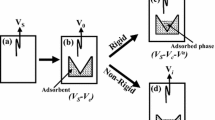
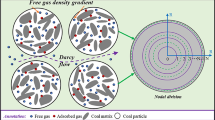
Abstract
A gravimetric method with in situ density measurement is used to determine the adsorption isotherms and kinetic characteristics of CO2 on Chinese dry coal plug at 293.29, 311.11, 332.79 and 352.57 K and pressures up to 19 MPa. The adsorption and desorption process is reversible, which shows that it is a process of physical adsorption for CO2 on coal. The excess adsorption increases with the increasing pressure at low pressures until the CO2 phase transitions pressure is reached. Above this pressure, the excess adsorption decreases with the increasing pressure. The adsorption behaviour is described using the CO2 density instead of pressure in four thermodynamic models such as modified Langmuir, Langmuir + k, DR and DR + k. It is found that the modified Langmuir + k and DR + k models are more suitable for liquid and supercritical CO2 adsorption, respectively. The adsorption kinetics data are also obtained during the measurement of adsorption isotherms. The experimental data are fluctuant at the initial time range due to the temperature variation in the adsorption cell after high-pressure CO2 is injected. The diffusivity is estimated using a modified unipore model. It is observed that the kinetic parameter C, accounting for the effect of gas diffusivity, increases with the increasing pressure at low pressures and has no obvious relations with pressure at high pressures. In this study, C value has no dependencies with temperature for CO2, and the order of magnitude of the effective diffusivity is approximately 10−5 to 10−4 s−1.









Similar content being viewed by others
References
Bae, J.S., Bhatia, S.K.: High-pressure adsorption of methane and carbon dioxide on coal. Energy Fuels 20, 2599–2607 (2006)
Bhowmik, S., Dutta, P.: Adsorption rate characteristics of methane and CO2 in coal samples from Raniganj and Jharia coalfields of India. Int. J. Coal Geol. 113, 50–59 (2013)
Busch, A., Gensterblum, Y., Krooss, B.M., Littke, R.: Methane and carbon dioxide adsorption-diffusion experiments on coal: upscaling and modeling. Int. J. Coal Geol. 60, 151–168 (2004)
Charrière, D., Pokryszka, Z., Behra, P.: Effect of pressure and temperature on diffusion of CO2 and CH4 into coal from the Lorraine basin (France). Int. J. Coal Geol. 81, 373–380 (2010)
Clarkson, C.R., Bustin, R.M.: The effect of pore structure and gas pressure upon the transport properties of coal: a laboratory and modeling study. 1. Isotherms and pore volume distributions. Fuel 78, 1333–1344 (1999a)
Clarkson, C.R., Bustin, R.M.: The effect of pore structure and gas pressure upon the transport properties of coal: a laboratory and modeling study. 2. Adsorption rate modeling. Fuel 78, 1345–1362 (1999b)
Cui, X., Bustin, M.C., Dipple, G.: Selective transport of CO2, CH4 and N2 in coals: insights from modeling of experimental gas adsorption data. Fuel 83, 293–303 (2004)
Day, S., Fry, R., Sakurovs, R.: Swelling of Australian coals in supercritical CO2. Int. J. Coal Geol. 74, 41–52 (2008)
Dreisbach, F., Seif, A.H., Lösch, H.W.: Gravimetric measurement of adsorption gas mixture CO/H2 with a magnetic suspension balance. Chem. Eng. Technol. 25, 1060–1065 (2002)
Dutta, P., Harpalani, S., Prusty, B.: Modeling of CO2 sorption on coal. Fuel 87, 2023–2036 (2008)
Fang, Z., Li, X.: A preliminary evaluation of carbon dioxide storage capacity in unmineable coalbeds in China. Acta Geotech. 9, 109–114 (2014)
Gensterblum, Y., van Hemert, P., Billemont, P., Battistutta, E., Busch, A., Krooss, B.M., Weireld, G.D., Wolf, K.H.A.A.: European inter-laboratory comparison of high pressure CO2 sorption isotherms II: natural coals. Int. J. Coal Geol. 84, 115–124 (2010)
Gensterblum, Y., Merkel, A., Busch, A., Krooss, B.M.: High-pressure CH4 and CO2 sorption isotherms as a function of coal maturity and the influence of moisture. Int. J. Coal Geol. 118, 45–57 (2013)
Harpalani, S., Basanta, K.P., Dutta, P.: Methane/CO2 sorption modeling for coalbed methane production and CO2 sequestration. Energy Fuels 20, 1591–1599 (2006)
Hol, S., Gensterblum, Y., Spiers, C.J.: Direct determination of total CO2 uptake by coal: a new technique compared with the manometric method. Fuel 105, 192–205 (2013)
Humayun, R., Tomasko, D.L.: High-resolution adsorption isotherms of supercritical carbon dioxide on activated carbon. AIChE J. 46, 2065–2075 (2000)
Kelemen, S.R., Kwiatek, L.M.: Physical properties of selected block Argonne Premium bituminous coal related to CO2, CH4, and N2 adsorption. Int. J. Coal Geol. 77, 2–9 (2009)
Krooss, B.M., van Bergen, F., Gensterblum, Y., Siemons, N., Pagnier, H.J.M., David, P.: High-pressure methane and carbon dioxide adsorption on dry and moisture-equilibrated Pennsylvanian coals. Int. J. Coal Geol. 51, 69–92 (2002)
Larsen, J.W.: The effects of dissolved CO2 on coal structure and properties. Int. J. Coal Geol. 57, 63–70 (2004)
Li, D., Liu, Q., Weniger, P., Gensterblum, Y., Busch, A., Krooss, B.M.: High-pressure sorption isotherms and sorption kinetics of CH4 and CO2 on coals. Fuel 89, 569–580 (2010)
Majewska, Z., Ziętek, J.: Changes of acoustic emission and strain in hard coal during gas sorption-desorption cycles. Int. J. Coal Geol. 70, 305–312 (2007)
Mianowski, A., Marecka, A.: The isokinetic effect as related to the activation energy for the gases diffusion in coal at ambient temperatures: part I. Fick’s diffusion parameter estimated from kinetic curves. J. Therm. Anal. Calorim. 95, 285–292 (2009)
Miknis, F.P., Netzel, D.A., Turner, T.F., Wallace, J.C., Butcher, C.H.: Effect of different drying methods on coal structure and reactivity toward liquefaction. Energy Fuels 10, 631–640 (1996)
Mohammad, S., Fitzgerald, J., Robinson, R.L., Gasem, K.A.M.: Experimental uncertainties in volumetric methods for measuring equilibrium adsorption. Energy Fuels 23, 2810–2820 (2009)
Nandi, S.P., Walker, P.L.: Activated diffusion of methane from coals at elevated pressures. Fuel 54, 81–86 (1975)
Ottiger, S., Pini, R., Storti, G., Mazzotti, M., Bencini, R., Quattrocchi, F., Sardu, G., Deriu, G.: Adsorption of pure carbon dioxide and methane on dry coal from the Sulcis coal province (SW Sardinia, Italy). Environ. Prog. 25, 355–364 (2006)
Ottiger, S., Pini, R., Storti, G., Mazzotti, M.: Competitive adsorption equilibria of CO2 and CH4 on a dry coal. Adsorption 14, 539–556 (2008)
Ozdemir, E., Morsi, B.I., Schroeder, K.: Importance of volume effects to adsorption isotherms of carbon dioxide on coals. Langmuir 19, 9764–9773 (2003)
Ozdemir, E., Morsi, B.I., Schroeder, K.: CO2 adsorption capacity of Argonne premium coals. Fuel 83, 1085–1094 (2004)
Pan, Z., Connell, L.D., Camilleri, M., Connelly, L.: Effects of matrix moisture on gas diffusion and flow in coal. Fuel 89, 3207–3217 (2010)
Pini, R., Ottiger, S., Rajendran, A., Storti, G., Mazzotti, M.: Reliable measurement of near-critical adsorption by gravimetric method. Adsorption 12, 393–403 (2006)
Romanov, V., Soong, Y.: Helium-volume dynamics of Upper Freeport coal powder and lumps. Int. J. Coal Geol. 77, 10–15 (2009)
Sakurovs, R., Day, S., Weir, S., Duffy, G.: Application of a modified Dubinin-Radushkevich equation to adsorption of gases by coals under supercritical conditions. Energy Fuels 21, 992–997 (2007)
Sakurovs, R., Day, S., Weir, S., Duffy, G.: Temperature dependence of sorption of gases by coals and charcoals. Int. J. Coal Geol. 73, 250–258 (2008)
Sakurovs, R., Day, S., Weir, S.: Relationships between the critical properties of gases and their high pressure sorption behavior on coals. Energy Fuels 24, 1781–1787 (2010)
Siemons, N., Busch, A.: Measurement and interpretation of supercritical CO2 sorption on various coals. Int. J. Coal Geol. 69, 229–242 (2007)
Siemons, N., Wolf, K.H.A.A., Bruining, J.: Interpretation of carbon dioxide diffusion behavior in coals. Int. J. Coal Geol. 72, 315–324 (2007)
Song, Y., Jian, W., Zhang, Y., Shen, Y., Zhan, Y., Zhao, J., Liu, Y., Wang, D.: Densities and volumetric characteristics of binary system of CO2 + Decane from (303.15 to 353.15) K and pressures up to 19 MPa. J. Chem. Eng. Data 57, 3399–3407 (2012)
St. George, J.D., Barakat, M.A.: The change in effective stress associated with shrinkage from gas desorption in coal. Int. J. Coal Geol. 45, 105–113 (2001)
Sudibandriyo, M., Pan, Z., Fitzgerald, J.E., Robinson, R.L., Gasem, K.A.M.: Adsorption of methane, nitrogen, carbon dioxide, and their binary mixtures on dry activated carbon at 318.2 K and pressures up to 13.6 MPa. Langmuir 9, 5323–5331 (2003)
Terzyk, A.P., Gauden, P.A.: The simple procedure of the calculation of diffusion coefficient for adsorption on spherical and cylindrical adsorbent particles. Sep. Sci. Technol. 36, 513–525 (2001)
Toribio, M.M., Oshima, Y., Shimada, S.: Evaluation of sequesterable carbon dioxide in Japanese coal samples at subcritical and supercritical conditions. Stud. Surf. Sci. Catal. 153, 375–380 (2004)
van Hemert, P., Bruining, H., Rudolph, E.S.J., Wolf, K.H.A.A., Maas, J.G.: Improved manometric setup for the accurate determination of supercritical carbon dioxide sorption. Rev. Sci. Instrum. (2009). doi:10.1063/1.3063064
Zhang, Y., Chang, F., Song, Y., Zhao, J., Zhan, Y., Jian, W.: Density of carbon dioxide + brine solution from Tianjin reservoir under sequestration conditions. J. Chem. Eng. Data 56, 565–573 (2011)
Acknowledgments
The authors thank the editor and the reviewers for the constructive comments that have helped to improve the quality of the paper. This work has been supported by the National Science and Technology Major Project of China (No. 2011ZX05026-004-07), the National Program on Key Basic Research Project (No. 2011CB707304) and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
See Table 5.
Rights and permissions
About this article
Cite this article
Song, Y., Xing, W., Zhang, Y. et al. Adsorption isotherms and kinetics of carbon dioxide on Chinese dry coal over a wide pressure range. Adsorption 21, 53–65 (2015). https://doi.org/10.1007/s10450-015-9649-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-015-9649-9