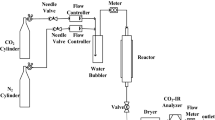
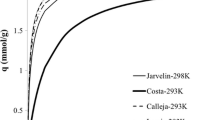
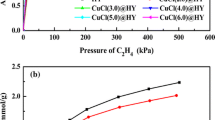
Abstract
Some potential adsorbents for ethylene/ethane separation are ethylene selective while the others are ethane selective. Among different adsorbents, i.e., zeolites and metal organic frameworks (MOFs), a comparative study is critical to find the more suitable adsorbent for the separation. In this paper, binary ethylene/ethane adsorption performances of zeolites and MOFs, i.e., equilibrium selectivities and adsorption capacities are investigated utilizing ideal adsorbed solution theory (IAST). IAST model is applied at different gas compositions (0.1–0.9 ethylene mole fractions) and pressures up to 100 kPa. The results revealed that the most selective adsorbent toward ethylene is 5A zeolite while MOFs have higher equilibrium adsorption capacities. Among zeolites and MOFs, 5A and Fe2(dobdc) have the highest selectivity (27.4 and 13.6) and capacity (≈2.8 and 5.8 mmol ethylene/g) at 100 kPa and 298 K for a 50/50 mixture. Among ethane selective adsorbents, Silicalite-1 zeolite and UTSA-33a (MOF) have the highest selectivity and capacity (≈2.9 and ≈1.5 mmol ethane/g) at 100 kPa and 298 K for a 50/50 mixture, respectively. Investigation showed that adsorption capacity of ethylene selective adsorbents is higher than that of ethane selective ones.







Similar content being viewed by others
Abbreviations
- A:
-
Adsorption surface (m2)
- b:
-
Affinity parameter of Langmuir, Toth and Sips isotherms (kPa−1)
- n:
-
Heterogeneity parameter in Sips isotherm (-)
- p:
-
Pressure (kPa)
- p0 :
-
Hypothetical pressure of the pure component (kPa−1)
- qsat :
-
Saturation adsorption capacity of adsorbent (mmol g−1)
- q:
-
Amount adsorbed (mmol g−1)
- Rg :
-
Universal gas constant (J K−1 mol−1)
- T:
-
Temperature (K)
- x:
-
Mole fraction of a pure component at the adsorbed phase (-)
- y:
-
Mole fraction of a pure component at the gas phase (-)
- z:
-
Reduced spreading pressure (mol cm−3)
- π:
-
Spreading pressure (J m−3)
References
Aguado, S., Bergeret, G.R., Daniel, C., Farrusseng, D.: Absolute molecular sieve separation of ethylene/ethane mixtures with silver zeolite A. J. Am. Chem. Soc. 134(36), 14635–14637 (2012)
Alexander, A.S., Erling, H.: Multicomponent Adsorption: principles and Models. Adsorption: theory, Modeling, and Analysis. Marcel Dekker Inc., New York (2002)
Berlier, K., Olivier, M.-G., Jadot, R.: Adsorption of methane, ethane, and ethylene on zeolite. J. Chem. Eng. Data 40(6), 1206–1208 (1995)
Bloch, E.D., Queen, W.L., Krishna, R., Zadrozny, J.M., Brown, C.M., Long, J.R.: Hydrocarbon separations in a metal-organic framework with open iron (II) coordination sites. Science 335(6076), 1606–1610 (2012)
Böhme, U., Barth, B., Paula, C., Kuhnt, A., Schwieger, W., Mundstock, A., Caro, J.R., Hartmann, M.: Ethene/ethane and propene/propane separation via the olefin and paraffin selective metal–organic framework adsorbents CPO-27 and ZIF-8. Langmuir 29(27), 8592–8600 (2013)
Choudhary, V.R., Mayadevi, S.: Adsorption of methane, ethane, ethylene, and carbon dioxide on silicalite-l. Zeolites 17(5), 501–507 (1996)
Da Silva, F.A., Rodrigues, A.E.: Adsorption equilibria and kinetics for propylene and propane over 13X and 4A zeolite pellets. Ind. Eng. Chem. Res. 38(5), 2051–2057 (1999)
Do, D.D.: Adsorption Analysis: Equilibria and Kinetics. Imperial College Press, London (1998)
Geier, S.J., Mason, J.A., Bloch, E.D., Queen, W.L., Hudson, M.R., Brown, C.M., Long, J.R.: Selective adsorption of ethylene over ethane and propylene over propane in the metal–organic frameworks M2(dobdc)(M = Mg, Mn, Fe Co, Ni, Zn). Chem. Sci. 4(5), 2054–2061 (2013)
Gücüyener, C., van den Bergh, J., Gascon, J., Kapteijn, F.: Ethane/ethene separation turned on its head: selective ethane adsorption on the metal–organic framework ZIF-7 through a gate-opening mechanism. J. Am. Chem. Soc. 132(50), 17704–17706 (2010)
Haynes, W.M.: CRC Handbook of Chemistry and Physics. CRC Press, Boca Raton (2014)
He, Y., Zhang, Z., Xiang, S., Fronczek, F.R., Krishna, R., Chen, B.: A microporous metal–organic framework for highly selective separation of acetylene, ethylene, and ethane from methane at room temperature. Chemistry 18(2), 613–619 (2012)
Lamia, N., Wolff, L., Leflaive, P., Sá Gomes, P., Grande, C.A., Rodrigues, A.E.: Propane/propylene separation by simulated moving bed I. Adsorption of propane, propylene and isobutane in pellets of 13X zeolite. Sep. Sci. Technol. 42(12), 2539–2566 (2007)
Lee, D.H., Yoon, K.B., Noh, S.K.: Polymerization of ethylene by using zirconocene catalyst anchored on silica with trisiloxane and pentamethylene spacers. Macromol. Rapid Commun. 18(5), 427–431 (1997)
Li, Y., Liang, F., Bux, H., Yang, W., Caro, J.: Zeolitic imidazolate framework ZIF-7 based molecular sieve membrane for hydrogen separation. J. Membr. Sci. 354(1), 48–54 (2010)
Maghsoudi, H.: Equilibrium adsorption analysis of microporous adsorbents in propene/propane binary mixture separation. Adsorption 21(6–7), 547–556 (2015)
Masoudi-Nejad, M., Fatemi, S.: Application of zeotype SAPO-34 molecular sieve as a selective adsorbent for separation of ethylene from ethane. Gas Process. J. 2(1), 1–7 (2014)
Moghadam, P.Z., Fairen-Jimenez, D., Snurr, R.Q.: Efficient identification of hydrophobic MOFs: application in the capture of toxic industrial chemicals. J. Mater. Chem. A 4(2), 529–536 (2016)
Myers, A., Prausnitz, J.M.: Thermodynamics of mixed-gas adsorption. AIChE J. 11(1), 121–127 (1965)
Narin, G., Martins, V.F., Campo, M., Ribeiro, A.M., Ferreira, A., Santos, J.C., Schumann, K., Rodrigues, A.E.: Light olefins/paraffins separation with 13X zeolite binderless beads. Sep. Purif. Technol. 133, 452–475 (2014)
Olson, D.H., Camblor, M.A., Villaescusa, L.A., Kuehl, G.H.: Light hydrocarbon sorption properties of pure silica Si-CHA and ITQ-3 and high silica ZSM-58. Microporous Mesoporous Mater. 67(1), 27–33 (2004)
Rege, S.U., Padin, J., Yang, R.T.: Olefin/paraffin separation by adsorption: π-complexation versus kinetic separation. AIChE J. 44(4), 799–809 (1998)
Ruthven, D.M.: Fundamentals of Adsorption Equilibrium and Kinetics in Microporous Solids. Adsorption and Diffusion, vol. 7. Springer, Berlin (2008)
Zhu, W., Kapteijn, F., Moulijn, J., Den Exter, M., Jansen, J.: Shape selectivity in adsorption on the all-silica DD3R. Langmuir 16(7), 3322–3329 (2000)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maghsoudi, H. Comparative study of adsorbents performance in ethylene/ethane separation. Adsorption 22, 985–992 (2016). https://doi.org/10.1007/s10450-016-9805-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-016-9805-x