Abstract
Research on human posture and balance control has grown in recent years, leading to continued advances in their understanding. The ability to maintain balance is attributed to the interplay of the visual, vestibular, and somatosensory systems, although an important role is also played by the auditory system. The lack or deficit in any of these systems leads to a reduced stability that may be counterbalanced by the integration of all the remaining sensory information. Auditory and vibratory stimulation have been found to be useful to enhance balance alongside daily activities either in healthy or pathological subjects; nevertheless, while widely investigated, the literature relating to these approaches is still fragmented. This review aims at addressing this by collecting, organising, and discussing all the literature to date on the effects of the various acoustic and vibratory stimulation techniques available on static upright posture in healthy subjects. In addition, this review intends to provide a solid and comprehensive starting point for all the researchers interested in these research areas. A systematic search of the literature was performed and a total of 33 articles (24 on vibratory stimulation and 9 on acoustic stimulation) were included in our analysis. For all articles, several elements were highlighted including: the study sample, the characteristics of the stimulations, the recording instruments, the experimental protocols, and outcomes. Overall, both stimulations analysed were found to have a positive effect on balance but more research is needed to align those alternative approaches to the traditional ones.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
From a pure mechanical perspective, human balance can be considered as equivalent to a condition of equilibrium, which is the state of an object when the resultant of the forces acting on it is zero.56 Human stance is however intrinsically unstable and constantly influenced by external and internal constraints, which make it necessary for the body to continuously control balance.45 This ability depends on sensory and motor processes through which the postural control mechanisms are performed.21 In more details: (i) the vestibular system provides the position of the head in space and its linear and angular acceleration; (ii) the visual system is responsible for providing information about the position of the body within the surrounding environment; (iii) the somatosensory system (or proprioception) records the position and movements of each body segment, playing a key role in maintaining balance12,19; (iv) finally the auditory system, which, even if rarely considered in balance control, contributes to the perception of the three-dimensionality of the surrounding space and is a supplementary source of information useful for maintaining balance.66
It is hypothesised that the integration among the above-mentioned systems76 enables balance control in different environmental conditions. However, with ageing the body undergoes physical and cognitive degenerative processes9,62 and the ability to integrate sensory information decreases, leading to a reduction in balance and therefore a higher risk of falling.
The risk of falling in the elderly has a considerable impact on their quality of life, both on social and economic aspects: hospitalisations due to falls count annually around 32.9% of the total.5 Moreover, after a fall, subjects report problems with mobility (70%), self-care (41%), daily activities (64%), and anxiety/depression (28%), showing how falls lead to functional limitation and a general detrimental impact on the quality of life.28
Interest on the body’s ability to maintain equilibrium has grown in recent years, leading to continued advances in the methods and approaches used to quantitatively assess it. From a biomechanical point of view, balance control is assessed by analysing the variation of the Centre of Mass (CoM), its relationship with the Base of Support (BoS), and the alignment of the Centre of Pressure (CoP) with respect to the Centre of Gravity (CoG).79 Traditional posturographic examination is performed on force platforms (considered as gold standard). Wearable inertial sensors have been increasingly used to provide similar metrics.62 Stereo-photogrammetric 3D motion capture systems are also used to investigate the control of the entire trunk posture and to obtain additional biomechanical measurements.64,72 Through these approaches, it is possible to observe and assess how the impairment of systems involved in human upright posture induces an increase in body sway and leads to greater instability.3,29,51,53,54
Sensory deficits lead to a reduction in stability, but can be re-balanced by an increase in sensory information, for example via additional auditory, visual or vibrotactile stimulations,14,31,47,73 as demonstrated by a vast body of literature, can be used as a complement to rehabilitation strategies to improve or partially restore balance control with minimal interference with common daily activities. In addition to the beneficial effects on pathological or neurological conditions such as Parkinson’s43 and Alzheimer’s23 diseases, stroke15 or sensory impairment,18,40 some evidence on the positive effect of sensory stimulation has also been reported for healthy subjects.1,16,39,55
Despite the vast literature on this topic however, the great variety of stimulation approaches available make it extremely varied and unstructured. A general uncertainty on the right protocol to use exists and is mainly related to the numerous stimuli characteristics (e.g., frequency, intensity, amplitude, association between different stimulation type).17,46,60 Moreover, it is not clear whether there is one stimulus that has a greater influence than another, or whether specific stimulation characteristics are eliciting better effects on balance than others. This uncertainty leads to poor or empirical, if non-existent use of additional sensorimotor stimulation in clinical rehabilitation of pathological conditions and moreover in healthy population.65 Acoustic and/or vibratory stimulations could be a significant aid for healthy population with increased risk47 (e.g., ageing), with minimal interference with common daily activities.
The authors of this work aimed therefore at collecting, organising, and discussing all the literature to date on the effect of the various acoustic and vibratory stimulation techniques, and the combination of both, available on static upright posture in healthy subjects. Furthermore, this work intends to highlight whether there is any key characteristic of those stimulations that may improve the effectiveness of the intervention on postural stability. The authors want to contribute to the development of innovative and comprehensive rehabilitation approaches combining new technologies alongside traditional rehabilitation protocols.
Methods
Literature Search
A database search to the latest available date (last search September 2022) was conducted to identify potentially relevant articles in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines.49 Four electronic databases (PubMed, Web of Science, Cochrane, and Scopus) were searched, using the following keywords and combination of them: Postural control, Postural stability, Balance, Upright stance, Auditory cue, Acoustic stimulation, Vibratory stimulation, Acoustic Cue, Vibratory Cue, Vibrotactile stimulation, Healthy, Vibration, Postural Response. The strings used for the PubMed database have been reported here: [(postural control) OR (postural stability) OR (postural response) OR (balance) AND (auditory cue) OR (acoustic stimulation) OR (acoustic cue) AND (vibratory cue) OR (vibratory stimulation) OR (vibrotactile stimulation) OR (vibration) AND (healthy)],[(postural control) OR (postural stability) OR (postural response) OR (balance) AND (auditory cue) OR (acoustic stimulation) OR (acoustic cue) AND (healthy)], [(postural control) OR (postural stability) OR (postural response) OR (balance) AND (vibratory cue) OR (vibratory stimulation) OR (vibrotactile stimulation) OR (vibration) AND (healthy)]. A hand search of reference lists of the retrieved papers was also additionally completed.
Study Selection and Screening Process
Studies analysing static balance control following vibratory or acoustic stimulation in healthy young and elderly adults were included in this review. Exclusion criteria include: (1) studies involving pathological subjects; (2) studies analysing the effect of sensory stimulation on gait; (3) studies evaluating the effects of stimulation on postural control in conjunction with other experimental conditions (e.g., dual task, sleep deprivation etc.). Non-English language papers, other reviews and studies published in books or conference proceedings were also excluded.
Results
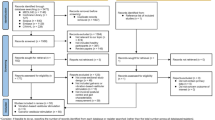
After the initial search, 932 articles were found (Fig. 1). The exclusion of duplicates reduced the number of potential articles to 631. From analysis of the titles and abstracts 33 articles were included in this review. To aid the organisation and further presentation of the literature, a subgroup analysis was carried out according to the sensory stimulation approach: retrieved studies include 24 articles utilising vibratory stimulation (see Table 1) and 9 utilising acoustic stimulation (see Table 2). No articles were found which investigated both stimulations.
For both vibratory and the auditory stimulation, the following information were retrieved and presented:
-
Participants number and cohort characteristics
-
Protocol type of session and experimental conditions
-
Characteristics of the stimulus stimulation device, positioning relative to the participant and environmental condition, stimulation intensity and frequency
-
Postural assessment device used, assessment parameters
Literature on Vibratory Stimulation
Participants
The sample size in these studies it is generally small, it ranges from 84,33 up to maximum of 70 participants.20,22,71,73 The overall age of the subjects recruited in the selected studies is also particularly heterogeneous. Most participants belonged to a middle/young age group (from 18 to 60 years). Two studies38,81 investigated the effects of vibratory stimulation on senior participants (90 years old), while in other two studies20,71 the cohorts included elderly adults (> 65 years old) with high risk of falling. Some of them compared an elderly population with an young one.2,20,22,58,71
Experimental Protocols
The current literature can be organised into three main groups, according by the number of carried trials: those with a single trial,27,30,34,69,73 two trials11,20,33,35,37,71 and four trials38,44,81 for each condition. Priplata et al. conducted their study using 10 trials for each condition in the young, and only 5 in the elderly.58 Our analyses highlighted that trials were generally conducted during the same day with non-substantial differences in duration and number or breaks between each trial; only in two studies the vibratory stimulation was applied for 1 h,27,34 while two other studies applied the stimulus according to a pseudorandom binary sequence (PRBS) providing different durations to each stimulation.7,22,25 Table 1 reports the details of all the studies.
Open eyes/closed eyes (OE-CE) approach was the most frequently adopted experimental condition to assess differences with and without the visual feedback. Some authors also explored additional experimental conditions, e.g., examining postural differences with and without vibratory stimulation or applying more than one frequency of stimulation to the subject. Furthermore, two studies included experimental conditions to alter proprioception, either through an oscillating surface or a sponge under the feet.32,68
Characteristics of the Stimulus
In the majority of the studies, the stimulation device is referred as vibrator,2,22,27,30,32,48,70 mechanical vibrators,11,50,75 focal vibrator20,71 or generically stimulator. In four articles33,35,36,44 the authors resorted to the use of particular types of tactors (C2-EAI Inc.) and tactaid: tactors attached to the subject's skin with medical tape. In four studies38,50,58,81 the vibratory stimulus was generated by a vibratory insole located under the subject’s foot plant.
Analysing the differences between the positioning of the stimulation device on to the subjects' bodies, essentially two areas of the body were subjected more to vibratory stimulation: the legs (mainly the gastrocnemius) and the trunk (on the neck, left and right internal/external oblique and erector spinae). Less commonly, other muscles exposed to the vibratory stimulation were the tensor of fasciae latae,27 the lumbar multifidus,30 the soleus,11,75 the tibialis anterior57,75 and the tricipes surae.32,50 Additionally, several studies applied the vibratory stimulations on the Achilles tendon directly.2,34,48,68
The range of frequencies adopted varied from 30 up to 500 Hz. In almost all the studies, the authors performed protocols of stimulation using different frequencies in different sessions.
The amplitude of the mechanical stimulus was not always reported. Of the selected articles, most authors used stimulation 1 mm.11,25,48,75 Ito et al. reported a vibratory stimulation with amplitude of 1.6 mm,30 and Thompson of 1.5 mm,68 while Kiers and Naka used an amplitude of 0.5 mm32,50; one study used a very small amplitude 200 µm.44 In two cases38,81 the authors applied vibratory stimulation at fraction of the stimulus perceptibility threshold (i.e. 0, 70, and 85%) for individual subjects but they did not specify any characteristics of the stimulus (e.g., frequency or amplitude).
Recording Equipment
Regarding the recording tools adopted in the studies, the force platform is the most widely used tool to assess postural information with different information analysed (e.g., stabilogram diffusion function). Other recording tools included: 3D motion systems58,68; an accelerometer48; a tri-axial gyroscope20,71; a WII balance platform30; a set of computerised dynamic posturography tests33; a potentiometer27; IMU (inertial measurement unit)35,36 and an electromyography (EMG).34
Outcomes
In a fair number of articles, the outcomes investigated were the variation of the CoP, its displacement in the anterior/posterior and medio/lateral direction2,11,30,48,50,57,68,75 and its velocity.32,38,48,75 Some outcomes investigated the CoG variations, such as CoG sway, the displacement of the projection of CoG,27 the sway velocity which analyses the CoG sway distance, divided by the test duration (cm/s) and the body tilt which represents the average of CoG location during the trial.71 Some other authors used other quantities, such as the root mean square of the A/P sways,33,35,36 the power spectral density of the sway (PSD)35,36 or the multiscale entropy (MSE), to quantify the complexity of the postural sway.81 Furthermore, Kinnaird33 and Lee36,37 used the 95% confidence interval ellipse to analyse postural sway by defining their outcomes postural shift vector33 and sway area35,36 respectively. Ehsani et al.20 evaluated parameters such as the local control slope and the central control slope which provided information about the characteristic of the body sway. Lastly, Gomez et al., evaluated the antero-posterior body position calculating the mean angular position of the head, shoulder, hip and knee.25
Literature on Auditory Stimulation
Participants
Unlike the articles on vibratory stimulation, the number of participants recruited was found to be slightly higher: samples included at least 11 subjects52 but no more than 50.74 There was a prevalent presence of healthy young group aged between 18 and 38 years old. Two studies analysed elderly people with a mean age of 68.9 + 4.0 years old67 and 78.67 years old.61 Furthermore, only one paper considered a control group, comparing the differences in postural responses between young and old subjects.67 Table 2 reports all the details of the selected papers.
Experimental Protocols
Focusing on the experimental execution, the experiments were generally conducted on the same day and the number of trials varied according to the different experimental conditions ranging from 152,67 to 6 trials.24 For 3 out of the 9 selected papers we found 5 trials for each condition.6,24,61 The duration of each trial did not exceed one minute. In each study, authors decided to adopt more than one experimental condition, which may be categorized into three main groups: (1) open eyes/closed eyes; (2) with and without auditory stimulation; (3) recording on a normal surface and on a foam surface to verify whether a change in proprioception may lead to a further alteration in the response to the auditory stimulus.
Characteristics of the Stimulus
The authors decided to pursue two different approaches: those who decided to use loudspeakers, which ensure the sound diffusion throughout the room, and those who used earphones, which isolate the subject from the external environment and produce sound only at the level of the subject's ears. The auditory stimulus is classified mostly according to its intensity, being for most of studies white noise (i.e. wideband). The analysis of the selected studies showed that some authors reported the auditory impulse at a specific decibel (Db) level, thus measuring the noise level in an absolute way.52,61,67,80 Others, such as Gandemer24 used DbA, which accounts for the distance of the subject from the sound source. In only three papers6,41,52 the authors specified specific frequencies for the auditory stimulus.
Recording Equipment
Even for auditory stimulation, it emerged that the force plate is by far the most widely adopted recording tool. Other recording instruments used were: Vertiguard system, which is a small box fixed to the subject’s waist trough an elastic band that measures the trunk’s momentary angular velocity in the A/P and M/L directions to the hip6; a cranio-corpography positioned on the participant’s head which provides an image of subject’s movement pattern63 and infrared-system for the position of the head.80
Outcomes
The majority of the outcomes assessed the different CoP variations depending on the acoustic stimulation such as the CoP path length,52,74 and the length of A/P and M/L sway,52 and the area within the sway path which assessed the position of the CoP.67 Other authors considered velocity as their principal outcome: Tanaka et al. analysed the mean sway velocity, while Anton et al.6 the angular velocity of trunk movements. Other outcomes included are: the distance of displacement, the angle of displacement and the angle of rotation which were the three main results of the Fukuda test.63
Discussion
The literature is quite varied: each author pursued different paths in terms of parameters assessed, stimulation instruments and their location, resulting in the development of extremely varied scenarios. In the following we have tried to extract clear take-aways from the existing literature for each of the stimulation approaches and the combination of both.
Effects of Vibratory Stimulation
Out of 24 papers analysed, different results emerged on the effect of vibratory stimulation on balance. Some studies shown that vibratory stimulation can promote a reduction of postural sway,38,70,71 others highlighted an increase in sway with forward or backward body tilt.2,7,11,81 Reductions in body sway are mainly appreciable in elderly population with high risk of falling, while less than 10% of young and older subjects showed a small reduction of post stimulation sways.20 Some authors found the most significant results when the frequency of stimulation applied, regardless of the location, was 30 Hz20,30,81 and this may be explained as lower frequencies seem to act directly on somatosensory system26 and especially the Meissner corpuscles.30 Kinnaird et al. highlighted reduced AP oscillation and a smaller 95th percentile confidence interval ellipse obtained using stimuli producing an opposite sway (i.e. when the subjects moved away from the vibratory stimulus33). This may be explained from a cognitive point of view, as the stimulation may have been perceived as a threat, so the natural reaction is to move away from it. Lee et al.,37 found that young adults respond with an increased postural shift (with higher RMS sways during vibration compared to pre and post vibration) in the direction of the stimulus but no change in CoP displacement. Similar results were also found by Martin et al.,44 who reported that vibration induced a trunk inclination, but neither the 95th percentile confidence interval ellipse of the CoP and the CoP shift vector changed significantly during vibration compared to the pre vibration period or between two consecutive stimulations. These results may seem in contrast one another, although an increase in CoP displacement may not necessarily be detrimental to stability, especially if this is accompanied by an increase in muscular activity. Increased muscular activation may allow for a stronger movement response (thus COP and COG displacement), while also contributing to increase stability with counteracting involvement during postural perturbations.
Stimulation Targets and Postural Response
Literature analysis also revealed a variety of target locations of vibratory stimulation, which influence the postural control response.25 All the article analysing the effect of vibration on the Achilles tendon highlighted a backward tilt of the body. According to Abrahamova et al., body tilt seems to depend on stimulation frequency and age.2 They showed that older participants respond to Achille’s tendon vibration with a greater inclination compared to young ones, and that this inclination increases with the increase of frequency of stimulation. This result is intuitively confirmed by the different trunk posture in the two groups. In fact, although the participants had a similar biomechanical response at leg level, the trunk position in elderly followed the direction of the tilt of the legs, while displaying a compensatory movement with an increased hip flexion in young participants. This compensation allowed the young group greater verticality during the stand position and a better postural adaptation.
Vibration of the distal tendon of tibialis anterior and extensor digitorum longus causes an altered proprioception (illusionary sensation) of the lower-extremities; this influences the nature of the information coming from the neuromuscular spindles to maintain balance59 and may be used as balance challenge.37 However, this seems to be age dependent: in the elderly population, as the spindle activity is weaker, vibration elicits less illusory disturbances, and therefore appear to act on a more tactile proprioceptive level. This might be also the reason why the older population respond better to this type of vibration in posture balance recovery approaches. Noteworthy, according to Ito et al., the older population rely much more on proprioceptive information derived from Meissner and Pacini corpuscles to regulate their postural responses with respect to the neuromuscular spindles. Therefore, a training program based on the reinforcement of the proprioceptive skills in the elderly may be helpful in fostering better postural control.
Stimulation at the level of the erector spinae and the internal oblique induce postural shifts oriented in the direction of the stimulus.35,36 Martin et al.44 proved that vibratory stimulation applied on the trunk also seem to elicit or enhance proprioceptive inputs. Cutaneous receptors act as a reference system of the upper body in the space.44
Feet plant stimulation has also been reported to reduce of postural sway as well as CoP displacement in several studies.13,58,77 In a particular case, vibratory insole stimulation has been found to promote a reduction in ML postural sway38,81 (found particularly sensitive to changes in skin somatosensitive sensibility) especially when the stimulation frequency was at 70 or 85% of each subject's threshold value.38,81 In this case a stochastic resonance (SR) stimulation was used (a particular low level of white noise to enhance the detection of a weak signal).13 In particular the SR emphasises the detection of sub-threshold signals maintaining the responsiveness of biological systems, such as the vestibular, the visual and the somatosensory systems, to external stimulations.78 Partial simulation of the foot’s sole produce a body reaction in the opposite direction to that of the stimulation (i.e. a rearfoot vibration produce a forward whole-body tilt with increased flexion in trunk, hip, and ankle).11 The nervous system may perceive the vibration as an increase in pressure, hence, responding with a body tilt in the opposite direction to rebalance it to maintain verticality.68 Postural adaptations seem faster when stimulations are applied at the neck compared to the calf (most notably with closed eyes).
The Long-Term Memory Effect of the Vibratory Stimulation
Tjernstorm et al. also evaluated the effect of repeated vibratory stimulation over time. In their study the vibration was applied for 5 consecutive days toward the calf muscle of both legs at a frequency of 85 Hz, and on each successive trial the subject performed better (reduction of the total and low frequency body sway) than the previous69 showing an habituation effect. In addition, their effect largely remained at 90 days. These results suggested that vibratory stimulation could promote the development of a long-term memory for postural adjustments.8,10
Auditory Stimulation
From the 9 papers related to the auditory stimulation included in this review, the main effect that has emerged is that an auditory stimulus plays an important role in postural control with a reduction of the sway oscillations.24 This could be considered for therapeutic purposes especially in elderly people at high risk of falling, considering the typical age-related changes in balance.
Source Information
Nevertheless, Anton et al. suggested that the effects of acoustic stimulation depend on a number of variables such as the structure of the auditory signal, the sensorimotor conditions of the subject and the nature of the surrounding environment (the greater the auditory environment the better the balance).6 Auditory stimuli, which provide information about the surrounding environment, can be used as an additional source of information. In fact, some of the studies analysed showed an increase in postural sway when auditory input was reduced or excluded through the use of headphones or soundproof rooms.24,41,63,74 According to Maheu and colleagues, participants using headphones to neutralise pink noise emitted by loudspeakers are inclined to engage in sensory reweighting, shifting more attention to visual inputs.41
Integration of Auditory and Other Sensory System
Several papers have investigated the influence of each of the different sensory systems during the use of an auditory stimulus. All the papers analysing the influence of vision on posture, through the condition of open and closed eyes (OE and CE), agreed that with closed eyes the sways were greater, both in the presence and in the absence of an acoustic stimulus.61,74,80 Ross61 and Zhong80 showed that although there is an increase in stability in the acoustic stimulus condition, both in the basic static condition61 and during the Tandem Romberg test and the Fukuda test,80 the impact on balance is less than in the visual system. Ross et al. also showed that the acoustic stimulus, with both OE and CE condition, had beneficial effects in both the young and elderly population.61 With regard to alterations of the somatosensory system, several studies investigated the condition of the foam under the feet in order to reduce proprioceptive information.6,24,41,67,74 A reduction in proprioception caused by the sponge, which in turn implies a further reduction in sensory information, led to an increase in postural oscillations. However, in the case of a concomitant auditory stimulus this instability decreases.74 Tanaka et al. showed a reduction in lateral oscillations in an elderly population subjected to a reduction in the sensory tactile and an acoustic stimulus.67 With regard to sensory interference on stability, a further study that is in agreement with the previous works is Kanegaonkar et al., according to which postural control is reduced following acoustic stimuli, even in the case of a reduction in other sensory inputs, suggesting that they can be a useful tool for improving the condition of global balance.31
Stimulus Characteristics
Gandemer et al. focused mainly on the type of acoustic stimulus emitted and the number of acoustic sources. In their work, divided into two experiments, using environmental stimuli that are often present in everyday life (e.g. the noise of a car motor, or the sounds of insects) they found that the greater the number of acoustic sources, the greater the stability.24 This is in line with the work of Easton et al., who confirmed that the more spatial information, the greater the ability to control posture.18 Furthermore, Gandemer et al., although they analysed a stationary acoustic stimulus, assumed that moving the head during acoustic delivery, recreating a moving stimulus, would result in more spatial information, increasing postural benefits.24 This assumption was studied by Vitkovic74 and Tanaka67 as well. Vitkovic et al. showed that among the four conditions tested (with headphones, environmental, stationary sound and moving sound), first of all the conditions of acoustic stimulation were those in which there was greater stability, and that the moving stimulus seems to have a more beneficial effect than the stationary one, even in conditions of sensory deprivation (eyes closed and on a foam).74 Tanaka et al. also conducted their experiment with a moving stimulus, clockwise and counterclockwise.67 As mentioned above, the beneficial effect of the stimulus is present in the elderly population by reducing lateral oscillations, which are the ones most likely to be associated with a high risk of falling.42 Although a beneficial effect of such a stimulus is clear, this study did not compare the moving stimulus with a stationary one, which might be able to support the thesis of Vitkovic et al.74 and Gandemer et al.24
Another characteristic of the acoustic stimulus investigated was the effect of a continuous or interrupted stimulus. Many of the reported articles showed that continuous noise (white or pink noise)41,63,74 is able to increase stability. In disagreement with this is the work of Anton et al. who reported an improvement in stability in the case of an interrupted stimulus, and a worsening of the continuous stimulus.6 However, this is probably due to the type of analysis performed. In fact, while most of the works carry out a posturographic examination by means of a force platform, they investigated angular velocity through an instrument that is placed on the torso, much closer to the centre of gravity of the body, and therefore probably less sensitive to body oscillations.
Stimulation Frequency
Finally, the work of Park et al. investigated different frequencies and stimulus pressure.52 Comparing four different types of frequencies (1000, 2000, 3000 and 4000 Hz) and three different sound intensities (45, 90 and 120 Db), antero-posterior oscillations increased with increasing frequency, although a stimulus with a frequency of 2000 Hz induced greater stability than all the others, including the lower one (of 1000 Hz). In contrast, sound pressure did not seem to interfere with postural control.
Conclusions
The literature found is extremely inhomogeneous, and further studies should refer to any review on these topics before designing similar trials. Overall, both stimulations analysed were found to have a positive effect on balance so more research is needed to align those alternative approaches to the traditional ones.
Regarding the vibratory stimulus, the main positive effects were found with a stimulation of the cutaneous receptors, rather than the deeper proprioceptive stimulation which appear to be destabilising. Indeed, better results were obtained with low frequency (30 Hz), which may promote earlier activation of skin receptors, in particular the Meissner corpuscles, than neuromuscular spindles, which are activated more slowly. Moreover, this can be a significant factor in the elderly population, as the neuromuscular spindles can often be compromised with the ageing process. Another advantageous approach could be the use of shoe insoles that exploit the principle of stochastic resonance, which could be beneficial without affecting daily activities. In contrast, other papers highlighted an increase of postural inclinations and sways during a vibratory stimulation. Although these results are usually considered detrimental to stability, it should be considered that, certain types of vibration cause the muscles contraction, which, on the one hand leads to increased movement during standing, but on the other hand leads to an increased ability to compensate and maintain balance during disturbances.
Acoustic stimulation was not found to have the same impact as a somatosensory or visual input, although was also found to improve postural control. Providing information about the environment, we found that the more acoustic information, and therefore the more acoustic sources present, the greater the effect of such a stimulation. Lower frequencies (e.g., 1000 Hz) were also shown to have greater efficacy. Therefore, although it is a useful tool for improving balance, it may be most helpful in case of people with sensory deficits, and therefore with reduced sensory information.
References
Abduljawad, K. A., R. W. Langley, C. M. Bradshaw, et al. The effects of attractive vs. repulsive instructional cuing on balance performance. Exp Brain Res. 13(1):61–66, 2014. https://doi.org/10.3233/VES-170601.
Abrahámová, D., M. Mancini, F. Hlavačka, and L. Chiari. The age-related changes of trunk responses to Achilles tendon vibration. Neurosci. Lett. 467(3):220–224, 2009. https://doi.org/10.1016/j.neulet.2009.10.041.
Agmon, M., L. Lavie, and M. Doumas. The association between hearing loss, postural control, and mobility in older adults: a systematic review. J. Am. Acad. Audiol. 28(6):575–588, 2017. https://doi.org/10.3766/jaaa.16044.
Allum, J. H. J. J., M. G. Carpenter, B. C. Horslen, et al. The effects of attractive vs repulsive instructional cuing on balance performance. Exp. Brain Res. 9(4):61–66, 2014. https://doi.org/10.3233/VES-170601.
Ambrose, A. F., G. Paul, and J. M. Hausdorff. Risk factors for falls among older adults: a review of the literature. Maturitas. 75(1):51–61, 2013.
Anton, K., A. Ernst, and D. Basta. Auditory influence on postural control during stance tasks in different acoustic conditions. J. Vestib. Res.-Equilib. Orientat. 29(6):287–294, 2019. https://doi.org/10.3233/VES-190674.
Barollo, F., R. Friðriksdóttir, K. J. Edmunds, et al. Postural control adaptation and habituation during vibratory proprioceptive stimulation: an HD-EEG investigation of cortical recruitment and kinematics. IEEE Trans. Neural Syst. Rehabil. Eng. 28(6):1381–1388, 2020. https://doi.org/10.1109/TNSRE.2020.2988585.
Bliss, T. V. P., and G. L. Collingridge. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 361(6407):31–39, 1993.
Borzuola, R., A. Giombini, G. Torre, et al. Central and peripheral neuromuscular adaptations to ageing. J. Clin. Med. 9(3):741, 2020.
Brashers-Krug, T., R. Shadmehr, and E. Bizzi. Consolidation in human motor memory. Nature. 382(6588):252–255, 1996.
Capicíková, N., L. Rocchi, F. Hlavacka, L. Chiari, and A. Cappello. Human postural response to lower leg muscle vibration of different duration. Physiol. Res. 55(Suppl 1):S129–S134, 2006. https://doi.org/10.33549/physiolres.930000.55.s1.129.
Cimadoro, G., C. Paizis, G. Alberti, and N. Babault. Effects of different unstable supports on EMG activity and balance. Neurosci. Lett. 548:228–232, 2013.
Collins, J. J., T. T. Imhoff, and P. Grigg. Noise-enhanced tactile sensation. Nature. 1996
Coste, A., R. N. Salesse, M. Gueugnon, L. Marin, and B. G. Bardy. Standing or swaying to the beat: discrete auditory rhythms entrain stance and promote postural coordination stability. Gait Posture. 2018(59):28–34, 2017. https://doi.org/10.1016/j.gaitpost.2017.09.023.
Cunha, B. P., S. R. Alouche, I. M. G. Araujo, and S. Freitas. Individuals with post-stroke hemiparesis are able to use additional sensory information to reduce postural sway. Neurosci. Lett. 513(1):6–11, 2012.
Dettmer, M., A. Pourmoghaddam, B. C. Lee, and C. S. Layne. Associations between tactile sensory threshold and postural performance and effects of healthy aging and subthreshold vibrotactile stimulation on postural outcomes in a simple dual task. Curr. Gerontol. Geriatr. Res. 2016. https://doi.org/10.1155/2016/9797369.
Di Iorio, F., M. Cesarelli, P. Bifulco, A. Fratini, E. Roveda, and M. Ruffo. The effect of whole body vibration on oxygen uptake and electromyographic signal of the rectus femoris muscle during static and dynamic squat. J. Exerc. Physiol. Online. 15(5):18–31, 2012.
Easton, R. D., A. J. Greene, P. DiZio, and J. R. Lackner. Auditory cues for orientation and postural control in sighted and congenitally blind people. Exp. Brain Res. 118(4):541–550, 1998.
Edmunds, K. J., H. Petersen, M. Hassan, et al. Cortical recruitment and functional dynamics in postural control adaptation and habituation during vibratory proprioceptive stimulation. J. Neural Eng. 16(2):026037, 2019. https://doi.org/10.1088/1741-2552/ab0678.
Ehsani, H., J. Mohler, V. Marlinski, E. Rashedi, and N. Toosizadeh. The influence of mechanical vibration on local and central balance control. J. Biomech. 71:59–66, 2018. https://doi.org/10.1016/j.jbiomech.2018.01.027.
Fransson, P. A., A. Hafström, M. Karlberg, M. Magnusson, A. Tjäder, and R. Johansson. Postural control adaptation during galvanic vestibular and vibratory proprioceptive stimulation. IEEE Trans. Biomed. Eng. 50(12):1310–1319, 2003. https://doi.org/10.1109/TBME.2003.819851.
Fransson, P. A., E. K. Kristinsdottir, A. Hafström, M. Magnusson, and R. Johansson. Balance control and adaptation during vibratory perturbations in middle-aged and elderly humans. Eur. J. Appl. Physiol. 91(5–6):595–603, 2004. https://doi.org/10.1007/s00421-003-1013-1.
Gago, M. F., V. Fernandes, J. Ferreira, et al. Role of the visual and auditory systems in postural stability in alzheimer’s disease. J. Alzheimers Dis. 46(2):441–449, 2015. https://doi.org/10.3233/JAD-150131.
Gandemer, L., G. Parseihian, R. Kronland-Martinet, and C. Bourdin. Spatial cues provided by sound improve postural stabilization: evidence of a spatial auditory map? Front Neurosci. 2017. https://doi.org/10.3389/fnins.2017.00357.
Gomez, S., M. Patel, M. Magnusson, L. Johansson, E. J. Einarsson, and P. A. Fransson. Differences between body movement adaptation to calf and neck muscle vibratory proprioceptive stimulation. Gait Posture. 30(1):93–99, 2009. https://doi.org/10.1016/j.gaitpost.2009.03.009.
Gravelle, D. C., C. A. Laughton, N. T. Dhruv, et al. Noise-enhanced balance control in older adults. Neuroreport. 13(15):1853–1856, 2002.
Gurfinkel, V. S., Y. P. Ivanenko, and Y. S. Levik. The influence of head rotation on human upright posture during balanced bilateral vibration. Neuroreport. 7(1):137–140, 1995.
Hartholt, K. A., E. F. van Beeck, S. Polinder, et al. Societal consequences of falls in the older population: injuries, healthcare costs, and long-term reduced quality of life. J. Trauma Acute Care Surg. 71(3):748–753, 2011.
Ishida, A., T. Masuda, H. Inaoka, and Y. Fukuoka. Stability of the human upright stance depending on the frequency of external disturbances. Med. Biol. Eng. Comput. 46(3):213–221, 2008.
Ito, T., Y. Sakai, A. Kubo, et al. The relationship between physical function and postural sway during local vibratory stimulation of middle-aged people in the standing position. J. Phys. Ther. Sci. 26(10):1627–1630, 2014. https://doi.org/10.1589/jpts.26.1627.
Kanegaonkar, R. G., K. Amin, and M. Clarke. The contribution of hearing to normal balance. J. Laryngol. Otol. 126(10):984–988, 2012. https://doi.org/10.1017/S002221511200179X.
Kiers, H., S. Brumagne, J. van Dieën, and L. Vanhees. Test–retest reliability of muscle vibration effects on postural sway. Gait Posture. 40(1):166–171, 2014. https://doi.org/10.1016/j.gaitpost.2014.03.184.
Kinnaird, C., J. Lee, W. J. Carender, M. Kabeto, B. Martin, and K. H. Sienko. The effects of attractive vs. repulsive instructional cuing on balance performance. J. NeuroEng. Rehabil. 13(1):1–5, 2016. https://doi.org/10.1186/s12984-016-0131-z.
Lapole, T., F. Canon, and C. Pérot. Acute postural modulation of the soleus H-reflex after Achilles tendon vibration. Neurosci. Lett. 523(2):154–157, 2012. https://doi.org/10.1016/j.neulet.2012.06.067.
Lee, B. C., B. J. Martin, and K. H. Sienko. Directional postural responses induced by vibrotactile stimulations applied to the torso. Exp. Brain Res. 222(4):471–482, 2012. https://doi.org/10.1007/s00221-012-3233-2.
Lee, B. C., B. J. Martin, and K. H. Sienko. The effects of actuator selection on non-volitional postural responses to torso-based vibrotactile stimulation. J. NeuroEng. Rehabil. 10(1):1–10, 2013. https://doi.org/10.1186/1743-0003-10-21.
Lee, B. C., B. J. Martin, and K. H. Sienko. Comparison of non-volitional postural responses induced by two types of torso based vibrotactile stimulations. In: 2012 IEEE Haptics Symposium, HAPTICS 2012; 2012, pp. 195–198.https://doi.org/10.1109/HAPTIC.2012.6183790
Lipsitz, L. A., M. Lough, J. Niemi, T. Travison, H. Howlett, and B. Manor. A shoe insole delivering subsensory vibratory noise improves balance and gait in healthy elderly people. Arch. Phys. Med. Rehabil. 96(3):432–439, 2015. https://doi.org/10.1016/j.apmr.2014.10.004.
Lubetzky, A. V., M. Gospodarek, L. Arie, J. Kelly, A. Roginska, and M. Cosetti. Auditory input and postural control in adults: a narrative review. JAMA Otolaryngol.-Head Neck Surg. 146(5):480–487, 2020. https://doi.org/10.1001/jamaoto.2020.0032.
Ma, C.Z., A. H. P. Wan, D. W. C. Wong, Y. P. Zheng, and W. C. C. Lee. Improving postural control using a portable plantar pressure-based vibrotactile biofeedback system. In: 3rd IEEE Conference on Biomedical Engineering and Sciences, IECBES 2014. Institute of Electrical and Electronics Engineers Inc.; 2014, pp. 855–860. https://doi.org/10.1109/IECBES.2014.7047632
Maheu, M., A. Sharp, S. P. Landry, and F. Champoux. Sensory reweighting after loss of auditory cues in healthy adults. Gait Posture. 53:151–154, 2017. https://doi.org/10.1016/j.gaitpost.2017.01.015.
Maki, B. E., P. J. Holliday, and A. K. Topper. A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. J. Gerontol. 49(2):M72–M84, 1994.
Marchese, R., M. Diverio, F. Zucchi, C. Lentino, and G. Abbruzzese. The role of sensory cues in the rehabilitation of parkinsonian patients: a comparison of two physical therapy protocols. Mov. Disord. 15(5):879–883, 2000.
Martin, B. J., B. C. Lee, and K. H. Sienko. A cutaneous positioning system. Exp. Brain Res. 233(4):1237–1245, 2015. https://doi.org/10.1007/s00221-014-4194-4.
Massion, J. Movement, posture and equilibrium: interaction and coordination. Prog. Neurobiol. 38(1):35–56, 1992.
Minino, R., E. Troisi Lopez, A. Polverino, A. Romano, L. Mandolesi, and M. Liparoti. Rhythmic acoustic stimulation and balance in a group of young athletes: a pilot study. J. Phys. Educ. Sport. 2022.
Minino, R., E. Troisi Lopez, P. Sorrentino, et al. The effects of different frequencies of rhythmic acoustic stimulation on gait stability in healthy elderly individuals: a pilot study. Sci. Rep. 11(1):19530, 2021. https://doi.org/10.1038/s41598-021-98953-2.
Mohapatra, S., V. Krishnan, and A. S. Aruin. Postural control in response to an external perturbation: effect of altered proprioceptive information. Exp. Brain Res. 217(2):197–208, 2012. https://doi.org/10.1007/s00221-011-2986-3.
Moher, D., A. Liberati, J. Tetzlaff, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement (Chinese edition). J. Chin. Integr. Med. 7(9):889–896, 2009.
Naka, M., K. Fujiwara, and N. Kiyota. Postural responses to various frequencies of vibration of the triceps surae and forefoot sole during quiet standing. Perception. 44(1):39–51, 2015. https://doi.org/10.1068/p7738.
Nashner, L. M., F. O. Black, and C. Wall. Adaptation to altered support and visual conditions during stance: patients with vestibular deficits. J. Neurosci. 2(5):536–544, 1982. https://doi.org/10.1523/jneurosci.02-05-00536.1982.
Park, S., K. Lee, T. Lockhart, and S. Kim. Effects of sound on postural stability during quiet standing. J. NeuroEng. Rehabil. 8(1):1–5, 2011. https://doi.org/10.1186/1743-0003-8-67.
Peterka, R. J., and M. S. Benolken. Role of somatosensory and vestibular cues in attenuating visually induced human postural sway. Exp. Brain Res. 105(1):101–110, 1995.
Peterka, R. J., and F. O. Black. Age-related changes in human posture control: sensory organization tests. J. Vestib. Res. 1(1):73–85, 1990.
Petersen, H., M. Magnusson, R. Johansson, M. Akesson, and P. A. Fransson. Acoustic cues and postural control. Scand. J. Rehabil. Med. 27(2):99–104, 1995.
Pollock, A. S., B. R. Durward, P. J. Rowe, and J. P. Paul. What is balance? Clin. Rehabil. 14(4):402–406, 2000.
Polónyová, A., and F. Hlava. Human postural responses to different frequency vibrations of lower leg muscles. Physiol. Res. 50:6, 2001.
Priplata, A., J. Niemi, M. Salen, J. Harry, L. A. Lipsitz, and J. J. Collins. Noise-enhanced human balance control. Phys. Rev. Lett. 89(23):238101, 2002.
Roll, J. P., J. P. Vedel, and E. Ribot. Alteration of proprioceptive messages induced by tendon vibration in man: a microneurographic study. Exp. Brain Res. 76(1):213–222, 1989.
Romano, M., A. Fratini, G. D. Gargiulo, M. Cesarelli, L. Iuppariello, and P. Bifulco. On the power spectrum of motor unit action potential trains synchronized with mechanical vibration. IEEE Trans. Neural. Syst. Rehabil. Eng. 26(3):646–653, 2018. https://doi.org/10.1109/TNSRE.2018.2803019.
Ross, J. M., O. J. Will, Z. McGann, and R. Balasubramaniam. Auditory white noise reduces age-related fluctuations in balance. Neurosci. Lett. 630:216–221, 2016. https://doi.org/10.1016/j.neulet.2016.07.060.
Rucco, R., A. Sorriso, M. Liparoti, et al. Type and location of wearable sensors for monitoring falls during static and dynamic tasks in healthy elderly: a review. Sensors. 18(5):1613, 2018.
Seiwerth, I., J. Jonen, T. Rahne, et al. Influence of hearing on vestibulospinal control in healthy subjects. Hno. 66(8):49–55, 2018. https://doi.org/10.1007/s00106-018-0520-7.
Sorrentino, P., A. Barbato, L. Del Gaudio, et al. Impaired gait kinematics in type 1 Gaucher’s disease. J. Park. Dis. 6(1):191–195, 2016. https://doi.org/10.3233/JPD-150660.
Stenhagen, M., H. Ekström, E. Nordell, and S. Elmståhl. Accidental falls, health-related quality of life and life satisfaction: a prospective study of the general elderly population. Arch. Gerontol. Geriatr. 58(1):95–100, 2014.
Stevens, M. N., D. L. Barbour, M. P. Gronski, and T. Hullar. Auditory contributions to maintaining balance. J. Vestib. Res. Equilib. Orientat. 26(5–6):433–438, 2017.
Tanaka, T., S. Kojima, H. Takeda, S. Ino, and T. Ifukube. The influence of moving auditory stimuli on standing balance in healthy young adults and the elderly. Ergonomics. 44(15):1403–1412, 2001. https://doi.org/10.1080/00140130110110601.
Thompson, C., M. Bélanger, and J. Fung. Effects of plantar cutaneo-muscular and tendon vibration on posture and balance during quiet and perturbed stance. Hum. Mov. Sci. 30(2):153–171, 2011. https://doi.org/10.1016/j.humov.2010.04.002.
Tjernström, F., P. A. Fransson, A. Hafström, and M. Magnusson. Adaptation of postural control to perturbations—a process that initiates long-term motor memory. Gait Posture. 15(1):75–82, 2002. https://doi.org/10.1016/s0966-6362(01)00175-8.
Tjernstrom, F., P. A. Fransson, and A. Magnusson. Improved postural control through repetition and consolidation. J. Vestib. Res.-Equilib. Orientat. 15(1):31–39, 2005.
Toosizadeh, N., H. Ehsani, M. Miramonte, and J. Mohler. Proprioceptive impairments in high fall risk older adults: the effect of mechanical calf vibration on postural balance. Biomed. Eng. 17(1):51, 2018. https://doi.org/10.1186/s12938-018-0482-8.
Troisi Lopez, E., R. Minino, P. Sorrentino, et al. A synthetic kinematic index of trunk displacement conveying the overall motor condition in Parkinson’s disease. Sci. Rep. 11(1):1–11, 2021. https://doi.org/10.1038/s41598-021-82348-4.
Uimonen, S., M. Sorri, K. Laitakari, and T. Jämsä. A comparison of three vibrators in static posturography: the effect of vibration amplitude on body sway. Med. Eng. Phys. 18(5):405–409, 1996. https://doi.org/10.1016/1350-4533(96)00079-3.
Vitkovic, J., C. Le, S. L. Lee, and R. A. Clark. The contribution of hearing and hearing loss to balance control. Audiol. Neurotol. 21(4):195–202, 2016. https://doi.org/10.1159/000445100.
Vuillerme, N., F. Danion, N. Forestier, and V. Nougier. Postural sway under muscle vibration and muscle fatigue in humans. Neurosci. Lett. 333(2):131–135, 2002. https://doi.org/10.1016/S0304-3940(02)00999-0.
Wade, M. G., and G. Jones. The role of vision and spatial orientation in the maintenance of posture. Phys. Ther. 77(6):619–628, 1997.
Wanderley, F. S., F. Alburquerque-Sendín, N. A. Parizotto, and J. R. Rebelatto. Effect of plantar vibration stimuli on the balance of older women: a randomized controlled trial. Arch. Phys. Med. Rehabil. 2(92):199–206, 2011. https://doi.org/10.1016/j.apmr.2010.10.014.
White, O., J. Babič, C. Trenado, L. Johannsen, and N. Goswami. The promise of stochastic resonance in falls prevention. Front. Physiol. 9:1865, 2019. https://doi.org/10.3389/fphys.2018.01865.
Winter, D. A., A. E. Patla, J. S. Frank, and S. E. Walt. Biomechanical walking pattern changes in the fit and healthy elderly. Phys. Ther. 70(6):340–347, 1990.
Zhong, X., and W. A. Yost. Relationship between postural stability and spatial hearing. J. Am. Acad. Audiol. 24(9):782–788, 2013. https://doi.org/10.3766/jaaa.24.9.3.
Zhou, J., L. Lipsitz, D. Habtemariam, and B. Manor. Sub-sensory vibratory noise augments the physiologic complexity of postural control in older adults. J. NeuroEng. Rehabil. 13(1):1–8, 2016. https://doi.org/10.1186/s12984-016-0152-7.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Stefan M. Duma oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Minino, R., Romano, A., Troisi Lopez, E. et al. The Effects of Vibratory and Acoustic Stimulations on Postural Control in Healthy People: A Systematic Review. Ann Biomed Eng 51, 643–659 (2023). https://doi.org/10.1007/s10439-023-03136-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-023-03136-x