Abstract
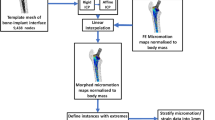
In silico clinical trials (ISCT) can contribute to demonstrating a device’s performance via credible computational models applied on virtual cohorts. Our purpose was to establish the credibility of a model for assessing the risk of humeral stem loosening in total shoulder arthroplasty, based on a twofold validation scheme involving both benchtop and clinical validation activities, for ISCT applications. A finite element model computing bone-implant micromotion (benchtop model) was quantitatively compared to a bone foam micromotion test (benchtop comparator) to ensure that the physics of the system was captured correctly. The model was expanded to a population-based approach (clinical model) and qualitatively evaluated based on its ability to replicate findings from a published clinical study (clinical comparator), namely that grit-blasted stems are at a significantly higher risk of loosening than porous-coated stems, to ensure that clinical performance of the stem can be predicted appropriately. Model form sensitivities pertaining to surgical variation and implant design were evaluated. The model replicated benchtop micromotion measurements (52.1 ± 4.3 µm), without a significant impact of the press-fit (“Press-fit”: 54.0 ± 8.5 µm, “No press-fit”: 56.0 ± 12.0 µm). Applied to a virtual population, the grit-blasted stems (227 ± 78µm) experienced significantly larger micromotions than porous-coated stems (162 ± 69µm), in accordance with the findings of the clinical comparator. This work provides a concrete example for evaluating the credibility of an ISCT study. By validating the modeling approach against both benchtop and clinical data, model credibility is established for an ISCT application aiming to enrich clinical data in a regulatory submission.








Similar content being viewed by others
References
Erickson, B. J., P. N. Chalmers, P. J. Denard, R. Gobezie, A. A. Romeo, and E. S. Lederman. Current state of short-stem implants in total shoulder arthroplasty: a systematic review of the literature. JSES Int. 4:114–119, 2020. https://doi.org/10.1016/j.jses.2019.10.112.
Australian Orthopaedic Association National Joint Replacement Registry. Hip, knee & shoulder arthroplasty: 2022 AOANJRR annual report n.d. https://aoanjrr.sahmri.com/annual-reports-2022.
Szerlip, B. W., B. J. Morris, M. S. Laughlin, C. M. Kilian, and T. B. Edwards. Clinical and radiographic outcomes after total shoulder arthroplasty with an anatomic press-fit short stem. J. Shoulder Elb. Surg. 27:10–16, 2018. https://doi.org/10.1016/j.jse.2017.08.012.
Morwood, M. P., P. S. Johnston, and G. E. Garrigues. Proximal ingrowth coating decreases risk of loosening following uncemented shoulder arthroplasty using mini-stem humeral components and lesser tuberosity osteotomy. J. Shoulder Elb. Surg. 26:1246–1252, 2017. https://doi.org/10.1016/j.jse.2016.11.041.
Zmistowski, B., D. P. Carpenter, P. N. Chalmers, M. J. Smith, and J. D. Keener. Symptomatic aseptic loosening of a short humeral stem following anatomic total shoulder arthroplasty. J. Shoulder Elb. Surg. 30:2738–2744, 2021. https://doi.org/10.1016/j.jse.2021.04.038.
Pilliar, R. M., J. M. Lee, and C. Maniatopoulos. Observations on the effect of movement on bone ingrowth into porous-surfaced implants. Clin. Orthop. Relat. Res. 208:108–113, 1986. https://doi.org/10.1097/00003086-198607000-00023.
Kohli, N., J. C. Stoddart, and R. J. van Arkel. The limit of tolerable micromotion for implant osseointegration: a systematic review. Sci. Rep. 11:1–11, 2021. https://doi.org/10.1038/s41598-021-90142-5.
Engh, C. A., P. Massin, and K. E. Suthers. Roentgenographic assessment of the biologic fixation of porous-surfaced femoral components. Clin. Orthop. Relat. Res. 257:107–128, 1990. https://doi.org/10.1097/00003086-199008000-00022.
Klim, S. M., F. Amerstorfer, G. A. Bernhardt, P. Sadoghi, G. Hauer, L. Leitner, et al. Excellent mid-term osseointegration and implant survival using metaphyseal sleeves in revision total knee arthroplasty. Knee Surg. Sport Traumatol. Arthrosc. 28:3843–3848, 2020. https://doi.org/10.1007/s00167-020-05865-1.
Sanchez-Sotelo, J., T. W. Wright, S. W. O’Driscoll, R. H. Cofield, and C. M. Rowland. Radiographic assessment of uncemented humeral components in total shoulder arthroplasty. J. Arthroplasty. 16:180–187, 2001. https://doi.org/10.1054/arth.2001.20905.
Throckmorton, T. W., P. C. Zarkadas, J. W. Sperling, and R. H. Cofield. Radiographic stability of ingrowth humeral stems in total shoulder arthroplasty. Clin. Orthop. Relat. Res. 468:2122–2128, 2010. https://doi.org/10.1007/s11999-010-1299-3.
ten Brinke, B., B. Hesseling, D. Eygendaal, M. A. Hoelen, and N. M. C. Mathijssen. Early fixation of the humeral component in stemless total shoulder arthroplasty A RADIOSTEREOMETRIC AND CLINICAL STUDY WITH 24-MONTH FOLLOW-UP. Bone Jt. J. 104:76–82, 2022. https://doi.org/10.1302/0301-620X.104B1.BJJ-2021-0945.R1.
Van de Kleut, M. L., X. Yuan, G. S. Athwal, and M. G. Teeter. Are short press-fit stems comparable to standard-length cemented stems in reverse shoulder arthroplasty? A prospective, randomized clinical trial. J. Shoulder Elb. Surg. 31:580–590, 2022. https://doi.org/10.1016/j.jse.2021.11.005.
Bieger, R., A. Ignatius, R. Decking, L. Claes, H. Reichel, and L. Dürselen. Primary stability and strain distribution of cementless hip stems as a function of implant design. Clin. Biomech. 27:158–164, 2012. https://doi.org/10.1016/j.clinbiomech.2011.08.004.
Favre, P., and A. D. Henderson. Prediction of stemless humeral implant micromotion during upper limb activities. Clin. Biomech. 36:46–51, 2016. https://doi.org/10.1016/j.clinbiomech.2016.05.003.
Al-Dirini, R. M. A., D. Huff, J. Zhang, T. Besier, J. G. Clement, and M. Taylor. Influence of collars on the primary stability of cementless femoral stems: a finite element study using a diverse patient cohort. J. Orthop. Res. 36:1185–1195, 2018. https://doi.org/10.1002/jor.23744.
Al-Dirini, R. M. A., S. Martelli, D. Huff, J. Zhang, J. G. Clement, T. Besier, et al. Evaluating the primary stability of standard vs lateralised cementless femoral stems—a finite element study using a diverse patient cohort. Clin. Biomech. 59:101–109, 2018. https://doi.org/10.1016/j.clinbiomech.2018.09.002.
Al-Dirini, R. M. A., S. Martelli, D. O’Rourke, D. Huff, J. Zhang, J. G. Clement, et al. Virtual trial to evaluate the robustness of cementless femoral stems to patient and surgical variation. J. Biomech. 82:346–356, 2019. https://doi.org/10.1016/j.jbiomech.2018.11.013.
Al-Dirini, R. M. A., S. Martelli, and M. Taylor. Computational efficient method for assessing the influence of surgical variability on primary stability of a contemporary femoral stem in a cohort of subjects. Biomech. Model. Mechanobiol. 19:1283–1295, 2020. https://doi.org/10.1007/s10237-019-01235-0.
Bischoff, J. E., O. C. O’Reilly, J. V. Nepola, and B. M. Patterson. The influence of over-reaming on stem stability in reverse shoulder arthroplasty. Semin. Arthroplast. JSES. 30:123–131, 2020. https://doi.org/10.1053/j.sart.2020.07.002.
Quental, C., J. Folgado, M. Comenda, J. Monteiro, and M. Sarmento. Primary stability analysis of stemless shoulder implants. Med. Eng. Phys. 81:22–29, 2020. https://doi.org/10.1016/j.medengphy.2020.04.009.
Bola, M., J. Simões, and A. Ramos. Finite element analysis to predict short and medium-term performance of the anatomical Comprehensive® Total Shoulder System. Comput. Methods Programs Biomed. 219:106751, 2022. https://doi.org/10.1016/j.cmpb.2022.106751.
Cunningham, D. E., G. W. Spangenberg, G. D. G. Langohr, G. S. Athwal, and J. A. Johnson. Stemless reverse humeral component neck shaft angle has an influence on initial fixation. J. Shoulder Elb. Surg. 2023. https://doi.org/10.1016/j.jse.2023.06.035.
Favre, P., G. Maquer, A. Henderson, D. Hertig, D. Ciric, and J. E. Bischoff. In silico clinical trials in the orthopedic device industry: from fantasy to reality? Ann. Biomed. Eng. 49:3213–3226, 2021. https://doi.org/10.1007/s10439-021-02787-y.
Favre, P., J. Seebeck, P. A. E. Thistlethwaite, M. Obrist, J. G. Steffens, A. R. Hopkins, et al. In vitro initial stability of a stemless humeral implant. Clin. Biomech. 32:113–117, 2016. https://doi.org/10.1016/j.clinbiomech.2015.12.004.
Awadalla, M., R. M. A. Al-Dirini, D. O’Rourke, L. B. Solomon, M. Heldreth, and M. Taylor. Influence of varying stem and metaphyseal sleeve size on the primary stability of cementless revision tibial trays used to reconstruct AORI IIA defects. A simulation study. J. Orthop. Res. 36:1876–1886, 2018. https://doi.org/10.1002/jor.23851.
Viceconti M, Henney A, Morley-Fletcher E. in silico Clinical Trials: How Computer Simulation will Transform the Biomedical Industry. Research and Technological Development Roadmap, Avicenna Consortium. Avicenna Consort 2016.
ENRICHMENT in Silico Clinical Trial Project n.d. https://mdic.org/project/enrichment-in-silico-clinical-trial-project/.
InSilicoWorld, Virtual Physiological Human institute, Avicenna Alliance. Good Simulation Practice (GSP) n.d. https://insilico.world/community/good-simulation-practice-gsp-task-force/.
Guidelines for Developing in silico evaluation 2019 (translated from Japanese) n.d. https://www.meti.go.jp/policy/mono_info_service/healthcare/iryou/downloadfiles/pdf/39_guideline.pdf.
Bodner J, Kaul V. A Framework for In Silico Clinical Trials for Medical Devices Using Concepts From Model Verification, Validation, and Uncertainty Quantification. J Verif Valid Uncertain Quantif 2022;7. https://doi.org/10.1115/1.4053565.
ASME V&V40-2018: Assessing credibility of computational modeling through verification and validation: Application to medical devices 2018.
Food and Drug Administration (FDA). Assessing the Credibility of Computational Modeling and Simulation in Medical Device Submissions 2021:1–35.
Bischoff, J. E., M. A. Dharia, and P. Favre. A risk and credibility framework for in silico clinical trials of medical devices. Comput. Methods Programs Biomed.242:107813, 2023. https://doi.org/10.1016/j.cmpb.2023.107813.
Favre, P., and J. Bischoff. Identifying the patient harms to include in an in silico clinical trial. Comput Methods Programs Biomed. 2023. https://doi.org/10.1016/j.cmpb.2023.107735.
ASTM F1839-08: Standard Specification for Rigid Polyurethane Foam for Use a Standard Material for Testing Orthopaedic Devices and Instruments. 2008.
Sawbones Biomechanical Products Catalog n.d. https://www.sawbones.com/media/assets/product/documents/biomechanical_catalog2020.pdf.
Alidousti, H., J. W. Giles, R. J. H. Emery, and J. Jeffers. Spatial mapping of humeral head bone density. J. Shoulder Elb. Surg. 26:1653–1661, 2017. https://doi.org/10.1016/j.jse.2017.03.006.
Favre, P., S. Perala, P. Vogel, S. F. Fucentese, J. R. Goff, C. Gerber, et al. In vitro assessments of reverse glenoid stability using displacement gages are misleading—recommendations for accurate measurements of interface micromotion. Clin. Biomech. 26:917–922, 2011. https://doi.org/10.1016/j.clinbiomech.2011.05.002.
Bergmann, G., F. Graichen, A. Bender, M. Kääb, A. Rohlmann, and P. Westerhoff. In vivo glenohumeral contact forces-measurements in the first patient 7 months postoperatively. J. Biomech. 40:2139–2149, 2007. https://doi.org/10.1016/j.jbiomech.2006.10.037.
Grant, J. A., N. E. Bishop, N. Götzen, C. Sprecher, M. Honl, and M. M. Morlock. Artificial composite bone as a model of human trabecular bone: the implant-bone interface. J. Biomech. 40:1158–1164, 2007. https://doi.org/10.1016/j.jbiomech.2006.04.007.
Dharia, M. A., S. Snyder, and J. E. Bischoff. Computational model validation of contact mechanics in total ankle arthroplasty. J. Orthop. Res. 38:1063–1069, 2020. https://doi.org/10.1002/jor.24551.
Hettich, G., J.-B. Weiß, B. Wünsch, and T. M. Grupp. Finite element analysis for pre-clinical testing of custom-made knee implants for complex reconstruction surgery. Appl. Sci. 12:4787, 2022. https://doi.org/10.3390/app12094787.
Luraghi, G., S. Bridio, C. Miller, A. Hoekstra, J. F. Rodriguez Matas, and F. Migliavacca. Applicability analysis to evaluate credibility of an in silico thrombectomy procedure. J. Biomech. 126:110631, 2021. https://doi.org/10.1016/j.jbiomech.2021.110631.
Aldieri, A., C. Curreli, J. A. Szyszko, A. A. La Mattina, and M. Viceconti. Credibility assessment of computational models according to ASME V&V40: application to the Bologna Biomechanical Computed Tomography solution. Comput. Methods Programs Biomed. 2023. https://doi.org/10.1016/j.cmpb.2023.107727.
Zimmer Biomet. Surgical Technique - Comprehensive Total Shoulder. 2018.
Niinomi, M., Y. Liu, M. Nakai, H. Liu, and H. Li. Biomedical titanium alloys with Young’s moduli close to that of cortical bone. Regen Biomater. 3:173–185, 2016. https://doi.org/10.1093/rb/rbw016.
Maquer, G., A. Bürki, K. Nuss, P. K. Zysset, and M. Tannast. Head-neck osteoplasty has minor effect on the strength of an ovine cam-FAI model: in vitro and finite element analyses. Clin Orthop Relat Res. 2016. https://doi.org/10.1007/s11999-016-5024-8.
Stadelmann, M. A., D. E. Schenk, G. Maquer, C. Lenherr, F. M. Buck, D. D. Bosshardt, et al. Conventional finite element models estimate the strength of metastatic human vertebrae despite alterations of the bone’s tissue and structure. Bone. 2020. https://doi.org/10.1016/j.bone.2020.115598.
Ovesy, M., J. D. Silva-Henao, J. W. A. Fletcher, B. Gueorguiev, P. K. Zysset, and P. Varga. Non-linear explicit micro-FE models accurately predict axial pull-out force of cortical screws in human tibial cortical bone. J. Mech. Behav. Biomed. Mater. 126:105002, 2022. https://doi.org/10.1016/j.jmbbm.2021.105002.
Prado, M., S. Khosla, C. Chaput, and H. Giambini. Opportunistic application of phantom-less calibration methods for fracture risk prediction using QCT/FEA. Eur. Radiol. 31:9428–9435, 2021. https://doi.org/10.1007/s00330-021-08071-w.
Ataei, A., J. Eikhout, R. G. H. van Leeuwen, E. Tanck, and F. Eggermont. The effect of variations in CT scan protocol on femoral finite element failure load assessment using phantomless calibration. PLoS ONE. 17:1–17, 2022. https://doi.org/10.1371/journal.pone.0265524.
Bayraktar, H. H., E. F. Morgan, G. L. Niebur, G. E. Morris, E. K. Wong, and T. M. Keaveny. Comparison of the elastic and yield properties of human femoral trabecular and cortical bone tissue. J. Biomech. 37:27–35, 2004. https://doi.org/10.1016/S0021-9290(03)00257-4.
Favre, P., R. Sheikh, S. F. Fucentese, and H. A. C. Jacob. An algorithm for estimation of shoulder muscle forces for clinical use. Clin. Biomech. 20:822–833, 2005. https://doi.org/10.1016/j.clinbiomech.2005.04.007.
Favre, P., M. Senteler, J. Hipp, S. Scherrer, C. Gerber, and J. G. Snedeker. An integrated model of active glenohumeral stability. J. Biomech. 45:2248–2255, 2012. https://doi.org/10.1016/j.jbiomech.2012.06.010.
Westerhoff, P., F. Graichen, A. Bender, A. Halder, A. Beier, A. Rohlmann, et al. In vivo measurement of shoulder joint loads during activities of daily living. J. Biomech. 42:1840–1849, 2009. https://doi.org/10.1016/j.jbiomech.2009.05.035.
Charlton, I. W., and G. R. Johnson. A model for the prediction of the forces at the glenohumeral joint. Proc. Inst. Mech. Eng. Part H. 220:801–812, 2006. https://doi.org/10.1243/09544119JEIM147.
Masjedi, M., and G. R. Johnson. Glenohumeral contact forces in reversed anatomy shoulder replacement. J. Biomech. 43:2493–2500, 2010. https://doi.org/10.1016/j.jbiomech.2010.05.024.
Pinto, M. C., A. T. Archie, Z. A. Mosher, E. F. Ransom, G. McGwin, E. V. Fehringer, et al. Radiographic restoration of native anatomy: a comparison between stemmed and stemless shoulder arthroplasty. J. Shoulder Elb. Surg. 28:1595–1600, 2019. https://doi.org/10.1016/j.jse.2019.01.015.
Levadnyi, I., J. Awrejcewicz, M. F. Goethel, and A. Loskutov. Influence of the fixation region of a press–fit hip endoprosthesis on the stress–strain state of the “bone–implant” system. Comput. Biol. Med. 84:195–204, 2017. https://doi.org/10.1016/j.compbiomed.2017.03.030.
Conlisk, N., C. R. Howie, and P. Pankaj. Computational modelling of motion at the bone–implant interface after total knee arthroplasty: the role of implant design and surgical fit. Knee. 24:994–1005, 2017. https://doi.org/10.1016/j.knee.2017.07.003.
Conlisk, N., C. R. Howie, and P. Pankaj. Quantification of interfacial motions following primary and revision total knee arthroplasty: a verification study versus experimental data. J. Orthop. Res. 36:387–396, 2018. https://doi.org/10.1002/jor.23653.
Wang, Y., M. Wang, C. Li, Y. Nakamura, L. Deng, G. Yamako, et al. Biomechanical effect of metal augment and bone graft on cup stability for acetabular reconstruction of total hip arthroplasty in hip dysplasia: a finite element analysis. BMC Musculoskelet. Disord. 23:1–9, 2022. https://doi.org/10.1186/s12891-022-05168-1.
Bernakiewicz, M., and M. Viceconti. The role of parameter identification in finite element contact analyses with reference to orthopaedic biomechanics applications. J. Biomech. 35:61–67, 2002. https://doi.org/10.1016/S0021-9290(01)00163-4.
Viceconti, M., R. Muccini, M. Bernakiewicz, M. Baleani, and L. Cristofolini. Large-sliding contact elements accurately predict levels of bone-implant micromotion relevant to osseointegration. J. Biomech. 33:1611–1618, 2000. https://doi.org/10.1016/S0021-9290(00)00140-8.
Berahmani, S., D. Janssen, S. van Kessel, D. Wolfson, Malefijt M. de Waal, P. Buma, et al. An experimental study to investigate biomechanical aspects of the initial stability of press-fit implants. J. Mech. Behav. Biomed. Mater. 42:177–185, 2015. https://doi.org/10.1016/j.jmbbm.2014.11.014.
ASTM F2028-17:Standard Test Methods for Dynamic Evaluation of Glenoid Loosening or Disassociation 2017.
MacLeod, A. R., G. Serrancoli, B. J. Fregly, A. D. Toms, and H. S. Gill. The effect of plate design, bridging span, and fracture healing on the performance of high tibial osteotomy plates. Bone Jt. Res. 7:639–649, 2018. https://doi.org/10.1302/2046-3758.712.BJR-2018-0035.R1.
Viceconti, M., F. Pappalardo, B. Rodriguez, M. Horner, J. Bischoff, and Tshinanu F. Musuamba. In silico trials: Verification, validation and uncertainty quantification of predictive models used in the regulatory evaluation of biomedical products. Methods. 2020. https://doi.org/10.1016/j.ymeth.2020.01.011.
Briant, P., J. E. Bischoff, M. A. Dharia, F. Le Naveaux, X. Li, S. Kulkarni, et al. Use of Real-World Data for Enhancing Model Credibility : Applications to Medical Device Development. 16:1–9, 2022. https://doi.org/10.1115/1.4053888.
Lopez Poncelas, M., L. La Barbera, J. J. Rawlinson, D. Crandall, and C. E. Aubin. Credibility assessment of patient-specific biomechanical models to investigate proximal junctional failure in clinical cases with adult spine deformity using ASME V&V40 standard. Comput. Methods Biomech. Biomed. Eng. 25:543–553, 2022. https://doi.org/10.1080/10255842.2021.1968380.
Stott, B., P. Afshari, J. Bischoff, J. Clin, A. Francois-Saint-Cyr, M. Goodin, et al. A critical comparison of comparators used to demonstrate credibility of physics-based numerical spine models. Ann. Biomed. Eng. 51:150–162, 2023. https://doi.org/10.1007/s10439-022-03069-x.
Viceconti, M., L. Emili, P. Afshari, E. Courcelles, C. Curreli, N. Famaey, et al. Possible contexts of use for in silico trials methodologies: a consensus-based review. IEEE J. Biomed. Health Inform. 25:3977–3982, 2021. https://doi.org/10.1109/JBHI.2021.3090469.
Norman, T. L., E. S. Ackerman, T. S. Smith, T. A. Gruen, A. J. Yates, J. D. Blaha, et al. Cortical bone viscoelasticity and fixation strength of press-fit femoral stems: an in-vitro model. J. Biomech. Eng. 128:13–17, 2006. https://doi.org/10.1115/1.2133766.
Dall’Ara, E., and G. Tozzi. Digital volume correlation for the characterization of musculoskeletal tissues: current challenges and future developments. Front. Bioeng. Biotechnol. 2022. https://doi.org/10.3389/fbioe.2022.1010056.
Solitro, G. F., K. Whitlock, F. Amirouche, and C. Santis. Measures of micromotion in cementless femoral stems-review of current methodologies. Biomater. Biomech. Bioeng. 3:85–104, 2016. https://doi.org/10.12989/bme.2016.3.2.085.
Denard, P. J., M. P. Noyes, J. B. Walker, Y. Shishani, R. Gobezie, A. A. Romeo, et al. Radiographic changes differ between two different short press-fit humeral stem designs in total shoulder arthroplasty. J. Shoulder Elb. Surg. 27:217–223, 2018. https://doi.org/10.1016/j.jse.2017.08.010.
Schnetzke, M., M. Loew, P. Raiss, and G. Walch. Short-stem anatomical shoulder replacement—a systematic review. Obere. Extrem. 14:139–148, 2019. https://doi.org/10.1007/s11678-019-0514-4.
Godenèche, A., J. Garret, J. Barth, A. Michelet, and L. Geais. Comparison of revision rates and radiographic observations of long and short, uncoated and coated humeral stem designs in total shoulder arthroplasty. EFORT Open Rev. 4:70–76, 2019. https://doi.org/10.1302/2058-5241.4.180046.
MacLeod, A. R., N. Peckham, G. Serrancolí, I. Rombach, P. Hourigan, V. I. Mandalia, et al. Personalised high tibial osteotomy has mechanical safety equivalent to generic device in a case–control in silico clinical trial. Commun. Med. 1:1–9, 2021. https://doi.org/10.1038/s43856-021-00001-7.
Sarrami-Foroushani, A., T. Lassila, M. MacRaild, J. Asquith, K. C. B. Roes, J. V. Byrne, et al. In-silico trial of intracranial flow diverters replicates and expands insights from conventional clinical trials. Nat. Commun. 12:1–12, 2021. https://doi.org/10.1038/s41467-021-23998-w.
Acknowledgments
The authors thank Sandeep Mani (Zimmer Biomet, Warsaw, Indiana USA) for his support with the benchtop testing.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by GM, CM, and AH. The first draft of the manuscript was written by GM, PF, and CM. Revisions were done by GM, JB, and PF. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors are employees of Zimmer Biomet, and therefore, they have received/will receive benefits for personal or professional use from Zimmer Biomet related directly or indirectly to the subject of this manuscript. The authors did not receive support from any organization for the submitted work.
Additional information
Associate Editor Elisabetta Zanetti oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix – Mesh convergence
Appendix – Mesh convergence
The mesh convergence was conducted according to the ASME V&V40 standard [32] on a representative bone model for the Abduction 60 load case. First, the element size on the contact surfaces between stem and bone, then the element size on the outer surface of the humerus, and finally, the element size of the volumes, were varied. For every level, the element size was reduced by half until mesh convergence was achieved, i.e., when the change in micromotion (QOI) compared to the next mesh iteration (Δ) reached 5% or less. The regions of the model whose element size was varied independently are presented in Fig.
9 and the impact of the element size on the QOI (micromotion) is presented in in Table
2.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maquer, G., Mueri, C., Henderson, A. et al. Developing and Validating a Model of Humeral Stem Primary Stability, Intended for In Silico Clinical Trials. Ann Biomed Eng 52, 1280–1296 (2024). https://doi.org/10.1007/s10439-024-03452-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-024-03452-w