Abstract
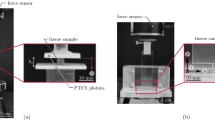
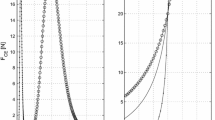
In the field of computational biomechanics, the experimental evaluation of the material properties is crucial for the development of computational models that closely reproduce real organ systems. When simulations of muscle tissue are concerned, stress/strain relations for both passive and active behavior are required. These experimental relations usually exhibit certain variability. In this study, a set of material parameters involved in a 3D skeletal muscle model are determined by using a system biology approach in which the parameters are randomly varied leading to a population of models. Using a set of experimental results from an animal model, a subset of the entire population of models was selected. This reduced population predicted the mechanical response within the window of experimental observations. Hence, a range of model parameters, instead of a single set of them, was determined. Rat Tibialis Anterior muscle was selected for this study. Muscles (\(n=6\)) were activated through the sciatic nerve and during contraction the tissue pulled a weight fixed to the distal tendon (concentric contraction). Three different weights 1, 2 and 3 N were used and the time course of muscle stretch was analyzed obtaining values of (mean \(\pm\) standard deviation): \(0.714\pm 0.01\), \(0.808\pm 0.060\) and \(0.851\pm 0.033\) respectively. A paired two-sided sign rank test showed significant differences between the muscle response for the three weights (\(p<0.01\)). This study shows that the Monte Carlo method could be used for determine muscle characteristic parameters considering the variability of the experimental population.









Similar content being viewed by others
References
Blemker, S. S., Pinsky, P. M., and Delp, S. L. A 3d model of muscle reveals the causes of nonuniform strains in the biceps brachii. J. Biomech. 38:657–665, 2005.
Böl, M., Kruse, R., Ehret, A. E., Leichsenring, K., and Siebert, T. Compressive properties of passive skeletal muscle-the impact of precise sample geometry on parameter identification in inverse finite element analysis. J. Biomech. 45:2673–2679, 2012.
Böl, M., and Reese, S. Micromechanical modelling of skeletal muscles based on the finite element method. Comput. Methods Biomech. Biomed. Eng. 11:489–504, 2008.
Britton, O. J., Bueno-Orovio, A., Van Ammel, K., Lu, H. R., Towart, R., Gallacher, D. J., and Rodriguez, B. Experimentally calibrated population of models predicts and explains intersubject variability in cardiac cellular electrophysiology. Proc. Natl. Acad. Sci. USA 110:E2098–105, 2013.
Calvo, B., Ramírez, A., Alonso, A., Grasa, J., Soteras, F., Osta, R., and Muñoz, M. J. Passive nonlinear elastic behaviour of skeletal muscle: experimental results and model formulation. J. Biomech. 43:318–325, 2010.
Cheung, J. T.-M., Zhang, M., Leung, A. K.-L., and Fan, Y.-B. Three-dimensional finite element analysis of the foot during standing-a material sensitivity study. J. Biomech. 38:1045–1054, 2005.
Everett, A. Muscle Mechanics. 2006.
Fiorentino, N. M., and Blemker, S. S. Musculotendon variability influences tissue strains experienced by the biceps femoris long head muscle during high-speed running. J. Biomech. 47:3325–3333, 2014.
Grasa, J., Ramírez, A., Osta, R., Muñoz, M. J., Soteras, F., and Calvo, B. A 3d active-passive numerical skeletal muscle model incorporating initial tissue strains. validation with experimental results on rat tibialis anterior muscle. Biomech. Model Mechanobiol. 10:779–787, 2011.
Grasa, J., Sierra, M., Muñoz, M. J., Soteras, F., Osta, R., Calvo, B., and Miana-Mena, F. J. On simulating sustained isometric muscle fatigue: a phenomenological model considering different fiber metabolisms. Biomech. Model Mechanobiol. 13:1373–1385, 2014.
Hedenstierna, S., Halldin, P., and Brolin, K. Evaluation of a combination of continuum and truss finite elements in a model of passive and active muscle tissue. Comput. Methods Biomech. Biomed. Eng. 11:627–639, 2008.
Hernández-Gascón, B., Grasa, J., Calvo, B., and Rodríguez, J. F. A 3d electro-mechanical continuum model for simulating skeletal muscle contraction. J. Theor. Biol. 335:108–118, 2013.
Hoang, P. D., Herbert, R. D., Todd, G., Gorman, R. B., and Gandevia, S. C. Passive mechanical properties of human gastrocnemius muscle tendon units, muscle fascicles and tendons in vivo. J. Exp. Biol. 210:4159–4168, 2007.
Hodgson, J. A., Chi, S.-W., Yang, J. P., Chen, J.-S., Edgerton, V. R., and Sinha, S. Finite element modeling of passive material influence on the deformation and force output of skeletal muscle. J. Mech. Behav. Biomed. Mater. 9:163–183, 2012.
Hsieh, A. H., Wagner, D. R., Cheng, L. Y., and Lotz, J. C. Dependence of mechanical behavior of the murine tail disc on regional material properties: a parametric finite element study. J. Biomech. Eng. 127:1158–1167, 2005.
Kumaresan, S., Yoganandan, N., and Pintar, F. A. Finite element analysis of the cervical spine: a material property sensitivity study. Clin. Biomech. (Bristol, Avon) 14:41–53, 1999.
Lapointe, B. M., and Côté, C. H. Anesthetics can alter subsequent in vitro assessment of contractility in slow and fast skeletal muscles of rat. Am. J. Physiol. 277:R917–921, 1999.
Loocke, M. V., Lyons, C. G., and Simms, C. K. A validated model of passive muscle in compression. J. Biomech. 39:2999–3009, 2006.
Magnusson, S. P. Passive properties of human skeletal muscle during stretch maneuvers. a review. Scand. J. Med. Sci. Sports, 8:65–77, 1998.
Mela, P., Veltink, P. H., Huijing, P. A., Salmons, S., and Jarvis, J. C. The optimal stimulation pattern for skeletal muscle is dependent on muscle length. IEEE Trans. Neural Syst. Rehabil. Eng. 10:85–93, 2002.
Osnes, H., and Sundnes, J. Uncertainty analysis of ventricular mechanics using the probabilistic collocation method. IEEE Trans. Biomed. Eng. 59:2171–2179, 2012.
Raikova, R., Krutki, P., Aladjov, H., and Celichowski, J. Variability of the twitch parameters of the rat medial gastrocnemius motor units-experimental and modeling study. Comput. Biol. Med. 37:1572–1581, 2007.
Ramírez, A., Grasa, J., Alonso, A., Soteras, F., Osta, R., Muñoz, M. J., and Calvo, B. Active response of skeletal muscle: in vivo experimental results and model formulation. J. Theor. Biol. 267:546–553, 2010.
Rehorn, M. R., Schroer, A. K., and Blemker, S. S. The passive properties of muscle fibers are velocity dependent. J. Biomech. 47:687–693, 2014.
Shin, R. H., Vathana, T., Giessler, G. A., Friedrich, P. F., Bishop, A. T., and Shin, A. Y. Isometric tetanic force measurement method of the tibialis anterior in the rat. Microsurgery 28:452–457, 2008.
Shin, D. D., Hodgson, J. A., Edgerton, V. R. and Sinha, S. In vivo intramuscular fascicle-aponeuroses dynamics of the human medial gastrocnemius during plantarflexion and dorsiflexion of the foot. J. Appl. Physiol. 107:1276–1284, 2009.
Sigal, I. A., Flanagan, J. G., Tertinegg, I., and Ethier, C. R. Modeling individual-specific human optic nerve head biomechanics. part ii: influence of material properties. Biomech. Model Mechanobiol. 8:99–109, 2009.
Silder, A., Reeder, S. B., and Thelen, D. G. The influence of prior hamstring injury on lengthening muscle tissue mechanics. J. Biomech. 43:2254–2260, 2010.
Song, B., Chen, W., Ge, Y., and Weerasooriya, T. Dynamic and quasi-static compressive response of porcine muscle. J. Biomech. 40:2999–3005, 2007.
Takaza, M., Moerman, K. M., Gindre, J., Lyons, G., and Simms, C. K. The anisotropic mechanical behaviour of passive skeletal muscle tissue subjected to large tensile strain. J. Mech. Behav. Biomed. Mater. 17:209–220, 2013.
Tang, C. Y., Tsui, C. P., Stojanovic, B., and Kojic, M. Finite element modelling of skeletal muscles coupled with fatigue. Int. J. Mech. Sci. 49:1179–1191, 2007.
Tang, C. Y., Zhang, G., and Tsui, C. P. A 3D skeletal muscle model coupled with active contraction of muscle fibres and hyperelastic behaviour. J. Biomech. 42:865–872, 2009.
Wu, J. Z., and Herzog, W. Modelling concentric contraction of muscle using an improved cross-bridge model. J. Biomech. 32:837–848, 1999.
Zhao, S., Li, W., and Gu, L. Biomechanical prediction of abdominal aortic aneurysm rupture risk: sensitivity analysis. J. Biomed. Sci. Eng. 5:664–671, 2012.
Acknowledgements
The authors gratefully acknowledge research support from the Spanish Ministry of Economy and Competitiveness (Projects DPI2011-27939-C02-01), the Department of Industry and Innovation (Government of Aragon) and also support from the University of Zaragoza through the research Project JIUZ-2012-TEC-05 and the predoctoral Grant of the Department of Industry and Innovation (Government of Aragon). The authors also want to thank the Tissue Characterization Platform of CIBER-BBN for technical support during the experimental tests. CIBER-BBN is an initiative funded by the VI National R&D&i Plan 2008–2011, Iniciativa Ingenio 2010, Consolider Program, CIBER Actions and financed by the Instituto de Salud Carlos III with assistance from the European Regional Development Fund.
Conflict of interest
There are no conflicts of interest to report in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Stefan M. Duma oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Sierra, M., Miana-Mena, F.J., Calvo, B. et al. On Using Model Populations to Determine Mechanical Properties of Skeletal Muscle. Application to Concentric Contraction Simulation. Ann Biomed Eng 43, 2444–2455 (2015). https://doi.org/10.1007/s10439-015-1279-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-015-1279-6