Abstract
Adsorption of acetazolamide (ACT) and formation of a mixed adsorption layers of acetazolamide (ACT)—sodium 1-decanesulfonate (SDS) and acetazolamide—hexadecyltrimethylammonium bromide (CTAB) at the R-AgLAFe/chlorates(VII) interface are described. The systems were characterized by the measurements of differential capacity, potential of zero charge, and surface tension at this potential. The adsorption parameters determined in the studied systems indicate the SDS domination in the adsorption equilibria and the competitive adsorption between the ACT—SDS or mixed micelles. However, acetazolamide dominates at adsorption equilibria of the ACT—CTAB mixture.
Similar content being viewed by others
1 Introduction
Explanation of the mixed adsorption layers formation is significant importance as it is the basis for the action of corrosion inhibitors [1, 10, 11, 18, 22, 32]. The mechanism of the corrosion inhibitor action may differ depending on the type of metal, the character of environment and the structure of the inhibitor [3, 4, 14, 15, 26, 29, 32]. One possible mechanism involves adsorption of the inhibitor, which blocks the metal surface and prevents the corrosion process. The use of corrosion inhibitors is to prevent the anodic and cathodic processes that may be triggered by an increase in the overvoltage or to block the active metal surface. Organic corrosion inhibitors belong to the same type of compounds as those preventing metal etching in acids or shine-formers [3, 4, 7, 14, 30]. Their effectiveness depends largely on their adsorption properties, as adsorption of such compounds changes the metal ability to resist corrosion. The process of adsorption usually involves electrostatic or covalent interactions between the atoms of the inhibitor and the metal surface. The adsorbed organic molecules form a protective layer on the surface that prevents or reduces the rate of metal dissolution.
Thanks to their great effectiveness, ease of production, relatively low price and, above all, no threat to the environment, ionic and nonionic surfactants have been generally appreciated corrosion inhibitors for steel, copper, aluminum and iron [2, 19, 21, 26, 32]. The use of mixtures of these compounds can significantly improve the corrosion prevention effectiveness [3, 12, 27, 31]. Thus, the adsorption mechanism is mainly related to competitive adsorption [5, 8, 9, 13, 24]. The degree of competitiveness of adsorption depends on the potential of the electrode, its charge as well as the interactions in the adsorbate layer and those at the adsorbate–metal (electrode) interface.
The adsorption of acetazolamide and formation of mixed adsorption layers of acetazolamide—sodium 1-decanesulfonate and acetazolamide—hexadecyltrimethylammonium bromide was studied. Experimental data indispensable for the determination of adsorptive properties of acetazolamide and mixed layers were obtained from measurements of differential capacity of the double layer, the potential of zero charge and the surface tension with this potential.
Earlier adsorption studies indicated a change in the structure and properties of the adsorption layer at the R-AgLAFe/aqueous chlorates(VII) solution interface in the presence of mixtures of an organic substance and a surfactant, which resulted in the mixed adsorption layer formation [5, 8, 9, 13, 24].
2 Experimental
2.1 Materials and methods
A cyclically renewable liquid silver amalgam film electrode (R-AgLAFe) was prepared by reproduction of the liquid amalgam layer prior to the series of measurements. The 1% liquid amalgam was prepared by immersion of the pieces of silver wire (of 0.5 mm diameter) in the 0.5 ml mercury for 2 weeks [25].
The automatic cell stand (Mtm Anko) included an electrode cell containing: a R-AgLAFE electrode along with the automatized steering system as well as the stable reproducible electrode surface (17.25 mm2), as a working electrode; an Ag/AgCl (3 M saturated solution of NaCl), as a reference electrode and a platinum spiral, as an auxiliary electrode at 298 K.
More detailed description of the R-AgLAFE electrode refreshment before each measurement, the operating principles, and the design are presented in the paper [25].
Acetazolamide (Sigma Aldrich) used in the study was of analytical purity. CTAB (Fluka) and SDS (Fluka) were used without further purification. The solutions were made using newly doubly–distilled water purified in a Millipore Milli-Q system. Before measurements, the solutions were deaerated using high–purity nitrogen (assay 99.999%, Air Liquid). Nitrogen was passed over the solution during the measurements.
The basic electrolyte contained 1 mol•dm−3 chlorates(VII) solution (mixed in the proportions 0.5 mol·dm−3 NaClO4 (Fluka) + 0.5 mol·dm−3 HClO4 (Fluka)), made of the reagents of analytical purity. Acetazolamide solutions were prepared directly before the measurements. Their concentration was in the interval from 1·10−5 to 1·10−3 mol·dm−3, but the concentration of CTAB ranged from 1.5•10–6 to 1.5•10–5 mol·dm−3, and that of SDS ranged from 1•10–5 to 9•10–5 mol·dm−3.
The double layer capacity (\(C_{d}\)) was measured using the AC impedance technique at several frequencies with the amplitude of 5 mV, using an electrochemical analyzer Autolab controlled by the GPES software (Version 4.9) (Eco Chemie, Utrecht Netherlands). The reproducibility of the average capacity measurements was ± 0.5%.
The double layer capacity (Cd) was measured using the impedance technique at several frequencies with the amplitude of 5 mV. For the whole polarization range, the capacity dispersion was tested at different frequencies changed between 200 and 1000 Hz. Adsorption equilibrium was not preserved in any of the systems. Therefore, to obtain the equilibrium values of differential capacity, the linear dependence of capacity on frequency was extrapolated to the zero frequency [23]. The reproducibility of the average capacity measurements was ± 0.5%.
The potential of zero charge (Ez) was measured for each solution by the method of streaming mercury electrode [24], (the accuracy of ± 0.1 mV).
The surface tension at the potential of zero charge (γz) was determined using the method of the highest pressure inside the mercury drop [24], (the accuracy of ± 0.2 mNm−1).
Viscosity measurements were performed using a rotational CVO 50 rheometer with the “double gap”; measuring system (Bohlin Instruments).
Applying the viscosity method [using a rotational CVO 50 rheometer (Bohlin Instruments)] [24] the critical micelle concentration (CMC) was determined. The measurements were made in the 1 mol·dm−3 chlorates(VII)—CTAB, 1 mol·dm−3 chlorates(VII)—SDS and in the presence of acetazolamide system.
3 Results and discussion
The evident changes in the differential capacity curves in the presence of acetazolamide indicate the adsorption of the substance at the R-AgLAFe/1 mol•dm−3 chlorates(VII) interface (Fig. 1). The possible partial charge transfer between the sulfur atom and the electrode due to π electrons suggests specific adsorption [5, 17, 28].
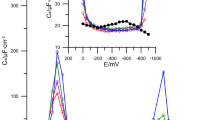
Differential capacity—potential curves of the double layer interface R-AgLAFe/1 mol•dm−3 chlorates(VII) (•) with various concentrations of ACT (1·10–5 mol•dm−3 (Red ◊); 1·10–4 mol•dm−3 (Grey ◊); 1·10–3 mol•dm−3 (Green ◊). Capacitance curves obtained after extrapolation to the zero frequency (Color figure online)
In the region of the hump potentials (≈ − 200 – − 800 mV), after the addition of ACT to the solution, the height of the hump increases. In the region of ≈ 100 mV potential, small adsorption peaks appear in the presence of ACT (Fig. 1), however, in the area of the most negative potentials about—1600 mV a desorption peak appears. It should be noted that the increase in ACT concentration in the basic electrolyte results in the evident decrease in the desorption peaks.
The addition of the surfactant to the basic electrolyte solution containing ACT (Fig. 2) changes the character of the capacity curves, particularly for the 5·10 −5 mol·dm−3 (CMC) concentration. It should be noted that the increase in the SDS concentration in the basic electrolyte results in the evident increase in adsorption and decrease in the desorption peaks. In turn, the appearance of a clear adsorption peak is definitely associated with the competitive adsorption between the ACT and SDS molecules or mixed micelles [5, 8, 9, 13, 24]. The surfactant molecules dominate in the formulation [13, 16, 24].
Differential capacity—potential curves of the double layer interface R-AgLAFe/1 mol•dm−3 chlorates(VII) (•) with 1·10–4 mol•dm−3ACT (Grey ◊) and with various concentrations of SDS (1.5·10–5 mol•dm−3 (Red ▲); 3·10–5 mol•dm−3 (Blue ▲); 5·10–5 mol•dm−3 (Green ▲); 7.5·10–5 mol•dm−3 (Purple ▲); 9·10–5 mol•dm−3 (Yellow ▲)). Capacitance curves obtained after extrapolation to the zero frequency (Color figure online)
However, the interactions of ACT molecules and the surfactant resulting in the formation of a more or less compact structure of mixed adsorption layers cannot be excluded [13, 16, 24].
Moreover, the presence of CTAB in the basic electrolyte solution containing 1•10–4 mol·dm−3 of ACT, does not change the character of capacity curves (Fig. 3). It should be noted that with increasing CTAB concentration, the height of the desorption peaks does not change. As CTAB is a cationic molecule, one can expect its different arrangements on the electrode surface. It should be mentioned that in the range of the studied concentrations in the mixture of ACT and cationic detergent, the critical micelle concentration CMC was not observed [20]. Definitely one can observe a change in the structure and properties of the adsorption layer at the R-AgLAFe/aqueous chlorates(VII) solution in the presence of ACT and CTAB solution, which results in the mixed adsorption layer formation. However, the predominance of acetazolamide in formation of adsorption equilibria of acetazolamide-CTAB should be pointed out [17, 24, 28]. However, such a predominance was not observed for acetazolamide-SDS.
Differential capacity—potential curves of the double layer interface. R-AgLAFe/1 mol•dm−3 chlorates(VII) (•) with 1·10−4 mol•dm−3 ACT (Grey ◊) and with various concentrations of CTAB (1.5·10−6 mol•dm−3 (Red •); 3·10−6 mol•dm−3 (Blue •); 6·10−6 mol•dm−3 (Green •); 1.5·10−5 mol•dm−3 (Purple •). Capacitance curves obtained after extrapolation to the zero frequency (Color figure online)
As follows from Table 1, the addition of ACT in concentrations increasing to 1•10–4 mol•dm−3, to a 1 mol·dm−3 chlorates(VII) solution causes a shift of Ez towards more positive potentials.
A further increase in the ACT concentration results in a change in the Ez value toward more negative potentials, which may suggest a change in the position of the ACT molecule on the electrode surface [13, 16, 24]. The trend of the value change indicates definitely the change in the orientation of the molecule from the positive to the negative direction relative to the electrode surface [6]. At lower concentrations, ACT molecules assume a more oblique orientation towards the electrode surface, so that their interaction with the electrode surface is weaker, making them more accessible to the electrode surface. At higher concentrations, on the other hand, the interaction becomes much stronger as a result of the transition of the molecule into a planar orientation, facilitating the interaction of the π electrons of sulfur present in the aromatic ring with the electrode surface [5, 17, 28] (Scheme 1).
The addition of SDS results in a shift of Ez towards the positive potentials. However, it should be noted that for the CMC concentration of 5·10–5 mol·dm−3 these changes are definitely significant (the displacement effect is much pronounced) and may indicate the aggregation phenomena at the Hg–aqueous electrolyte solution interface in the presence of surfactant (Table 2) [20].
The increase in CMC value in the presence of ACT (CMC for SDS = 3·10–5 mol dm−3) and in the presence of ACT (CMC = 5·10–5 mol dm−3) should be associated with competitive adsorption but also between the mixed micelles [5, 8, 9, 13, 24]. A synergistic effect should not be excluded [5, 8, 9, 13, 24]. This observation is consistent with the conclusions resulting from the differential capacitance curves analysis (Fig. 2).
Moreover, in the presence of CTAB in the basic electrolyte solution containing 1•10–4 mol dm−3 of ACT, Ez is shifted towards more positive potentials (Table 3). No evident anomalies were observed in the changing values.
The values of surface tension γz (Tables 1, 2, 3) decrease for all studied systems as proved by the adsorption phenomenon.
4 Conclusions
The results presented in this paper show specific adsorption of ACT at the R-AgLAFe/1 mol•dm−3 chlorates(VII) interface. Changes in Ez with increasing ACT concentration suggest a change in the orientation of the ACT molecule on the electrode surface. Formation of the mixed adsorption layers of acetazolamide and an ionic surfactant at the R-AgLAFe/chlorates(VII) interface was proved. In the systems containing ACT and SDS, the surfactant dominance and competitive adsorption [appearance of a well-developed adsorption peak (Fig. 2)] or the formation of mixed micelles should be considered.
However, acetazolamide dominates in the formation of adsorption equilibria of the ACT-CTAB mixture. The absence of a well-developed adsorption peak in this system (Fig. 3) suggests the co-adsorption of ACT and CTAB, with the CTAB interactions with the electrode taking place through the adsorbed molecule, which can be partially inclined in these conditions. In both mixed systems interactions the role of ClO4− ions should be neglected.
Data availability
At the author of correspondence.
References
Algaber, A.S., El-Nemma, E.M., Saleh, M.M.: Effect of octylphenol polyethylene oxide on the corrosion inhibition of steel in 0.5 M H2SO4. Mater. Chem. Phys. 86(1), 26–32 (2004)
Antonijevic, M.M., Petrovic, M.B.: Copper corrosion inhibitors. A review. Int. J. Electrochem. Sci. 3(1), 1–28 (2008)
Asefi, D., Mahmoodi, N.M., Arami, M.: Effect of nonionic co-surfactants on corrosion inhibition effect of cationic Gemini surfactant. Colloids Surf A Physicochem Eng Asp 355(1–3), 183–186 (2010)
Asefi, D., Arami, M., Mahmoodi, N.M.: Electrochemical effect of cationic gemini surfactant and halide salts on corrosion inhibition of low carbon steel in acid medium. ECS Trans. 33(30), 1 (2011)
Avranas, A., Papadopoulos, N., Papoutsi, D., Sotiropoulos, S.: Adsorption of the neutral macromonomeric surfactant Tween-80 at the mercury/electrolyte solution interface as a function of electrode potential and time. Langmuir 16, 6043–6053 (2000)
Brycht, M., Skrzypek, S., Nosal-Wiercińska, A., Smarzewska, S., Guziejewski, D., Ciesielski, W., Burnat, B., Leniart, A.: The new application of renewable silver amalgam film electrode for the electrochemical reduction of nitrile, cyazofamid, and its voltammetric determination in the real samples and in a commercial formulation. Electrochim. Acta 134, 302–308 (2014)
Brycki, B.E., Kowalczyk, I.H., Szulc, A., Kaczerewska, O., Pakiet, M.: Organic corrosion inhibitors. Corrosion inhibitors, principles and recent applications 3, 33 (2018)
Chen, X., Mccrum, I.T., Schwarz, K.A., Janik, M.J., Koper, M.T.: Co-adsorption of cations as the cause of the apparent pH dependence of hydrogen adsorption on a stepped platinum single-crystal electrode. Angew. Chem. Int. Ed. 56, 15025–15029 (2017)
Diehl, R.D., Mcgrath, R.: Structural studies of alkali metal adsorption and coadsorption on metal surfaces. Surf. Sci. Rep. 23, 43–171 (1996)
El-Tabei, A.S., Hegazy, M.A.: A corrosion inhibition study of a novel synthesized gemini nonionic surfactant for carbon steel in 1 M HCl solution. J. Surfactants Deterg. 16(5), 757–766 (2013)
Fuchs-Godec, R.: Inhibitory effect of non-ionic surfactants of the TRITON-X series on the corrosion of carbon steel in sulphuric acid. Electrochim. Acta 52(15), 4974–4981 (2007)
Fuchs-Godec, R.: Effects of surfactants and their mixtures on inhibition of the corrosion process of ferritic stainless steel. Electrochim. Acta 54(8), 2171–2179 (2009)
Gugała-Fekner, D., Nieszporek, J., Sieńko, D.: Adsorption of anionic surfactant at the electrode–NaClO 4 solution interface. Monatsh. Chem. 146, 541–545 (2015)
Hegazy, M.A., Badawi, A.M., Abd El Rehim, S.S., Kamel, W.M.: Corrosion inhibition of carbon steel using novel N-(2-(2-mercaptoacetoxy) ethyl)-N, N-dimethyl dodecan-1-aminium bromide during acid pickling. Corros. Sci. 69, 110–122 (2013)
Huang, Z., Gu, T.: Mixed adsorption of nonionic and cationic surfactants on silica gel and methylated silica gel. Colloids Surf. 28, 159–168 (1987)
Klin, M., Nieszporek, J., Sieńko, D., Gugała-Fekner, D., Saba, J.: Adsorption of tetramethylthiourea in the presence of cationic detergent at interface electrode/aqueous perchlorate solutions. Croat. Chem. Acta 84, 475–480 (2011)
Kumar, D., Rub, M.A.: Role of cetyltrimethylammonium bromide (CTAB) surfactant micelles on kinetics of [Zn (II)-Gly-Leu]+ and ninhydrin. J. Mol. Liq. 274, 639–645 (2019)
Li, X., Deng, S., Mu, G., Fu, H., Yang, F.: Inhibition effect of nonionic surfactant on the corrosion of cold rolled steel in hydrochloric acid. Corros. Sci. 50(2), 420–430 (2008)
Malik, M.A., Hashim, M.A., Nabi, F., Al-Thabaiti, S.A., Khan, Z.: Anti-corrosion ability of surfactants: a review. Int. J. Electrochem. Sci. 6(6), 1927–1948 (2011)
Martyna, M., Grochowski, M., Urban, T., Nosal-Wiercińska, A.: Study of 2–thiocytosine/surfactant adsorption at the R-AgLAFe/chlorate (VII) interface–impact of surfactant ionic character. Physicochem. Probl. Miner. Process 58, 144322 (2022)
Mobin, M., Zehra, S., Parveen, M.: L-Cysteine as corrosion inhibitor for mild steel in 1 M HCl and synergistic effect of anionic, cationic and non-ionic surfactants. J. Mol. Liq. 216, 598–607 (2016)
Negm, N.A., Kandile, N.G., Badr, E.A., Mohammed, M.A.: Gravimetric and electrochemical evaluation of environmentally friendly nonionic corrosion inhibitors for carbon steel in 1 M HCl. Corros. Sci. 65, 94–103 (2012)
Nosal-Wiercińska, A.: The influence of water activity on double layer parameters on the interface mercury/chlorates (VII) in the presence of cysteine. Croat. Chem. Acta 86(2), 159–164 (2013)
Nosal-Wiercińska, A., Kaliszczak, W., Grochowski, M., Wiśniewska, M., Klepka, T.: Effects of mixed adsorption layers of 6-mercaptopurine–Triton X-100 and 6-mercaptopurine–Tween 80 on the double layer parameters at the mercury/chlorates (VII) interface. J. Mol. Liq. 253, 143–148 (2018)
Nosal-Wiercińska, A., Martyna, M., Grochowski, M., Baś, B.: First electrochemical studies on “CAP—PAIR” effect for Bi (III) ion electroreduction in the presence of 2-Thiocytosine on novel cyclically renewable liquid silver amalgam film electrode (R-AgLAFE). J. Electrochem. Soc. 168, 066504 (2021)
Raja, P.B., Ismail, M., Ghoreishiamiri, S., Mirza, J., Ismail, M.C., Kakooei, S., Rahim, A.A.: Reviews on corrosion inhibitors: a short view. Chem. Eng. Commun. 203(9), 1145–1156 (2016)
Singh, A., Quraishi, M.A.: Acidizing corrosion inhibitors: a review. J. Mater. Environ. Sci 6(1), 224–235 (2015)
Sotiropulo, S., Nikitas, P., Papadopoulo, N.: Interfacial micellization of cetyl–dimethyl–benzylammonium chloride and Tween 80 at the Hg/electrolyte solution interphase. J. Electroanal. Chem. 356, 225–243 (1993)
Umoren, S.A., Eduok, U.M.: Application of carbohydrate polymers as corrosion inhibitors for metal substrates in different media: a review. Carbohydr. polym 140, 314–341 (2016)
Winkler, D.A.: Predicting the performance of organic corrosion inhibitors. Metals 7(12), 553 (2017)
Yousefi, A., Javadian, S., Neshati, J.: A new approach to study the synergistic inhibition effect of cationic and anionic surfactants on the corrosion of mild steel in HCl solution. Ind. Eng. Chem. Res. 53(13), 5475–5489 (2014)
Zhu, Y., Free, M.L., Woollam, R., Durnie, W.: A review of surfactants as corrosion inhibitors and associated modeling. Prog. Mater. Sci. 90, 159–223 (2017)
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
ANW and MM wrote the main manuscript text, AP and ABW prepared figures and scheme, ANW and RP authors reviewed the manuscript, MM, AP and ABW made the experimental part.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martyna, M., Pawlak, A., Bazan-Wozniak, A. et al. Impact of acetazolamide on the double layer parameters at the R-AgLAFe/chlorates(VII) interface in the presence of ionic surfactants. Adsorption 30, 329–335 (2024). https://doi.org/10.1007/s10450-023-00416-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-023-00416-9