Abstract
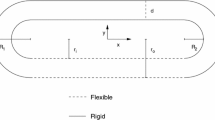
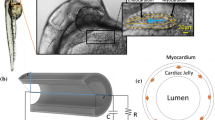
The embryonic heart of vertebrate embryos, including humans, has a tubular thick-wall structure when it first starts to beat. The tubular embryonic heart (TEH) does not have valves, and yet, it produces an effective unidirectional blood flow. The actual pumping mechanism of the TEH is still controversial with pros and cons for either peristaltic pumping (PP) or impedance pumping (IP). On the other hand, observation of movies of the contractile TEH of the quail revealed a propagating wave from the venous end towards the arterial end that occludes the lumen behind the leading edge. This pattern of contraction represents a complex PP with a duty cycle, and was defined here as biological pumping (BP). In this work we developed a heart-like model that represents the main features of the chick TEH and allows for numerical analysis of all the three pumping mechanisms (i.e., IP, PP, and BP) as well as a comprehensive sensitivity evaluation of the structural, operating, and mechanical parameters. The physical model also included components representing the whole circulatory system of the TEH. The simulations results revealed that the BP mechanism yielded the level and time-dependent pattern of blood flow and blood pressure, as well as contractility that were observed in experiments.










Similar content being viewed by others
References
Adina R&D Inc. ADINA theory and modeling guides: Adina CFD & FSI. Report ARD 13-10, 2013.
Avrahami, I., and M. Gharib. Computational studies of resonance wave pumping in compliant tubes. J. Fluid Mech. 608:139–160, 2008.
Barry, A. The intrinsic pulsation rates of fragments of the embryonic chick heart. J. Exp. Zool. 91:119–130, 1942.
Bartman, T., E. C. Walsh, K.-K. Wen, M. McKane, J. Ren, J. Alexander, P. A. Rubenstein, and D. Y. R. Stainier. Early myocardial function affects endocardial cushion development in Zebrafish. PLoS Biol. 2:e129, 2004.
Broekhuizen, M. L., B. Hogers, M. C. DeRuiter, R. E. Poelmann, A. C. Gittenberger-de Groot, and J. W. Wladimiroff. Altered hemodynamics in chick embryos after extraembryonic venous obstruction. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. 13:437–445, 1999.
Campbell, K. A., N. Hu, E. B. Clark, and B. B. Keller. Analysis of dynamic atrial dimension and function during early cardiac development in the chick embryo. Pediatr. Res. 32:333–337, 1992.
Chi, N. C., R. M. Shaw, B. Jungblut, J. Huisken, T. Ferrer, R. Arnaout, I. Scott, D. Beis, T. Xiao, H. Baier, L. Y. Jan, M. Tristani-Firouzi, and D. Y. R. Stainier. Genetic and physiologic dissection of the vertebrate cardiac conduction system. PLoS Biol. 6:e109, 2008.
Davis, A. M., F. G. Rothenberg, N. Shepherd, and J. A. Izatt. In vivo spectral domain optical coherence tomography volumetric imaging and spectral Doppler velocimetry of early stage embryonic chicken heart development. J. Opt. Soc. Am. A 25:3134–3143, 2008.
de Jong, F., T. Opthof, A. A. Wilde, M. J. Janse, R. Charles, W. H. Lamers, and A. F. Moorman. Persisting zones of slow impulse conduction in developing chicken hearts. Circ. Res. 71:240–250, 1992.
Forouhar, A. S., M. Liebling, A. Hickerson, A. Nasiraei-Moghaddam, H. J. Tsai, J. R. Hove, S. E. Fraser, M. E. Dickinson, and M. Gharib. The embryonic vertebrate heart tube is a dynamic suction pump. Science 312:751–753, 2006.
Goenezen, S., M. Y. Rennie, and S. Rugonyi. Biomechanics of early cardiac development. Biomech. Model. Mechanobiol. 11:1187–1204, 2012.
Groenendijk, B. C. W., B. P. Hierck, J. Vrolijk, M. Baiker, M. J. B. M. Pourquie, A. C. Gittenberger-de Groot, and R. E. Poelmann. Changes in shear stress-related gene expression after experimentally altered venous return in the chicken embryo. Circ. Res. 96:1291–1298, 2005.
Groenendijk, B. C. W., K. Van der Heiden, B. P. Hierck, and R. E. Poelmann. The role of shear stress on ET-1, KLF2, and NOS-3 expression in the developing cardiovascular system of chicken embryos in a venous ligation model. Physiol. Bethesda Md. 22:380–389, 2007.
Heebøll-Christensen, J. Mathematical modeling of flow characteristics in the embryonic chick heart, 2011.
Hou, J. H., J. M. Kralj, A. D. Douglass, F. Engert, and A. E. Cohen. Simultaneous mapping of membrane voltage and calcium in zebrafish heart in vivo reveals chamber-specific developmental transitions in ionic currents. Front. Physiol. 5:344, 2014.
Hove, J. R., R. W. Köster, A. S. Forouhar, G. Acevedo-Bolton, S. E. Fraser, and M. Gharib. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 421:172–177, 2003.
Hu, N., and E. B. Clark. Hemodynamics of the stage 12 to stage 29 chick embryo. Circ. Res. 65:1665–1670, 1989.
Jenkins, M. W., D. C. Adler, M. Gargesha, R. Huber, F. Rothenberg, J. Belding, M. Watanabe, D. L. Wilson, J. G. Fujimoto, and A. M. Rollins. Ultrahigh-speed optical coherence tomography imaging and visualization of the embryonic avian heart using a buffered Fourier domain mode locked laser. Opt. Exp. 15:6251–6267, 2007.
Johnson, B. M., D. M. Garrity, and L. P. Dasi. The transitional cardiac pumping mechanics in the embryonic heart. Cardiovasc. Eng. Technol. 4:246–255, 2013.
Keller, B. B., N. Hu, and E. B. Clark. Correlation of ventricular area, perimeter, and conotruncal diameter with ventricular mass and function in the chick embryo from stages 12 to 24. Circ. Res. 66:109–114, 1990.
Kozlovsky, P., M. Rosenfeld, A. J. Jaffa, and D. Elad. Dimensionless analysis of valveless pumping in a thick-wall elastic tube: application to the tubular embryonic heart. J. Biomech. 48:1652–1661, 2015.
Latacha, K. S., M. C. Rémond, A. Ramasubramanian, A. Y. Chen, E. L. Elson, and L. A. Taber. Role of actin polymerization in bending of the early heart tube. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 233:1272–1286, 2005.
Lee, J., M. E. Moghadam, E. Kung, H. Cao, T. Beebe, Y. Miller, B. L. Roman, C.-L. Lien, N. C. Chi, A. L. Marsden, and T. K. Hsiai. Moving domain computational fluid dynamics to interface with an embryonic model of cardiac morphogenesis. PLoS One 8:e72924, 2013.
Liu, A., S. Rugonyi, J. O. Pentecost, and K. L. Thornburg. Finite element modeling of blood flow-induced mechanical forces in the outflow tract of chick embryonic hearts. Comput. Struct. 85:727–738, 2007.
Liu, A., X. Yin, L. Shi, P. Li, K. L. Thornburg, R. Wang, and S. Rugonyi. Biomechanics of the chick embryonic heart outflow tract at HH18 using 4D optical coherence tomography imaging and computational modeling. PLoS One 7:e40869, 2012.
Loumes, L., I. Avrahami, and M. Gharib. Resonant pumping in a multilayer impedance pump. Phys. Fluids 20:023103, 2008.
Maes, F., B. Chaudhry, P. Van Ransbeeck, and P. Verdonck. The Pumping Mechanism of Embryonic Hearts. Berlin: Springer, 2011.
Männer, J. Cardiac looping in the chick embryo: a morphological review with special reference to terminological and biomechanical aspects of the looping process. Anat. Rec. 259:248–262, 2000.
Männer, J., L. Thrane, K. Norozi, and T. M. Yelbuz. High-resolution in vivo imaging of the cross-sectional deformations of contracting embryonic heart loops using optical coherence tomography. Dev. Dyn. 237:953–961, 2008.
Männer, J., L. Thrane, K. Norozi, and T. M. Yelbuz. In vivo imaging of the cyclic changes in cross-sectional shape of the ventricular segment of pulsating embryonic chick hearts at stages 14 to 17: a contribution to the understanding of the ontogenesis of cardiac pumping function. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 238:3273–3284, 2009.
Männer, J., A. Wessel, and T. M. Yelbuz. How does the tubular embryonic heart work? Looking for the physical mechanism generating unidirectional blood flow in the valveless embryonic heart tube. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 239:1035–1046, 2010.
Manopoulos, C. G., D. S. Mathioulakis, and S. G. Tsangaris. One-dimensional model of valveless pumping in a closed loop and a numerical solution. Phys. Fluids 18:017106, 2006.
Marom, G., R. Haj-Ali, E. Raanani, H.-J. Schäfers, and M. Rosenfeld. A fluid–structure interaction model of the aortic valve with coaptation and compliant aortic root. Med. Biol. Eng. Comput. 50:173–182, 2012.
Martinsen, B. J. Reference guide to the stages of chick heart embryology. Dev. Dyn. 233:1217–1237, 2005.
Meier, G. Viscous Flow in the Embryonic Heart Geometry. Embryol. Hefte 1:1–19, 1987.
Ramasubramanian, A., K. S. Latacha, J. M. Benjamin, D. A. Voronov, A. Ravi, and L. A. Taber. Computational model for early cardiac looping. Ann. Biomed. Eng. 34:1355–1369, 2006.
Ramasubramanian, A., N. L. Nerurkar, K. H. Achtien, B. A. Filas, D. A. Voronov, and L. A. Taber. On modeling morphogenesis of the looping heart following mechanical perturbations. J. Biomech. Eng. 130:061018, 2008.
Rideout, V. C. Mathematical and Computer Modeling of Physiological Systems. Englewood Cliffs: Prentice Hall, 1991.
Rosenfeld, M., and I. Avrahami. Net flow rate generation by a multi-pincher impedance pump. Comput. Fluids 39:1634–1643, 2010.
Rugonyi, S. On finite element analysis of fluid flows fully coupled with structural interactions. CMES 2:195–212, 2001.
Santhanakrishnan, A., and L. A. Miller. Fluid dynamics of heart development. Cell Biochem. Biophys. 61:1–22, 2011.
Taber, L. A., and R. Perucchio. Modeling heart development. J. Elast. Phys. Sci. Solids 61:165–197, 2000.
Taber, L. A., J. Zhang, and R. Perucchio. Computational model for the transition from peristaltic to pulsatile flow in the embryonic heart tube. J. Biomech. Eng. 129:441, 2007.
Ursem, N. T. C., S. Stekelenburg-de Vos, J. W. Wladimiroff, R. E. Poelmann, A. C. Gittenberger-de Groot, N. Hu, and E. B. Clark. Ventricular diastolic filling characteristics in stage-24 chick embryos after extra-embryonic venous obstruction. J. Exp. Biol. 207:1487–1490, 2004.
Waldrop, L., and L. Miller. Large amplitude, short wave peristalsis and its implications for transport, 2015. doi:10.7287/peerj.preprints.906v1.
Wisser, J., and P. Dirschedl. Embryonic heart rate in dated human embryos. Early Hum. Dev. 37:107–115, 1994.
Zamir, E. A., and L. A. Taber. Material properties and residual stress in the stage 12 chick heart during cardiac looping. J. Biomech. Eng. 126:823–830, 2004.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interests
None.
Additional information
Associate Editor Jane Grande-Allen oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Video S1. Variation of normalized functions that represent the TEH contraction for the reference case for IP, PP, and BP mechanisms in space and time. The video is 1/5 of the original speed. Supplementary material 1 (WMV 662 kb).
Video S2. The calculated deformation of the TEH wall and blood velocity vectors for the reference cases for the IP, PP, and BP mechanisms. The video is 1/5 of the original speed. Supplementary material 2 (WMV 2771 kb).
Video S3. The calculated deformations of lumen heart-wall interface of the TEH for the reference case for IP, PP, and BP mechanisms and of motion of lumen heart-wall interface of HH-10 quail TEH traced from published videos (Jenkins et al., 2007). Supplementary material 3 (WMV 1692 kb).
Rights and permissions
About this article
Cite this article
Kozlovsky, P., Bryson-Richardson, R.J., Jaffa, A.J. et al. The Driving Mechanism for Unidirectional Blood Flow in the Tubular Embryonic Heart. Ann Biomed Eng 44, 3069–3083 (2016). https://doi.org/10.1007/s10439-016-1620-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-016-1620-8