Abstract
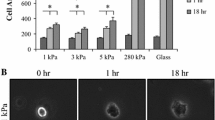
Macrophage to foam cell transition and their accumulation in the arterial intima are the key events that trigger atherosclerosis, a multifactorial inflammatory disease. Previous studies have linked arterial stiffness and cardiovascular disease and have highlighted the use of arterial stiffness as a potential early-stage marker. Yet the relationship between arterial stiffness and atherosclerosis in terms of macrophage function is poorly understood. Thus, it is pertinent to understand the mechanobiology of macrophages to clarify their role in plaque advancement. We explore how substrate stiffness affects proliferation of macrophages and foam cells, traction forces exerted by macrophages and uptake of native and oxidized low-density lipoproteins. We demonstrate that stiffness influences foam cell proliferation under both naïve and inflammatory conditions. Naïve foam cells proliferated faster on the 4 kPa polyacrylamide gel and glass whereas under inflammatory conditions, maximum proliferation was recorded on glass. Macrophage and foam cell traction forces were positively correlated to the substrate stiffness. Furthermore, the influence of stiffness was demonstrated on the uptake of lipoproteins on macrophages treated with lipopolysaccharide + interferon gamma. Cells on softer 1 kPa substrates had a significantly higher uptake of low-density lipoproteins and oxidized low-density lipoproteins compared to stiffer substrates. The results herein indicate that macrophage function is modulated by stiffness and help better understand ways in which macrophages and foam cells could contribute to the development and progression of atherosclerotic plaque.






Similar content being viewed by others
References
Adlerz, K. M., H. Aranda-Espinoza, and H. N. Hayenga. Substrate elasticity regulates the behavior of human monocyte-derived macrophages. Eur. Biophys. J. 45(4):301–309, 2016. https://doi.org/10.1007/s00249-015-1096-8.
Avolio, A. P., S. G. Chen, R. P. Wang, C. L. Zhang, M. F. Li, and M. F. O’Rourke. Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation. 68(1):50–58, 1983. https://doi.org/10.1161/01.CIR.68.1.50.
Benjamin, E. J., et al. Heart Disease and Stroke Statistics ’2017 Update: a report from the American Heart Association. Circulation. 135(10):e146–e603, 2017.
Blacher, J., B. Pannier, A. P. Guerin, S. J. Marchais, M. E. Safar, and G. M. London. Carotid arterial stiffness as a predictor of cardiovascular and all-cause mortality in end-stage renal disease. Hypertension. 32(3):570–574, 1998. https://doi.org/10.1161/01.HYP.32.3.570.
Cecelja, M., and P. Chowienczyk. Role of arterial stiffness in cardiovascular disease. JRSM Cardiovasc. Dis. 1(4):1–10, 2012. https://doi.org/10.1258/cvd.2012.012016.
Chen, M., et al. Substrate stiffness modulates bone marrow-derived macrophage polarization through NF-κB signaling pathway. Bioact. Mater. 5(4):880–890, 2020. https://doi.org/10.1016/j.bioactmat.2020.05.004.
Drew, A. F., and P. G. Tipping. T helper cell infiltration and foam cell proliferation are early events in the development of atherosclerosis in cholesterol-fed rabbits. Arterioscler. Thromb. Vasc. Biol. 15(10):1563–1568, 1995. https://doi.org/10.1161/01.ATV.15.10.1563.
Engler, A. J., M. A. Griffin, S. Sen, C. G. Bönnemann, H. L. Sweeney, and D. E. Discher. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J. Cell Biol. 166(6):877–887, 2004. https://doi.org/10.1083/jcb.200405004.
Frank, P. G., H. Lee, D. S. Park, N. N. Tandon, P. E. Scherer, and M. P. Lisanti. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 24(1):98–105, 2004. https://doi.org/10.1161/01.ATV.0000101182.89118.E5.
Goswami, R., et al. TRPV4 calcium-permeable channel is a novel regulator of oxidized LDL-induced macrophage foam cell formation. Free Radic. Biol. Med. 110(April):142–150, 2017. https://doi.org/10.1016/j.freeradbiomed.2017.06.004.
Gotschy, A., et al. Local arterial stiffening assessed by MRI precedes atherosclerotic plaque formation. Circ. Cardiovasc. Imaging. 6(6):916–923, 2013. https://doi.org/10.1161/CIRCIMAGING.113.000611.
Hall, M. S., et al. Fibrous nonlinear elasticity enables positive: mechanical feedback between cells and ECMs. Proc. Natl Acad. Sci. U. S. A. 113(49):14043–14048, 2016. https://doi.org/10.1073/pnas.1613058113.
Hansen, L., and W. R. Taylor. Is increased arterial stiffness a cause or consequence of atherosclerosis? Atherosclerosis. 249:226–227, 2016. https://doi.org/10.1016/j.atherosclerosis.2016.04.014.
Hind, L. E., M. Dembo, and D. A. Hammer. Macrophage motility is driven by frontal-towing with a force magnitude dependent on substrate stiffness. Integr. Biol. (U.K.). 7(4):447–453, 2015. https://doi.org/10.1039/c4ib00260a.
Holvoet, P., et al. Identifying patients with coronary artery disease. Atheroscler. Thromb. Vasc. Biol. 21:844–848, 2001.
Hui, K. L., L. Balagopalan, L. E. Samelson, and A. Upadhyaya. Cytoskeletal forces during signaling activation in Jurkat T-cells. Mol. Biol. Cell. 26(4):685–695, 2015. https://doi.org/10.1091/mbc.E14-03-0830.
Irwin, E. F., K. Saha, M. Rosenbluth, L. J. Gamble, D. G. Castner, and K. E. Healy. Modulus-dependent macrophage adhesion and behavior. J. Biomater. Sci. Polym. Ed. 19(10):1363–1382, 2008.
Kraning-Rush, C. M., J. P. Califano, and C. A. Reinhart-King. Cellular traction stresses increase with increasing metastatic potential. PLoS ONE. 2012. https://doi.org/10.1371/journal.pone.0032572.
Lamharzi, N., et al. Hyperlipidemia in concert with hyperglycemia stimulates the proliferation of macrophages in atherosclerotic lesions: Potential role of glucose-oxidized LDL. Diabetes. 53(12):3217–3225, 2004. https://doi.org/10.2337/diabetes.53.12.3217.
Le Master, E., S. J. Ahn, and I. Levitan. Mechanisms of endothelial stiffening in dyslipidemia and aging: Oxidized lipids and shear stress. Curr. Top. Membr. 86:185–215, 2020. https://doi.org/10.1016/bs.ctm.2020.08.006.
Li, J., et al. miRNA-mediated macrophage behaviors responding to matrix stiffness and ox-LDL. J. Cell. Physiol. 235(9):6139–6153, 2020. https://doi.org/10.1002/jcp.29543.
Libby, P., P. M. Ridker, and A. Maseri. Inflammation and atherosclerosis. Circulation. 105(9):1135–1143, 2002. https://doi.org/10.1161/hc0902.104353.
Lo Sardo, V., et al. Unveiling the role of the most impactful cardiovascular risk locus through haplotype editing. Cell. 175(7):1796-1810.e20, 2018. https://doi.org/10.1016/j.cell.2018.11.014.
London, G. M., et al. Cardiac and arterial interactions in end-stage renal disease. Kidney Int. 50(2):600–608, 1996. https://doi.org/10.1038/ki.1996.355.
Mangge, H. Antioxidants, inflammation and cardiovascular disease. World J. Cardiol. 6(6):462, 2014. https://doi.org/10.4330/wjc.v6.i6.462.
Marinkovic, A., J. D. Mih, J. A. Park, F. Liu, and D. J. Tschumperlin. Improved throughput traction microscopy reveals pivotal role for matrix stiffness in fibroblast contractility and TGF-β responsiveness. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012. https://doi.org/10.1152/ajplung.00108.2012.
Martinez, F. O., et al. Genetic programs expressed in resting and IL-4 alternatively activated mouse and human macrophages: similarities and differences. Blood. 121(9):57–69, 2013. https://doi.org/10.1182/blood-2012-06-436212.
Mathur, A. B., A. M. Collinsworth, W. M. Reichert, W. E. Kraus, and G. A. Truskey. Endothelial, cardiac muscle and skeletal muscle exhibit different viscous and elastic properties as determined by atomic force microscopy. J. Biomech. 34(12):1545–1553, 2001. https://doi.org/10.1016/S0021-9290(01)00149-X.
Mattace-Raso, F. U. S., et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 113(5):657–663, 2006. https://doi.org/10.1161/CIRCULATIONAHA.105.555235.
McKenzie, A. J., S. R. Hicks, K. V. Svec, H. Naughton, Z. L. Edmunds, and A. K. Howe. The mechanical microenvironment regulates ovarian cancer cell morphology, migration, and spheroid disaggregation. Sci. Rep. 8(1):1–21, 2018. https://doi.org/10.1038/s41598-018-25589-0.
Mekhdjian, A. H., et al. Integrin-mediated traction force enhances paxillin molecular associations and adhesion dynamics that increase the invasiveness of tumor cells into a three-dimensional extracellular matrix. Mol. Biol. Cell. 28(11):1467–1488, 2017. https://doi.org/10.1091/mbc.E16-09-0654.
Moeller, A., K. Ask, D. Warburton, J. Gauldie, and M. Kolb. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int. J. Biochem. Cell Biol. 40(3):362–382, 2008. https://doi.org/10.1016/j.biocel.2007.08.011.
Moore, K. J., F. J. Sheedy, and E. A. Fisher. Macrophages in atherosclerosis: a dynamic balance. Nat. Rev. Immunol. 13(10):709–721, 2013. https://doi.org/10.1038/nri3520.
Müller, C., and T. Pompe. Distinct impacts of substrate elasticity and ligand affinity on traction force evolution. Soft Matter. 12(1):272–280, 2015. https://doi.org/10.1039/c5sm01706h.
Okamoto, T., et al. Reduced substrate stiffness promotes M2-like macrophage activation and enhances peroxisome proliferator-activated receptor γ expression. Exp. Cell Res. 367(2):264–273, 2018. https://doi.org/10.1016/j.yexcr.2018.04.005.
Patel, N. R., et al. Cell elasticity determines macrophage function. PLoS ONE. 7(9):1–10, 2012. https://doi.org/10.1371/journal.pone.0041024.
Pelham, R. J., and Y. L. Wang. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl Acad. Sci. U. S. A. 94(25):13661–13665, 1997. https://doi.org/10.1073/pnas.94.25.13661.
Peloquin, J., J. Huynh, R. M. Williams, and C. A. Reinhart-King. Indentation measurements of the subendothelial matrix in bovine carotid arteries. J. Biomech. 44(5):815–821, 2011. https://doi.org/10.1016/j.jbiomech.2010.12.018.
Radmacher, M., M. Fritz, C. M. Kacher, J. P. Cleveland, and P. K. Hansma. Measuring the viscoelastic properties of human platelets with the atomic force microscope. Biophys. J. 70(1):556–567, 1996. https://doi.org/10.1016/S0006-3495(96)79602-9.
Razafiarison, T., et al. Biomaterial surface energy-driven ligand assembly strongly regulates stem cell mechanosensitivity and fate on very soft substrates. Proc. Natl Acad. Sci. U. S. A. 115(18):4631–4636, 2018. https://doi.org/10.1073/pnas.1704543115.
Robbins, C. S., et al. Accumulation in atherosclerosis. Nat. Med. 19(9):1166–1172, 2014. https://doi.org/10.1038/nm.3258.Local.
Ross, R. Inflammation or atherogenesis. N. Engl. J. Med. 340(1999):115–126, 1999.
Rougerie, P., and D. Cox. Spatio-temporal mapping of mechanical force generated by macrophages during FcγR-dependent phagocytosis reveals adaptation to target stiffness. bioRxiv. 2020. https://doi.org/10.1101/2020.04.14.041335.
Sakai, M., S. Kobori, A. Miyazaki, and S. Horiuchi. Macrophage proliferation in atherosclerosis. Curr. Opin. Lipidol. 11(5):503–509, 2000.
Sato, M., K. Nagayama, N. Kataoka, M. Sasaki, and K. Hane. Local mechanical properties measured by atomic force microscopy for cultured bovine endothelial cells exposed to shear stress. J. Biomech. 33(1):127–135, 2000. https://doi.org/10.1016/S0021-9290(99)00178-5.
Scott, R. A., K. L. Kiick, and R. E. Akins. Substrate stiffness directs the phenotype and polarization state of cord blood derived macrophages. Acta Biomater. 122:220–235, 2021. https://doi.org/10.1016/j.actbio.2020.12.040.
Shiffman, D., et al. Large scale gene expression analysis of cholesterol-loaded macrophages. J. Biol. Chem. 275(48):37324–37332, 2000. https://doi.org/10.1074/jbc.M004732200.
Song, Z., et al. Identification of foam cell biomarkers by microarray analysis. BMC Cardiovasc. Disord. 20(1):1–9, 2020. https://doi.org/10.1186/s12872-020-01495-0.
Spann, N. J., et al. “Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 151(1):138–152, 2013. https://doi.org/10.1016/j.cell.2012.06.054.Regulated.
Spiller, K. L., et al. Differential gene expression in human, murine, and cell line-derived macrophages upon polarization. Exp. Cell Res. 347(1):1–13, 2016. https://doi.org/10.1016/j.yexcr.2015.10.017.
Sridharan, R., B. Cavanagh, A. R. Cameron, D. J. Kelly, and F. J. O’Brien. Material stiffness influences the polarization state, function and migration mode of macrophages. Acta Biomater. 89:47–59, 2019. https://doi.org/10.1016/j.actbio.2019.02.048.
Stehouwer, C. D. A., R. M. A. Henry, and I. Ferreira. Arterial stiffness in diabetes and the metabolic syndrome: a pathway to cardiovascular disease. Diabetologia. 51(4):527–539, 2008. https://doi.org/10.1007/s00125-007-0918-3.
Tedla, Y. G., Y. Yano, M. Carnethon, and P. Greenland. Association between long-term blood pressure variability and 10-year progression in arterial stiffness. Hypertension. 69(1):118–127, 2017. https://doi.org/10.1161/HYPERTENSIONAHA.116.08427.
Thumkeo, D., et al. Targeted disruption of the mouse rho-associated kinase 2 gene results in intrauterine growth retardation and fetal death. Mol. Cell. Biol. 23(14):5043–5055, 2003. https://doi.org/10.1128/mcb.23.14.5043-5055.2003.
Tracqui, P., A. Broisat, J. Toczek, N. Mesnier, J. Ohayon, and L. Riou. Mapping elasticity moduli of atherosclerotic plaque in situ via atomic force microscopy. J. Struct. Biol. 174(1):115–123, 2011. https://doi.org/10.1016/j.jsb.2011.01.010.
Trichet, L., et al. Evidence of a large-scale mechanosensing mechanism for cellular adaptation to substrate stiffness. Proc. Natl Acad. Sci. U. S. A. 109(18):6933–6938, 2012. https://doi.org/10.1073/pnas.1117810109.
Vappou, J., J. Luo, and E. E. Konofagou. Pulse wave imaging for noninvasive and quantitative measurement of arterial stiffness in vivo. Am. J. Hypertens. 23(4):393–398, 2010. https://doi.org/10.1038/ajh.2009.272.
Wada, T., et al. Correlation of ultrasound-measured common carotid artery stiffness with pathological findings. Arterioscler. Thromb. 14(3):479–482, 1994. https://doi.org/10.1161/01.atv.14.3.479.
Wang, A. Y. M. Cardiovascular risk in diabetic end-stage renal disease patients. J. Diabetes. 3(2):119–131, 2011. https://doi.org/10.1111/j.1753-0407.2011.00113.x.
Wilkinson, I. B., and C. M. Mceniery. Arterial stiffness, endothelial function and novel pharmacological approaches. Pulse. 31(11):795–799, 2003.
Zhou, D. W., T. T. Lee, S. Weng, J. Fu, and A. J. García. Effects of substrate stiffness and actomyosin contractility on coupling between force transmission and vinculin-paxillin recruitment at single focal adhesions. Mol. Biol. Cell. 28(14):1901–1911, 2017. https://doi.org/10.1091/mbc.E17-02-0116.
Acknowledgments
The authors thank laboratory members Maziyar Keshavarzian, Alyssa Lamberti, Kaylie Kruppa, Dhivya Addula, Julia Mach for their assistance in imaging and FACS experimentation and analysis. We also thank Jacob Henderson from the Flow cytometry Core for helping with the FACS experiments. Lastly, we are extremely grateful to Dr. Adam J. Engler and Jaimie Mayner for providing us with the TimelapseTFMcode for the traction force data analysis.
Funding
This work was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health (NIH) under award number R01HL136776, and American Heart Association (AHA) under Award No. 17SDG33400239. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or AHA.
Conflict of Interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Stefan M. Duma oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ammanamanchi, M., Maurer, M. & Hayenga, H.N. Inflammation Drives Stiffness Mediated Uptake of Lipoproteins in Primary Human Macrophages and Foam Cell Proliferation. Ann Biomed Eng 49, 3425–3437 (2021). https://doi.org/10.1007/s10439-021-02881-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-021-02881-1