Abstract
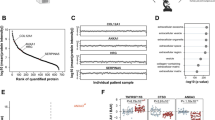
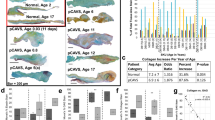
Aortic valve (AV) disease involves stiffening of the AV cusp with progression characterized by inflammation, fibrosis, and calcification. Here, we examine the relationship between biomechanical valve function and proteomic changes before and after the development of AV pathology in the Emilin1−/− mouse model of latent AV disease. Biomechanical studies were performed to quantify tissue stiffness at the macro (micropipette) and micro (atomic force microscopy (AFM)) levels. Micropipette studies showed that the Emilin1−/− AV annulus and cusp regions demonstrated increased stiffness only after the onset of AV disease. AFM studies showed that the Emilin1−/− cusp stiffens before the onset of AV disease and worsens with the onset of disease. Proteomes from AV cusps were investigated to identify protein functions, pathways, and interaction network alterations that occur with age- and genotype-related valve stiffening. Protein alterations due to Emilin1 deficiency, including changes in pathways and functions, preceded biomechanical aberrations, resulting in marked depletion of extracellular matrix (ECM) proteins interacting with TGFB1, including latent transforming growth factor beta 3 (LTBP3), fibulin 5 (FBLN5), and cartilage intermediate layer protein 1 (CILP1). This study identifies proteomic dysregulation is associated with biomechanical dysfunction as early pathogenic processes in the Emilin1−/− model of AV disease.








Similar content being viewed by others
Abbreviations
- AFM:
-
Atomic force microscopy
- AV:
-
Aortic valve
- ECM:
-
Extracellular matrix
- TGFB1:
-
Transforming growth factor beta 1
- VIC:
-
Valve interstitial cell
- WT:
-
Wild type
References
Aikawa, E., et al. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation 113(10):1344–1352, 2006.
Akhtar, S., K. M. Meek, and V. James. Immunolocalization of elastin, collagen type i and type iii, fibronectin, and vitronectin in extracellular matrix components of normal and myxomatous mitral heart valve chordae tendineae. Cardiovasc. Pathol. 8(4):203–211, 1999.
Alvarez-Llamas, G., et al. Modification of the secretion pattern of proteases, inflammatory mediators, and extracellular matrix proteins by human aortic valve is key in severe aortic stenosis. Mol. Cell. Proteomics 12(9):2426–2439, 2013.
Angel, P. M., et al. Networked-based characterization of extracellular matrix proteins from adult mouse pulmonary and aortic valves. J. Proteome Res. 10(2):812–823, 2011.
Aronow, W. S. Valvular aortic stenosis in the elderly. Cardiol. Rev. 15(5):217–225, 2007.
Balasubramanian, S., et al. mTOR in growth and protection of hypertrophying myocardium. Cardiovasc. Hematol. Agents Med. Chem. 7(1):52–63, 2009.
Barrick, C. J., et al. Reduced egfr causes abnormal valvular differentiation leading to calcific aortic stenosis and left ventricular hypertrophy in c57bl/6j but not 129s1/svimj mice. Am. J. Physiol. Heart Circ. Physiol. 297(1):H65–H75, 2009.
Benjamini, Y., and Y. Hochberg. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57(1):289–300, 1995.
Chu, Y., et al. Pioglitazone attenuates valvular calcification induced by hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 33(3):523–532, 2013.
Dabovic, B., et al. Bone abnormalities in latent tgf-β binding protein (ltbp)-3–null mice indicate a role for ltbp-3 in modulating tgf-β bioavailability. J. Cell Biol. 156(2):227–232, 2002.
Duncan, D., N. Prodduturi, and B. Zhang. Webgestalt2: an updated and expanded version of the web-based gene set analysis toolkit. BMC Bioinform. 11(Suppl 4):P10, 2010.
Guilak, F., L. G. Alexopoulos, M. A. Haider, H. P. Ting-Beall, and L. A. Setton. Zonal uniformity in mechanical properties of the chondrocyte pericellular matrix: micropipette aspiration of canine chondrons isolated by cartilage homogenization. Ann. Biomed. Eng. 33(10):1312–1318, 2005.
Harikrishnan, K., et al. Fibulin-1 suppresses endothelial to mesenchymal transition in the proximal outflow tract. Mech. Dev. 136:123–132, 2015.
Hinton, Jr, R. B., et al. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ. Res. 98(11):1431–1438, 2006.
Hwang, S.-K., and H.-H. Kim. The functions of mtor in ischemic diseases. BMB Rep. 44(8):506–511, 2011.
Iwamoto, R., and E. Mekada. Erbb and hb-egf signaling in heart development and function. Cell Struct. Funct. 31(1):1–14, 2006.
Jiang, X., et al. Modularity in the genetic disease-phenotype network. FEBS Lett. 582(17):2549–2554, 2008.
Johnson, K., D. Farley, S. I. Hu, and R. Terkeltaub. One of two chondrocyte-expressed isoforms of cartilage intermediate-layer protein functions as an insulin-like growth factor 1 antagonist. Arthritis Rheum. 48(5):1302–1314, 2003.
Jones, W. R., et al. Alterations in the young’s modulus and volumetric properties of chondrocytes isolated from normal and osteoarthritic human cartilage. J. Biomech. 32(2):119–127, 1999.
Koli, K., M. J. Ryynänen, and J. Keski-Oja. Latent tgf-β binding proteins (ltbps)-1 and-3 coordinate proliferation and osteogenic differentiation of human mesenchymal stem cells. Bone 43(4):679–688, 2008.
Krishnamurthy, V. K., F. Guilak, D. A. Narmoneva, and R. B. Hinton. Regional structure-function relationships in mouse aortic valve tissue. J. Biomech. 44(1):77–83, 2011.
Krishnamurthy, V. K., et al. Maladaptive matrix remodeling and regional biomechanical dysfunction in a mouse model of aortic valve disease. Matrix Biol. 31(3):197–205, 2012.
Li, F., et al. Pioglitazone attenuates progression of aortic valve calcification via down-regulating receptor for advanced glycation end products. Basic Res. Cardiol. 107(6):1–14, 2012.
Liu, H., R. G. Sadygov, and J. R. Yates. A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal. Chem. 76(14):4193–4201, 2004.
Loeys, B., et al. Homozygosity for a missense mutation in fibulin-5 (fbln5) results in a severe form of cutis laxa. Hum. Mol. Genet. 11(18):2113–2118, 2002.
Makki, N., K. W. Thiel, and F. J. Miller. The epidermal growth factor receptor and its ligands in cardiovascular disease. Int. J. Mol. Sci. 14(10):20597–20613, 2013.
Martín-Rojas, T., et al. Proteomic profile of human aortic stenosis: insights into the degenerative process. J. Proteome Res. 11(3):1537–1550, 2012.
Merryman, W. D., and F. J. Schoen. Mechanisms of calcification in aortic valve disease: role of mechanokinetics and mechanodynamics. Curr. Cardiol. Rep. 15(5):1–7, 2013.
Miller, J. D., R. M. Weiss, and D. D. Heistad. Calcific aortic valve stenosis: methods, models, and mechanisms. Circ. Res. 108(11):1392–1412, 2011.
Moremen, K. W., M. Tiemeyer, and A. V. Nairn. Vertebrate protein glycosylation: diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 13(7):448–462, 2012.
Munjal, C., et al. Tgf-β mediates early angiogenesis and latent fibrosis in an emilin1-deficient mouse model of aortic valve disease. Dis. Models Mech. 7(8):987–996, 2014.
Munjal, C., et al. Inhibition of mapk-erk pathway in vivo attenuates aortic valve disease processes in emilin1-deficient mouse model. Physiol. Rep. 5(5):e13152, 2017.
Nkomo, V. T., et al. Burden of valvular heart diseases: a population-based study. Lancet 368(9540):1005–1011, 2006.
Pezet, M., et al. Elastin haploinsufficiency induces alternative aging processes in the aorta. Rejuvenation Res. 11(1):97–112, 2008.
Rajamannan, N. M., et al. Calcific aortic valve disease: not simply a degenerative process a review and agenda for research from the national heart and lung and blood institute aortic stenosis working group executive summary: calcific aortic valve disease: 2011 update. Circulation 124(16):1783–1791, 2011.
Robertson, I. B., et al. Latent tgf-β-binding proteins. Matrix Biol. 47:44–53, 2015.
Saeed, A. I., et al. Tm4 microarray software suite. Methods Enzymol. 411:134–193, 2006.
Seki, S., et al. Cartilage intermediate layer protein promotes lumbar disc degeneration. Biochem. Biophys. Res. Commun. 446(4):876–881, 2014.
Sewell-Loftin, M. K., C. B. Brown, H. S. Baldwin, and W. D. Merryman. A novel technique for quantifying mouse heart valve leaflet stiffness with atomic force microscopy. J. Heart Valve Dis. 21:513–520, 2012.
Sharan, R., I. Ulitsky, and R. Shamir. Network-based prediction of protein function. Mol. Syst. Biol. 3:1–13, 2007.
Sibilia, M., et al. Mice humanised for the egf receptor display hypomorphic phenotypes in skin, bone and heart. Development 130(19):4515–4525, 2003.
Steitz, S. A., et al. Osteopontin inhibits mineral deposition and promotes regression of ectopic calcification. Am. J. Pathol. 161(6):2035–2046, 2002.
Sullivan, K. M., R. Bissonnette, H. Yanagisawa, S. N. Hussain, and E. C. Davis. Fibulin-5 functions as an endogenous angiogenesis inhibitor. Lab. Invest. 87(8):818–827, 2007.
Theret, D. P., M. J. Levesque, M. Sato, R. M. Nerem, and L. T. Wheeler. The application of a homogeneous half-space model in the analysis of endothelial cell micropipette measurements. J. Biomech. Eng. 110(3):190–199, 1988.
Varki, A. Biological roles of glycans. Glycobiology 27(1):3–49, 2017.
Vehviläinen, P., et al. Latent tgf-β binding proteins (ltbps) 1 and 3 differentially regulate transforming growth factor-β activity in malignant mesothelioma. Hum. Pathol. 42(2):269–278, 2011.
Weichhart, T., M. Hengstschläger, and M. Linke. Regulation of innate immune cell function by mtor. Nat. Rev. Immunol. 15(10):599–614, 2015.
Wiltz, D., et al. Extracellular matrix organization, structure, and function. In: Calcific Aortic Valve Disease, edited by E. Aikawa. Hicksville: InTech, 2013.
Wirrig, E. E., R. B. Hinton, and K. E. Yutzey. Differential expression of cartilage and bone-related proteins in pediatric and adult diseased aortic valves. J. Mol. Cell. Cardiol. 50(3):561–569, 2011.
Xu, L., and M. Brink. mTOR, cardiomyocytes and inflammation in cardiac hypertrophy. Biochim. Biophys. Acta Mol. Cell Res. 1863:1894–1903, 1863.
Yoshioka, M., et al. Chondromodulin-i maintains cardiac valvular function by preventing angiogenesis. Nat. Med. 12(10):1151–1159, 2006.
Zacchigna, L., et al. Emilin1 links tgfbeta maturation to blood pressure homeostasis. Cell 124(5):929–942, 2006.
Zanetti, M., et al. Emilin-1 deficiency induces elastogenesis and vascular cell defects. Mol. Cell. Biol. 24(2):638–650, 2004.
Zhang, B., S. Kirov, and J. Snoddy. Webgestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res. 33(Web Server Issue):W741, 2005.
Acknowledgments
We thank Aaron Reed for help in microscopy work and Susana Comte-Walters for help in proteomics data analysis. This study was supported by the National Center for Advancing Translational Sciences of the NIH (P.M.A., UL1 TR000445), National Institute of General Medical Sciences (P.M.A., P20 GM103542-06) the National Heart Lung and Blood Institute of the NIH (R.B.H., HL117851) an Institutional Clinical and Translational Science Award (R.B.H., NIH/NCRR 8UL1TR000077), and the Cincinnati Children’s Research Foundation (R.B.H.).
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Scott I Simon oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Angel, P.M., Narmoneva, D.A., Sewell-Loftin, M.K. et al. Proteomic Alterations Associated with Biomechanical Dysfunction are Early Processes in the Emilin1 Deficient Mouse Model of Aortic Valve Disease. Ann Biomed Eng 45, 2548–2562 (2017). https://doi.org/10.1007/s10439-017-1899-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-017-1899-0