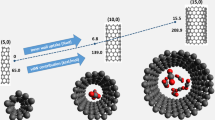
Abstract
Single-walled carbon nanotube (SWCNT) based materials are considered as one of the most promising adsorbents for adsorption-based technology for methane storage. Molecular dynamic simulations are employed to investigate the assembling of single-walled carbon nanotubes into an array via toluene coordinator-molecules. The smallest number of toluene molecules sufficient to maintain the ASWCNT + nC7H8 supramolecular structure is defined. The potential, kinetic and total energies of the simulated system plotted as a function of toluene molecules are used to evaluate the stability condition for the supramolecular structure. Methane adsorption onto the model ASWCNT + nC7H8 adsorbent is simulated to assess the contributions of toluene molecules to its adsorption capacity. The radial and angular probability density distributions of methane molecules in the ASWCNT + nC7H8 adsorbent reveal the most probable location of adsorbed methane near the SWCNT walls and toluene molecules as centers of adsorption. A SWCNT-based adsorbent is prepared by the saturation with toluene and subsequent two-step regeneration with a slow-rate increase in temperature. The content of toluene of 5wt.% in the adsorbent thus obtained is evaluated from a gain in weight observed at 338 K. Methane adsorption on the initial SWCNT and SWCNT + 5%C7H8 adsorbents is measured at 178 and 273 K up to 101 kPa. A comparison of the initial and differential molar heats of methane adsorption on the initial SWCNT and ASWCNT + nC7H8 adsorbents points to the role of toluene molecules as additional centers of adsorption. We estimate the contribution of toluene molecules to the porosity of the ASWCNT + nC7H8 adsorbent from the methane adsorption data.











Similar content being viewed by others
References
Redlin, M., Gries, T.: Anthropogenic climate change: the impact of the global carbon budget. Theor. Appl. Climatol. 146, 713–721 (2021). https://doi.org/10.1007/s00704-021-03764-0
Tsivadze, A.Y., Aksyutin, O.E., Ishkov, A.G., Men’shchikov, I.E., Fomkin, A.A., Shkolin, A.V., Khozina, E.V., Grachev, V.A.: Porous carbon-based adsorption systems for natural gas (methane) storage. Russ. Chem. Rev. 87(10), 950–983 (2018). https://doi.org/10.1070/RCR4807
Tsivadze, AYu., Aksyutin, O.E., Ishkov, A.G., Knyazeva, M.K., Solovtsova, O.V., Menshchikov, I.E., Fomkin, A.A., Shkolin, A.V., Khozina, E.V., Grachev, V.A.: Metal-organic framework structures: adsorbents for natural gas storage. Russ. Chem. Rev. 88(9), 925–978 (2019). https://doi.org/10.1070/RCR4873
Boateng, E., Chen, A.: Recent advances in nanomaterial-based solid-state hydrogen storage. Mater. Today Adv. 6, 100022 (2020). https://doi.org/10.1016/j.mtadv.2019.100022
Wu, Y., Weckhuysen, B.M.: Separation and purification of hydrocarbons with porous materials. Angew. Chem. Int. Ed. 60, 18930–18949 (2021). https://doi.org/10.1002/anie.202104318
Hinkov, I., Lamari, F.D., Langlois, P., Dicko, M., Chilev, C., Pentchev, I.: Carbon dioxide capture by adsorption (review). J. Chem. Technol 51(6), 609–626 (2016)
Patel, S.V., Patel, J.M.: Separation of high purity nitrogen from air by pressure swing S.V. adsorption on carbon molecular sieves. Int. J. Eng. Res. Technol. 3(3), 450–454 (2014)
Chuah, C.Y., Goh, K., Bae, T.-H.: Enhanced performance of carbon molecular sieve membranes incorporating zeolite nanocrystals for air separation. Membranes 11, 489 (2021). https://doi.org/10.3390/membranes11070489
Thommes, M., Kaneko, K., Neimark, A.V., Oliver, J.P., Rodrigues-Reinoso, F., Rouquerol, J., Sing, K.: Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 87, 1051–1069 (2015). https://doi.org/10.1515/pac-2014-1117
Dubinin, M.M.: Physical adsorption of gases and vapors in micropores. In: Cadenhead, D.D. (ed.) Progress in Surface and Membrane Science. Academic Press, London (1975)
Tománek, D., Jorio, A., Dresselhaus, M.S., Dresselhaus, G.: In Carbon nanotubes: advanced topics in the synthesis, structure, properties and applications. In: Jorio, A., Dresselhaus, G., Dresselhaus, M.S. (eds.) Series Topics in Applied Physics. Springer, Berlin (2008)
Radushkevich, L.V., Luk’yanovich, V.M.: The structure of carbon forming in thermal decomposition of carbon monoxide on an iron catalyst. Sov. J. Chem. Phys. 26, 88–95 (1952)
Iijima, S.: Helical microtubules of graphitic carbon. Nature 354, 56–58 (1991). https://doi.org/10.1038/354056a0
Iijima, S., Ichihashi, T.: Single shell carbon nanotubes of 1-nm diameter. Nature 363, 603–605 (1993). https://doi.org/10.1038/363603a0
Bethune, D.S., Klang, C.H., de Vries, M.S., Gorman, G., Savoy, R., Vazquez, J., Beyers, R.: Cobalt-catalyzed growth of carbon nanotubes with single-atomic-layer walls. Nature 363, 605–607 (1993). https://doi.org/10.1038/363605a0
Peigney, A., Laurent, C., Flahaut, E., Bacsa, R., Rousset, A.: Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 39, 507–514 (2001). https://doi.org/10.1016/S0008-6223(00)00155-X
Popov, V.N., Van Doren, V.E., Balkanski, M.: Elastic properties of crystals of single-walled carbon nanotubes. Solid State Commun. 114, 395–399 (2000). https://doi.org/10.1016/S0038-1098(00)00070-3
Balandin, A.A.: Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 10, 569–581 (2011). https://doi.org/10.1038/nmat3064
Wei, X., Wang, M.S., Bando, Y., Golberg, D.: Thermal stability of carbon nanotubes probed by anchored tungsten nanoparticles. Sci. Technol. Adv. Mater. (2011). https://doi.org/10.1088/1468-6996/12/4/044605
Dillon, A.C., Jones, K.M., Bekkedahl, T.A., Kiang, C.H., Bethune, D.S., Heben, M.J.: Storage of hydrogen in single-walled carbon nanotubes. Nature 386, 377–379 (1997). https://doi.org/10.1038/386377a0
Darkrim, F.L., Malbrunot, P., Tartaglia, G.P.: Review of hydrogen storage by adsorption in carbon nanotubes. Int. J. Hydrogen Energy 27, 193–202 (2002). https://doi.org/10.1016/S0360-3199(01)00103-3
Mosquera-Vargas, E., Tamayo, R., Morel, M., Roble, M., Díaz-Droguett, D.E.: Hydrogen storage in purified multi-walled carbon nanotubes: gas hydrogenation cycles effect on the adsorption kinetics and their performance. Heliyon 7, e08494 (2021). https://doi.org/10.1016/j.heliyon.2021.e08494
Targets for Onboard Hydrogen Storage Systems for Light-Duty Vehicles, https://www.energy.gov/eere/fuelcells/doe-technical-targets-onboard-hydrogen-storage-light-duty-vehicles. Accessed January 30 2022
Froudakis, G.E.: Hydrogen storage in nanotubes & nanostructures. Mater. Today 14, 324–328 (2011). https://doi.org/10.1016/S1369-7021(11)70162-6
Anikina, E., Banerjee, A., Beskachko, V., Ahuja, R.: Li-functionalized carbon nanotubes for hydrogen storage: importance of size effects. ACS Appl. Nano Mater. 2, 3021–3030 (2019). https://doi.org/10.1021/acsanm.9b00406
Global and Russian Energy Outlook 2019. Makarov, A., Mitrova, T., Kulagin, V. (eds); ERI RAS – Moscow School of Management SKOLKOVO, Moscow (2019)
Nie, Z., Lin, Y., Jin, X.: Research on the theory and application of adsorbed natural gas used in new energy vehicles: a review. Front. Mech. Eng. 11, 258–274 (2016). https://doi.org/10.1007/s11465-016-0381-2
Kumar, K.V., Preuss, K., Titirici, M.-M., Rodríguez-Reinoso, F.: Nanoporous materials for the onboard storage of natural gas. Chem. Rev. 117, 1796–1825 (2017). https://doi.org/10.1021/acs.chemrev.6b00505
Tanaka, H., El-Merraoui, E., Steele, W.A., Kaneko, K.: Methane adsorption on single-walled carbon nanotube: a density functional theory model. Chem. Phys. Lett. 352, 334–341 (2002). https://doi.org/10.1016/S0009-2614(01)01486-5
Methane Opportunities for Vehicular Energy, ARPA-E|MOVE. https://arpa-e.energy.gov/?q=arpa-e-programs/move
Bekyarova, E., Murata, K., Yudasaka, M., Kasuya, D., Iijima, S., Tanaka, H., Kahoh, H., Kaneko, K.: Single-wall nanostructured carbon for methane storage. J. Phys. Chem. B 107, 4681–4684 (2003). https://doi.org/10.1021/jp0278263)
Feng, Y., Li, Y., Yang, W., Yang, D., Ye, G., Zhang, S.: Open-tip carbon nanotubes for enhanced methane adsorption performance: a comparative study. J. Nanotechnol. (2018). https://doi.org/10.1155/2018/8905204)
Levchenko, I., Han, Z.-J., Kumar, S., Yick, S., Fang, J., Ostrikov, K. Large Arrays and Networks of Carbon Nanotubes: Morphology Control by Process Parameters, in Synthesis, and Applications of Carbon Nanotubes and Their Composites, IntechOpen, (2013) https://doi.org/10.5772/52674. Available from: https://www.intechopen.com/chapters/43663. Accessed December 01 2021.
Rybolt, T., Jordan, H.: Interactions and Binding energies in carbon nanotube bundles. Appl. Nano 2, 128–147 (2021). https://doi.org/10.3390/applnano2020011
Cao, D., Zhang, X., Chen, J., Wang, W., Yun, J.: Optimization of single-walled carbon nanotube arrays for methane storage at room temperature. J. Phys. Chem. B 107, 13286–13292 (2003). https://doi.org/10.1021/jp036094r
Duren, T., Sarkisov, L., Yaghi, O.M., Snurr, R.Q.: Design of new materials for methane storage. Langmuir 20, 2683–2689 (2004). https://doi.org/10.1021/la0355500
Shkolin, A.V., Fomkin, A.A., Strizhenov, E.M., Pulin, A.L.: Adsorption of methane on model adsorbents formed from single-wall carbon nanotubes. Prot. Met. Phys. Chem. Surf. 50, 279–286 (2014). https://doi.org/10.1134/S2070205114030186
Guo, T., Nikolaev, P., Thess, A., Colbert, D.T., Smalley, R.E.: Catalytic growth of single-walled nanotubes by laser vaporization. Chem. Phys. Lett. 243, 49 (1995). https://doi.org/10.1016/0009-2614(95)00825-O
Li, W.Z., Xie, S.S., Qian, L.X., Chang, B.H., Zou, B.S., Zhou, W.Y., Zhao, R.A., Wang, G.: Large-scale synthesis of aligned carbon nanotubes. Science 274, 1701 (1996). https://doi.org/10.1126/science.274.5293.1701
Xie, S., Li, W., Pan, Z., Chang, B., Sun, L.: Carbon nanotube arrays. Mater. Sci. Eng. A 286, 11–15 (2000). https://doi.org/10.1016/S0921-5093(00)00657-2
Prasek, J., Drbohlavova, J., Chomoucka, J., Hubalek, J., Jasek, O., Adam, V., Kizek, R.: Methods for carbon nanotubes synthesi—review. J. Mater. Chem. 21, 15872–15884 (2011). https://doi.org/10.1039/C1JM12254A
Ibrahim, K.S.: Carbon nanotubes–properties and applications: a review. Carbon Lett. 14, 131–144 (2013). https://doi.org/10.5714/CL.2013.14.3.131
Liu, T.L., Wu, H.W., Wang, C.Y., Chen, S.-Y., Hung, M.-H., Yew, T.-R.: A method to form self-aligned carbon-nanotube-vias using a Ta-cap layer on a Co-catalyst. Carbon 56, 366–373 (2013). https://doi.org/10.1016/j.carbon.2013.01.036
Georgakilas, V., Perman, J.A., Tucek, J., Zboril, R.: Broad family of carbon nanoallotropes: classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem. Rev. 115, 4744–4822 (2015). https://doi.org/10.1021/cr500304f
Shkolin, A.V., Fomkin A.A. A method for obtaining an ordered array of carbon nanotubes by using coordinator-molecules, the development of the secondary porosity in the resulting supramolecular structures, and the material obtained by this method. RF Patent 2714350. Published: 14.02.2020, Bulletin of Inventions No. 5.
Shkolin, A.V., Fomkin, A.A.: Self-organization of supramolecular microporous structures based on carbon nanotubes and benzene. Colloid. J. 78, 800–807 (2016). https://doi.org/10.1134/s1061933x16060144
Shkolin, A.V., Fomkin, A.A., Yakovlev, V.Y., Men’shchikov, I.E.: Model nanoporous supramolecular structures based on carbon nanotubes and hydrocarbons for methane and hydrogen adsorption. Colloid J. 80, 739–750 (2018). https://doi.org/10.1134/S1061933X18060157
Sone, H., Fugetsu, B., Tsukada, T., Endoc, M.: Affinity-based elimination of aromatic VOCs by highly crystalline multi-walled carbon nanotubes. Talanta 74, 1265–1270 (2008). https://doi.org/10.1016/j.talanta.2007.08.041
Bina, B., Amin, M.M., Rashidi, A., Pourzamani, H.: Benzene and toluene removal by carbon nanotubes from aqueous solution. Arch. Environ. Prot. 38, 3–25 (2012). https://doi.org/10.2478/v10265-012-0001-0
Zhou, L., Zhang, X., Yang, H., Peng, B.: Adsorption and ability to carry catalysts of carbon nanotubes for destructing dioxins. Recent Pat. Mater. Sci. 2, 226–231 (2009). https://doi.org/10.2174/1874464810902030226
Vagraftic, N.B. Spravochnik po Teplofizicheskim svoistvam gazov i zhidkostei (Handbook on thermophysical properties of gases and liquids), Nauka, Moscow, 1972, 721p. (in Russian).
Pauling, L. The Nature of the Chemical Bond and the Structure of Molecules and Crystals: An Introduction to Modern Structural Chemistry. Cornell University Press, 1960, 644p.
Kel’tsev, N.V. Osnovy adsorbtsionnoy tekhniki (Fundamental of adsorption techniques), Publisher: Khimiya, Moscow, 1976, 512p. (in Russian).
Sychev, V.V., Vasserman, A.A., Zagoruchenko, V.A., Kozlov, A.D., Spiridonov, G.A., Tzymarnyi, V.A.: Thermodynamic Properties of Methane. Izd. Standartov, Moscow (1979). (in Russian)
Rackers, J.A., Laury, M.L., Lu, C., Wang, Z., Lagardère, L., Piquemal, J.P., Ren, P., Ponder, J.W.: TINKER 8: a modular software package for molecular design and simulation. J. Chem. Theory. Comput. 14, 5273–5289 (2018). https://doi.org/10.1021/acs.jctc.8b00529
Jorgensen, W.L., Maxwell, D.-R., J.: Development and testing of the OPLS All-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 118, 11225–11236 (1996). https://doi.org/10.1021/ja9621760
Tolmachev, A.M., Anuchin, K.M., Kryuchenkova, N.G., Fomkin, A.A.: Theoretical calculation of the isotherms of adsorption on active coals using the molecular dynamics method. Prot. Met. Phys. Chem. Surf. 47, 150–155 (2011). https://doi.org/10.1134/S2070205111020195
Andersen, H.C.: Molecular dynamics simulations at constant pressure and/or temperature. J. Chem. Phys. 72, 2384–2393 (1980). https://doi.org/10.1063/1.439486
Shkolin, A.V., Fomkin, A.A.: Measurement of carbon-nanotube adsorption of energy-carrier gases for alternative energy systems. Meas. Tech. 61, 395–401 (2018)
Shkolin, A.V., Fomkin A.A., Men’shchikov, I.E., Kharitonov, V.M., Pulin, A.L. A bench for adsorption measurements of gases and vapors by the gravimetric method and the method of its operating. RF Patent 2732199. Published: 14.09.2020, Bulletin of Inventions No. 26.
GOST 34100.3-2017/ISO/IEC Guide 98-3:2008. Part 3. Uncertainty of measurement. Part 3. Guide to the expression of uncertainty in measurement. Available online: https://files.stroyinf.ru/Data/651/65118.pdf. Accessed on 18 June 2020.
Shkolin, A.V., Fomkin, A.A.: Supramolecular microporous structures based on carbon nanotubes and coordinating cumene (C9H12) molecules. Colloid. J. 79, 701–706 (2017). https://doi.org/10.1134/S1061933X17050131
Shkolin, A.V., Fomkin, A.A., Men’shchikov, I.E., Yakovlev, VYu.: Supramolecular nanoporous carbon materials based on the arrays of carbon nanotubes, ordered by cyclic hydrocarbons for methane and hydrogen storage. Mater. Today 5, 25911–25915 (2018). https://doi.org/10.1016/j.matpr.2018.08.002
Ganji, M.D., Mirnejad, A., Najafi, A.: Theoretical investigation of methane adsorption onto boron nitride and carbon nanotubes. Sci. Technol. Adv. Mat. 11, 045001 (2010).
Zhu, X., Zhao, Y.P.: Atomic mechanisms and equation of state of methane adsorption in carbon nanopores. J. Phys. Chem. C. 118, 17737–17744 (2014). https://doi.org/10.1021/jp5047003
Dubinin, M.M., Adsorbtsiya i poristost' (Adsorption and Porosity), VaKhZ, Moscow, 172p. (1972) (in Russian)
Schauer, M., Law, K.S., Bernstein, E.A.: Molecular jet study of the solvation of toluene by methane, ethane, and propane. J. Chem. Phys. 82, 736–746 (1985). https://doi.org/10.1063/1.448497
Yang, Y., Kumar, A., Nair, N., Sun, Sh.: Adsorption and diffusion of carbon dioxide, methane, and their mixture in carbon nanotubes in the presence of water. J. Phys. Chem. C. 124, 16478–16487 (2020). https://doi.org/10.1021/acs.jpcc.0c04325
Hoory, S.E., Prausnitz, J.M.: Adsorption of hydrocarbons on graphitized carbon. Trans. Faraday Soc. 63, 455–460 (1967). https://doi.org/10.1039/TF9676300455
Shkolin, A.V., Fomkin, A.A., Tsivadze, A.Y., Anuchin, K.M., Men’shchikov, I.E., Pulin, A.L.: Experimental study and numerical modeling: methane adsorption in microporous carbon adsorbent over the subcritical and supercritical temperature regions. Prot. Met. Phys. Chem. Surf. 52, 955–963 (2016). https://doi.org/10.1134/S2070205116060186
Shkolin, A.V., Fomkin, A.A.: Thermodynamics of methane adsorption on the microporous carbon adsorbent ACC. Russ. Chem. Bull. 57, 1799–1805 (2008). https://doi.org/10.1007/s11172-008-0242-1
McClellan, A.L., Harnsberger, H.F.: Cross-sectional areas of molecules adsorbed on solid surfaces. J. Colloid Interf. Sci. 23, 577–599 (1967). https://doi.org/10.1016/0021-9797(67)90204-4
Funding
The research was carried out within the State Assignment of the Russian Federation (Project No. 0081-2019-0018).
Author information
Authors and Affiliations
Contributions
Conceptualization, AVS, VVG, methodology, AVA, VVG; software, AVS, validation, AAF, IEM; formal analysis, IEM, VVG; investigation, VVG, AVS; resources, AVS; data curation, AVS, EVK; writing—original draft preparation, AVS, AAF; writing—review and editing, EVK, AVS; visualization, AVS, VVG; supervision, AAF; project administration, AAF, IEM; funding acquisition, AAF All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gaidamavichute, V.V., Shkolin, A.V., Men’shchikov, I.E. et al. A novel type microporous adsorbent based on single-walled carbon nanotubes assembled by toluene molecules for methane storage. Adsorption 29, 183–198 (2023). https://doi.org/10.1007/s10450-023-00388-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-023-00388-w