Abstract
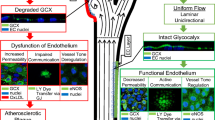
Therapies for atherosclerotic cardiovascular disease should target early disease stages and specific vascular sites where disease occurs. Endothelial glycocalyx (GCX) degradation compromises endothelial barrier function and increases vascular permeability. This initiates pro-atherosclerotic lipids and inflammatory cells to penetrate vessel walls, and at the same time this can be leveraged for targeted drug delivery. In prior cell culture studies, GCX degradation significantly increased endothelial cell uptake of nanoparticle vehicles that are designed for drug delivery, compared to the effects of intact GCX. The present study assessed if the cell culture findings translate to selective nanoparticle uptake in animal vessels. In mice, the left carotid artery (LCA) was partially ligated to disturb blood flow, which induces GCX degradation, endothelial dysfunction, and atherosclerosis. After ligation, the LCA vessel wall exhibited a loss of continuity of the GCX layer on the intima. 10-nm gold nanospheres (GNS) coated with polyethylene glycol (PEG) were delivered intravenously. GCX degradation in the ligated LCA correlated to increased GNS infiltration of the ligated LCA wall. This suggests that GCX dysfunction, which coincides with atherosclerosis, can indeed be targeted for enhanced drug delivery, offering a new approach in cardiovascular disease therapy.








Similar content being viewed by others
Abbreviations
- ANOVA:
-
Analysis of variance
- BSA:
-
Bovine serum albumin
- DAPI:
-
4′,6-diamidino-2-phenylindole
- DLS:
-
Dynamic light scattering
- ECA:
-
External carotid artery
- EGM-2:
-
Endothelial cell growth medium-2
- GCX:
-
Glycocalyx
- GNS:
-
Gold nanospheres
- HepIII:
-
Heparinase III
- HS:
-
Heparan sulfate
- HUVEC:
-
Human umbilical vein endothelial cells
- IACUC:
-
Institutional Animal Care and Use Committee
- ICA:
-
Internal carotid artery
- LCA:
-
Left carotid artery
- NHS:
-
N-Hydroxysuccinimide
- OA:
-
Occipital artery
- OCT:
-
Optimal cutting temperatire
- PBS:
-
Phosphate buffered saline
- PEG:
-
Polyethylene glycol
- RCA:
-
Right carotid artery
- SEM:
-
Standard error of the mean
- SH–PEG–COOH:
-
PEG with a thiol (SH) group and a carboxyl (COOH) group
- SH–PEG–NH2 :
-
PEG with a thiol (SH) group and an amide (NH2) group
- SH–PEG–OCH3 :
-
PEG with a thiol (SH) group and a methoxy (OCH3) group
- TEM:
-
Transmission electron microscopy
- THPC:
-
Tetrakis-(hydroxymethyl)-phosphonium chloride
- TSA:
-
Tyramide signal amplification
- WGA:
-
Wheat germ agglutinin
References
Alexis, F., E. Pridgen, L. K. Molnar, and O. C. Farokhzad. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 5:505–515, 2008.
Becker, B. F., M. Jacob, S. Leipert, A. H. Salmon, and D. Chappell. Degradation of the endothelial glycocalyx in clinical settings: searching for the sheddases. Br. J. Clin. Pharmacol. 80:389–402, 2015.
Bennett, K. M., K. C. Kent, J. Schumacher, C. C. Greenberg, and J. E. Scarborough. Targeting the most important complications in vascular surgery. J. Vasc. Surg. 65:793–803, 2017.
Bhadra, D., S. Bhadra, P. Jain, and N. K. Jain. Pegnology: a review of PEG-ylated systems. Pharmazie 57:5–29, 2002.
Cheng, M. J., N. N. Bal, P. Prabakaran, R. Kumar, T. J. Webster, S. Sridhar, and E. E. Ebong. Ultrasmall gold nanorods: synthesis and glycocalyx-related permeability in human endothelial cells. Int. J. Nanomed. 319–333:2019, 2019.
Cheng, M. J., R. Kumar, S. Sridhar, T. J. Webster, and E. E. Ebong. Endothelial glycocalyx conditions influence nanoparticle uptake for passive targeting. Int. J. Nanomed. 11:3305–3315, 2016.
Cheng, M. J., P. Prabakaran, R. Kumar, S. Sridhar, and E. E. Ebong. Synthesis of functionalized 10-nm polymer-coated gold particles for endothelium targeting and drug delivery. J. Vis. Exp. 313:56760, 2018.
DeBakey, M. E. Surgical treatment of atherosclerotic heart disease. Am. J. Cardiol. 63:9H–11H, 1989.
Duwayri, Y., J. Goss, W. Knechtle, R. K. Veeraswamy, S. Arya, R. R. Rajani, L. P. Brewster, T. F. Dodson, and J. F. Sweeney. The readmission event after vascular surgery: causes and costs. Ann. Vasc. Surg. 36:7–12, 2016.
Ebong, E. E., S. Kim, and N. DePaola. Flow regulates intercellular communication in HAEC by assembling functional Cx40 and Cx37 gap junctional channels. Am. J. Physiol. Heart Circ. Physiol. 290:H2015–2023, 2006.
Ebong, E. E., F. P. Macaluso, D. C. Spray, and J. M. Tarbell. Imaging the endothelial glycocalyx in vitro by rapid freezing/freeze substitution transmission electron microscopy. Arterioscler. Thromb. Vasc. Biol. 31:1908–1915, 2011.
Galyfos, G., K. Filis, F. Sigala, and G. Geropapas. Beta-blockers in vascular surgery patients: is the debate still going on? J. Anesth. 30:1031–1036, 2016.
Gomez-Garcia, M. J., A. L. Doiron, R. R. M. Steele, H. I. Labouta, B. Vafadar, R. D. Shepherd, I. D. Gates, D. T. Cramb, S. J. Childs, and K. D. Rinker. Nanoparticle localization in blood vessels: dependence on fluid shear stress, flow disturbances, and flow-induced changes in endothelial physiology. Nanoscale 10:15249–15261, 2018.
Harding, I. C., R. Mitra, S. A. Mensah, A. Nersesyan, N. N. Bal, and E. E. Ebong. Endothelial barrier reinforcement relies on flow-regulated glycocalyx, a potential therapeutic target. Biorheology 56:131–149, 2019.
Kumar, S., D.-W. Kang, A. Rezvan, and H. Jo. Accelerated atherosclerosis development in C57Bl6 mice by overexpressing AAV-mediated PCSK9 and partial carotid ligation. Lab. Invest. 97:935, 2017.
Kumase, F., Y. Morizane, S. Mohri, I. Takasu, A. Ohtsuka, and H. Ohtsuki. Glycocalyx degradation in retinal and choroidal capillary endothelium in rats with diabetes and hypertension. Acta Med. Okayama 64:277–283, 2010.
Lewis, J. C., R. G. Taylor, N. D. Jones, R. W. St Clair, and J. F. Cornhill. Endothelial surface characteristics in pigeon coronary artery atherosclerosis. I. Cellular alterations during the initial stages of dietary cholesterol challenge. Lab. Invest. 46:123–138, 1982.
Mathers, C. D., T. Boerma, and D. Ma Fat. Global and regional causes of death. Br. Med. Bull. 92:7–32, 2009.
Mensah, S. A., I. C. Harding, M. Zhang, M. P. Jaeggli, V. P. Torchilin, M. J. Niedre, and E. E. Ebong. Metastatic cancer cell attachment to endothelium is promoted by endothelial glycocalyx sialic acid degradation. AIChE J. 65:e16634, 2019.
Mitra, R., G. L. O’Neil, I. C. Harding, M. J. Cheng, S. A. Mensah, and E. E. Ebong. Glycocalyx in atherosclerosis-relevant endothelium function and as a therapeutic target. Curr. Atheroscler. Rep. 19:63, 2017.
Mitra, R., J. Qiao, S. Madhavan, G. L. O’Neil, B. Ritchie, P. Kulkarni, S. Sridhar, A. L. van de Ven, E. M. C. Kemmerling, C. Ferris, J. A. Hamilton, and E. E. Ebong. The comparative effects of high fat diet or disturbed blood flow on glycocalyx integrity and vascular inflammation. Trans. Med. Commun. 2018. https://doi.org/10.1186/s41231-018-0029-9.
Mockl, L., S. Hirn, A. A. Torrano, B. Uhl, C. Brauchle, and F. Krombach. The glycocalyx regulates the uptake of nanoparticles by human endothelial cells in vitro. Nanomedicine (Lond.) 12:207–217, 2017.
Nam, D., C.-W. Ni, A. Rezvan, J. Suo, K. Budzyn, A. Llanos, D. Harrison, D. Giddens, and H. Jo. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am. J. Physiol. 297:H1535–H1543, 2009.
Newman, C. B., D. Preiss, J. A. Tobert, T. A. Jacobson, R. L. Page II, L. B. Goldstein, C. Chin, L. R. Tannock, M. Miller, G. Raghuveer, P. B. Duell, E. A. Brinton, A. Pollak, L. T. Braun, F. K. Welty, et al. Statin safety and associated adverse events: a scientific statement from the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 39:e38–e81, 2019.
Nieuwdorp, M., M. C. Meuwese, H. Vink, J. B. Hoekstra, J. J. Kastelein, and E. S. Stroes. The endothelial glycocalyx: a potential barrier between health and vascular disease. Curr. Opin. Lipidol. 16:507–511, 2005.
Oohira, A., T. N. Wight, and P. Bornstein. Sulfated proteoglycans synthesized by vascular endothelial cells in culture. J. Biol. Chem. 258:2014–2021, 1983.
Potteaux, S., H. Ait-Oufella, and Z. Mallat. Mouse models of atherosclerosis. Drug Discov. Today 4:165–170, 2007.
Pries, A. R., T. W. Secomb, and P. Gaehtgens. The endothelial surface layer. Pflugers Arch. 440:653–666, 2000.
Prydz, K. Determinants of glycosaminoglycan (GAG) structure. Biomolecules 5:2003–2022, 2015.
Rahbar, E., J. C. Cardenas, G. Baimukanova, B. Usadi, R. Bruhn, S. Pati, S. R. Ostrowski, P. I. Johansson, J. B. Holcomb, and C. E. Wade. Endothelial glycocalyx shedding and vascular permeability in severely injured trauma patients. J. Trans. Med. 13:117, 2015.
Rehm, M., D. Bruegger, F. Christ, P. Conzen, M. Thiel, M. Jacob, D. Chappell, M. Stoeckelhuber, U. Welsch, B. Reichart, K. Peter, and B. F. Becker. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation 116:1896–1906, 2007.
Reitsma, S., D. W. Slaaf, H. Vink, M. van Zandvoort, and M. G. A. oude Egbrink. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 454:345–359, 2007.
Singh, A., S. C. Satchell, C. R. Neal, E. A. McKenzie, J. E. Tooke, and P. W. Mathieson. Glomerular endothelial glycocalyx constitutes a barrier to protein permeability. J. Am. Soc. Nephrol. 18:2885–2893, 2007.
Tarbell, J. M., and L. M. Cancel. The glycocalyx and its significance in human medicine. J. Intern. Med. 280:97–113, 2016.
Tarbell, J. M., Z. D. Shi, J. Dunn, and H. Jo. Fluid mechanics, arterial disease, and gene expression. Annu. Rev. Fluid Mech. 46:591–614, 2014.
Uhl, B., S. Hirn, R. Immler, K. Mildner, L. Mockl, M. Sperandio, C. Brauchle, C. A. Reichel, D. Zeuschner, and F. Krombach. The endothelial glycocalyx controls interactions of quantum dots with the endothelium and their translocation across the blood-tissue border. ACS Nano 11:1498–1508, 2017.
Ushiyama, A., H. Kataoka, and T. Iijima. Glycocalyx and its involvement in clinical pathophysiologies. J. Intensive Care 4:59, 2016.
Wang, H., Y. Lin, K. Nienhaus, and G. U. Nienhaus. The protein corona on nanoparticles as viewed from a nanoparticle-sizing perspective. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 10:e1500, 2018.
You, J., J. Zhou, M. Zhou, Y. Liu, J. D. Robertson, D. Liang, C. Van Pelt, and C. Li. Pharmacokinetics, clearance, and biosafety of polyethylene glycol-coated hollow gold nanospheres. Part Fibre Toxicol. 11:26, 2014.
Acknowledgements
Solomon Mensah, Bijay Singh, Maeve X Enright, Paige Baldwin, Bailey L Ritchie, and James Lister of Northeastern University provided technical assistance and feedback. Northeastern University’s Professor Thomas Webster, Professor Heather Clark, Assistant Professor Adam Ekenseair, the Department of Physics, and the Electronic Materials Research Institute shared equipment.
Author Contributions
All authors contributed to data analysis, drafted and revised the article, and gave final approval of the version to be published. Specifically: M.J.C. and E.E.E. designed the experiments. M.J.C, R.M., C.C.O., A.A.N., I.C.H, and R.K. performed the experiments and analyzed the data. M.J.C. and E.E.E. interpreted the results of the experiments. M.J.C., R.M., N.N.B, and E.E.E drafted the figures and manuscript. M.J.C., R.M., C.C.O., A.A.N., I.C.H, N.N.B, R.K., H.J., S.S., and E.E.E. edited, revised, and approved the final manuscript. H.J., S.S., and E.E.E. supervised the project. All authors agree to be accountable for all aspects of the work.
Funding
This work was funded by the National Institutes of Health (K01 HL125499 granted to EEE), the National Science Foundation (DGE-0965843 granted to SS and CMMI-1846962 granted to EEE), and the American Heart Association (18PRE33960461 granted to ICH). The funders had no role in data or information collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
Ming Cheng, Chinedu Okorafor, Alina Nersesyan, Ian Harding, Nandita Bal, Rajiv Kumar, Hanjoong Jo, Srinivas Sridhar, and Eno Ebong declare that they have no conflict of interest.
Research Involving Human and Animal Rights
No human studies were carried out by the authors for this article. All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the appropriate institutional committees.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Debra T. Auguste oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cheng, M.J., Mitra, R., Okorafor, C.C. et al. Targeted Intravenous Nanoparticle Delivery: Role of Flow and Endothelial Glycocalyx Integrity. Ann Biomed Eng 48, 1941–1954 (2020). https://doi.org/10.1007/s10439-020-02474-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-020-02474-4