20328 (2021). https://doi.org/10.1039/d1cp02805g
Rajabi, H.R., Shamsipur, M., Pourmortazavi, S.M.: Preparation of a novel potassium ion imprinted polymeric nanoparticles based on dicyclohexyl 18C6 for selective determination of K + ion in different water samples. Mater. Sci. Eng. C-Materials Biol. Appl. 33, 3374–3381 (2013). https://doi.org/10.1016/j.msec.2013.04.022
Wang, J.L., Guo, X.: Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 390, 18 (2020). https://doi.org/10.1016/j.jhazmat.2020.122156
Zhou, L., et al.: Dual ion-imprinted mesoporous silica for selective adsorption of U(VI) and cs(I) through multiple interactions. ACS Appl. Mater. Interfaces. 13, 6322–6330 (2021). https://doi.org/10.1021/acsami.0c21207
Zhang, Z.L., Xu, X.H., Yan, Y.S.: Kinetic and thermodynamic analysis of selective adsorption of cs(I) by a novel surface whisker-supported ion-imprinted polymer. Desalination. 263, 97–106 (2010). https://doi.org/10.1016/j.desal.2010.06.044
Meng, X.G., et al.: Synthesis of novel ion-imprinted polymers by two different RAFT polymerization strategies for the removal of cs(I) from aqueous solutions. RSC Adv. 5, 12517–12529 (2015). https://doi.org/10.1039/c4ra11459k
Xia, T.T., Yin, L.L., Xie, Y.H., Ji, Y.Q.: Efficiently remove of cs(I) by metals hexacyanoferrate modified magnetic Fe3O4-chitosan nanoparticles. Chem. Phys. Lett. 746, 8 (2020). https://doi.org/10.1016/j.cplett.2020.137293
Xia, T.T., Wu, H.Y., Yin, L.L., Ji, Y.Q.: Selective removal of cesium by ammonium molybdophosphate-magnetic Fe3O4-chitosan composites. J. Mater. Res. 36, 2926–2935 (2021). https://doi.org/10.1557/s43578-021-00279-2
He, J.T., et al.: Highly-efficient adsorptive separation of cs + from aqueous solutions by porous polyimide membrane containing Dibenzo-18-Crown-6. Sep. Purif. Technol. 299, 10 (2022). https://doi.org/10.1016/j.seppur.2022.121757
Funding
Beijing Natural Science Foundation (2232067), Research Fund of State Key Laboratory of Mesoscience and Engineering (MESO-23-A06).
Author information
Authors and Affiliations
Contributions
L.M. and J.Q. analyzed the experimental data, discussed the results, and wrote the manuscript. T.W. and Y.G. provided the resources of Cs(I). L.M. conceived the study design. J.Q. and L.G Investigated the experimental section and performed the measurements. L.M. provided funding support. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Conflict of interest
The authors have no relevant fnancial or on-fnancial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, L., Qi, J., Gao, L. et al. 18-Crown-6-ether assembly of cesium ion-imprinted polymer enabling efficiently selective separation of cs(I) from aqueous solution. Adsorption (2024). https://doi.org/10.1007/s10450-024-00481-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10450-024-00481-8