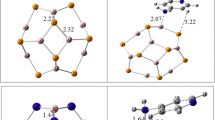
Abstract
In this work, the interaction of pyrazinamide (Pyr) drug with pristine, Sc, Ti, V and Cr-doped B12P12 nanocage is investigated by using density functional theory (DFT) at the cam-B3LYP/Lanl2DZ level of theory. From optimized structure, the adsorption energy, deformation energy, thermodynamic parameters, quantum parameters, reduced density gradient (RDG), natural bond orbital (NBO) and atom in molecule (AIM) parameters are calculated at the above level of theory. The calculated results demonstrate that with doping Ti atom the adsorption and deformation energy of Pyr/BP nanocage complex increase significantly from original values. The thermodynamic parameters revealed that adsorption of Pyr on the surface of doped models of B12P12 nanocage is more favorable than the pristine model. On the other hand, the ΔΔG(sol) values of water and ethanol solvent for adsorption of Pyr drug on the surface of pristine nanocage is negative and for Sc, Ti, V, and Cr doped B12P12 nanocage models are positive. The band gap of all adsorption models are in range 0.97–2.52 eV and the electrical and optical properties of system alter significantly from pristine models. The values of ▽2ρ and HBCP for all adsorption models are positive and negative respectively, it refers to medium strength or partially covalent bond and this result is an agreement with RDG and NBO outputs. The calculated results demonstrate that the Sc, Ti, V, and Cr doped B12P12 nanocages are a good candidate for deliver Pyr drug in the biological system.







Similar content being viewed by others
References
Bader, R.F.W.: Atoms in Molecules: A Quantum Theory. Oxford University Press, Oxford (1990)
Baei, M.T., Soltani, A.R., Torabi, P., Moradi, A.V.: Adsorption properties of SCN− on (6,0), (7,0), (8,0), and Al-doped (6,0) zigzag single-walled carbon nanotubes: a density functional study. Monatschefte Für Chem. 142, 979–984 (2011)
Beheshtian, J., Ahmadi Peyghan, A., Bagheri, Z.: Quantum chemical study of fluorinated AlN nano-cage. Appl. Surf. Sci. 259, 631–636 (2012a)
Beheshtian, J., Bagheri, Z., Kamfiroozi, M., Ahmadi, A.: A comparative study on the B12 N12, Al12 N12, B12P12 and Al12P12 fullerene-like cages. J. Mol. Model. 18, 2653–2658 (2012b)
Beheshtian, J., Kamfiroozi, M., Bagheri, Z., Ahmadi, A.: Theoretical study of hydrogen adsorption on the B12P12 fullerene-like nanocluster. Comput. Mater. Sci. 54, 115–118 (2012c)
Bulat, F.A., Toro-Labbé, A., Brinck, T., Murray, J.S., Politzer, P.: Quantitative analysis of molecular surfaces: areas, volumes, electrostatic potentials and average local ionization energies. J. Mol. Model. 16(11), 1679–1691 (2010)
Bulat, F.A., Burgess, J.S., Matis, B.R., Baldwin, J.W., Macaveiu, L., Murray, J.S., Politzer, P.: Hydrogenation and fluorination of graphene models: analysis via the average local ionization energy. J. Phys. Chem. A 116(33), 8644–8652 (2012)
Cossi, M., Barone, V.: Time-dependent density functional theory for molecules in liquid solutions. J. Chem. Phys. 115, 4708–4717 (2001)
Costales, A., Kandalam, A.K., Franco, R., Pandey, R.: Theoretical study of structural and vibrational properties of (AlP)n, (AlAs)n,(GaP)n, (GaAs)n, (InP)n, and (InAs)n clusters with n = 1, 2, 3. J. Phys. Chem. B 106, 1940–1944 (2002)
de Assis, J.L., Grobas, P.V.P., Signoretti, A.M., Fernandes, M.A.C., Miranda, B.F., Silva, R.H.F., Valverde, M., Einicker-Lamas, P.A., Beule, D.: Lipoplexes for gene delivery characterized by fluorescence correlation spectroscopy. Biophys. J. 110, 489–490 (2016)
Frisch, M.J.: GAUSSIAN 09, Revision D.01. Gaussian, Inc., Wallingford CT (2009)
Glendening, E., Reed, A., Carpenter, J., Weinhold, F.: NBO Version 3.1. Gaussian Inc., Pittsburg, PA (2003)
Hsieh, S.C., Wang, S.M., Li, F.Y.: A theoretical investigation of the effect of adsorbed NO2 molecules on electronic transport in semiconducting single-walled carbon nanotubes. Carbon 49, 955–965 (2011)
Ichida, K., Hosoyamada, M., Hisatome, I., Enomoto, A., Hikita, M., Endou, H., Hosoya, T.: Clinical and molecular analysis of patients with renal hypouricemia in Japan-influence of URAT1 gene on urinary urate excretion. J. Am. Soc. Nephrol. 15(1), 164–173 (2004)
Iqbal, M.J., Ayub, K.: Enhanced electronic and non-linear optical properties of alkali metal (Li, Na, K) doped boron nitride nano-cages. J. Alloys Compd. 687, 976–983 (2016)
Iqbal, M.J., Ludwigd, R., Ayub, K.: Phosphides or nitrides for better NLO properties? A detailed comparative study of alkali metal doped nano-cages. Mater. Res. Bull. 92, 113–122 (2017)
Johnson, E.R., Keinan, S., Mori-Sanchez, P., Contreras-Garcia, J., Cohen, A.J., Yang, W.: Revealing noncovalent interactions. J. Am. Chem. Soc. 132, 6498–6506 (2010)
Kandalam, A.K., Blanco, M.A., Pandey, R.: Theoretical study of AlnNn, GanNn, and InnNn (n = 4, 5, 6) clusters. J. Phys. Chem. B 106, 1945–1953 (2002a)
Kandalam, A.K., Blanco, M.A., Pandey, R.: Theoretical study of AlnNn, GanNn, and InnNn (n = 4, 5, 6) clusters. J. Phys. Chem. B 106, 1945–1953 (2002b)
Keresztury, G., Holly, S., Varga, J., Besenyei, G., Wang, A.V., Durig, J.R.: Vibrational spectra of monothiocarbamates-II IR and Raman spectra, vibrational assignment, conformational analysis and ab initio calculations of S-methyl-N, N. Spectrochim. Chim. Acta. 49, 2007–2017 (1993)
Li, S.: Semiconductor physical electronics, 2nd edn. Springer, Berlin (2006)
Lu, T., Chen, F.: Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012)
Na, L.J., Rang, C.Z., Fang, Y.S.: Study on the prediction of visible absorption maxima of azobenzene compounds. J. Zhejiang. Univ. Sci. 6, 584–589 (2005)
Najafi, M.: The SH functionalized B24N24 and B24P24 nanocages as potential sensor for oxygen difluoride (OF2) detection in the gas phase and methanol. Vacuum 135, 18–21 (2017)
Padash, R., Rahimi-Nasrabadi, M., Rad, A.S., Sobhani-Nasab, A., Jesionowski, T., Ehrlich, H.: Comparative computational investigation of phosgene adsorption on (XY)12 (X = Al, B and Y = N, P) nanoclusters: DFT investigations. J. Clust Sci. 30, 203–218 (2019)
Palmer, S., Sokolovski, S.G., Rafailov, E., Nabi, G.: Technologic developments in the field of photonics for the detection of urinary bladder cancer. Clin. Genitourin. Cancer 11, 390–396 (2013)
Qiang, Y., Antony, J., Sharma, A., Nutting, J., Sikes, D., Meyer, D.: Iron/iron oxide core-shell nanoclusters for biomedical applications. J. Nanoparticle Res. 8, 489–496 (2006)
Rad, A.S.: Study on the surface interaction of Furan with X12Y12 (X = B, Al, and Y = N, P) semiconductors. Heteroat. chem. 27, 316–322 (2016)
Rad, A.S., Ayub, K.: Ni adsorption on Al12P12 nano-cage: a DFT study. J. Alloys Compd. 678, 317–324 (2016)
Rad, A.S., Shabestari, S.S., Mohseni, S., Aghouzi, S.A.: Study on the adsorption properties of O3, SO2, and SO3 on B-doped graphene using DFT calculations. J. Solid State Chem. 237, 204–210 (2016)
Rad, A.S., Aghaei, S.M., Poralijan, V., Peyravi, M., Mirzae, M.: Application of pristine and Ni-decorated B12P12 nano-clusters as superior media for acetylene and ethylene adsorption: DFT calculations. Comput. Theor. Chem. 1109, 1–9 (2017)
Rakhshi, M., Mohsennia, M., Rasa, H., Rezaei Sameti, M.: First-principle study of ammonia molecules adsorption on boron nitride nanotubes in presence and absence of static electric field and ion field. Vacuum 155, 456–464 (2018)
Rezaei-Sameti, M., Amirian, B.: A quantum, NBO, RDG study of interaction cadmium ion with the pristine, C, P and C&P doped (4, 4) armchair boron nitride nanotube (BNNTs). Asian J. Nanosci. Mater. 1(4), 262–270 (2018)
Rezaei-Sameti, M., Yaghoobi, S.: Theoretical study of adsorption of CO gas on pristine and AsGa-doped (4, 4) armchair models of BPNTs. Comput. Condens. Matter. 3, 21–29 (2015)
Rezaei-Sameti, M., Zanganeh, F.: A computational study of adsorption H2S gas on the surface of the pristine, Al&P-doped armchair and zigzag BNNTs. J. Sulfur Chem. 38, 384–400 (2017)
Rezaei-Sameti, M., Zarei, P.: NBO, AIM, HOMO–LUMO and thermodynamic investigation of the nitrate ion adsorption on the surface of pristine. Al and Ga doped BNNTs: a DFT study. Adsorption 24(8), 757–767 (2018)
Rule, A.M.: American society of health-system pharmacists’ pain management network. J. Pain Palliat Care Pharmacother. 18(3), 59–62 (2004)
Shokuhi Rad, A., Ayub, K.: A comparative density functional theory study of guanine chemisorption on Al12N12, Al12P12, B12N12, and B12P12 nano-cages. J. Alloys. Compd. 672, 161–169 (2016a)
Shokuhi Rad, A., Ayub, K.: Adsorption of pyrrole on Al12N12, Al12P12, B12N12, and B12P12 fullerene-like nano-cages; a first principles study. Vacuum 131, 135–141 (2016b)
Soltani, A., Baei, M.T., Mirarab, M., Sheikhi, M., Lemeski, E.T.: The electronic and structural properties of BN and BP nano-cages interacting with OCN−: A DFT study. J. Phys. Chem. Solids 75, 1099–1105 (2014)
Spaia, S., Magoula, I., Tsapas, G., Vayonas, G.: Effect of pyrazinamide and probenecid on peritoneal urate transport kinetics during continuous ambulatory peritoneal dialysis. Perit. Dial. Int. 20(1), 47–52 (2000)
Stuart, M.C., Kouimtzi, M., Hill, S.R.: WHO Model Formulary, 136, 140, 594 (2009)
Sun, Y.T., Huang, P.Y., Lin, C.H., Lee, K.R., Lee, M.T.: Studying antibiotic-membrane interactions via X-Ray diffraction and fluorescence microscopy. Biophys. J. 110, 414–418 (2015)
Talla, J.A.: Ab initio simulations of doped single-walled carbon nanotube sensors. Chem. Phys. 392, 71–77 (2012)
Varghese, S.S., Lonkar, S., Singh, K.K., Swaminathan, S., Abdala, A.: Recent advances in graphene based gas sensors. Sens. Actuators B Chem. 218, 160–183 (2015)
Wu, H., Fan, X., Kuo, J.L.: Metal free hydrogenation reaction on carbon doped boron nitride fullerene: a DFT study on the kinetic issue. Int. J. Hydrog. Energy 37, 14336–14342 (2012)
Yong, Y., Liu, K., Song, B., He, P., Wang, P., Li, H.: Coalescence of BnNn fullerenes: a new pathway to produce boron nitride nanotubes with small diameter. Phys. Lett. A 376, 1465–1467 (2012)
Acknowledgment
The author thanks the Computational information center of Malayer University for providing the necessary facilities to carry out the research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rezaei-Sameti, M., Shiravand, E. The thermodynamic, quantum, AIM and NBO study of the interaction of pyrazinamide drug with the pristine and transition metal-doped B12P12. Adsorption 26, 955–970 (2020). https://doi.org/10.1007/s10450-019-00181-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-019-00181-8