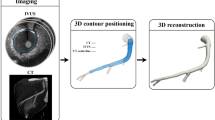
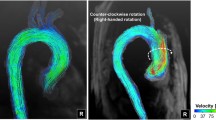
Abstract
Helical flow in the human aorta is possibly a typical example of ‘form follows function’ in the vascular system. The helical blood flow may provide guaranties for the inner surface of the ascending aortic wall to get smooth and even washing by the blood so that atherosclerotic plaques can hardly form in the area of the ascending aorta. It has been documented that the phenomenon of helical flow of blood is not just localized in the ascending aorta, it also exists in several large arteries and veins as well. Preliminary studies demonstrated the widely existing helical flow might play positive physiological roles in facilitating blood flow transport, suppressing disturbed blood flow, preventing the accumulation of atherogenic low density lipoproteins on the luminal surfaces of arteries, enhancing oxygen transport from the blood to the arterial wall and reducing the adhesion of blood cells on the arterial surface. These roles of helical blood flow may lessen the burden of arteries and protect the arteries from the pathology of atherosclerosis, thrombosis, and intimal hyperplasia. The great development of time-resolved three-dimensional phase contrast MRI (flow-sensitive 4D-MRI) and the advent of dimensionless indices such as helical flow index proposed to characterize helical flow make clinic quantification of the helical flow in the human large arteries possible. Moreover, researchers probed into the possibility to apply the mechanism of helical flow to the design of vascular interventions to reduce thrombus formation and intimal hyperplasia caused by abnormal flow conditions.




Similar content being viewed by others
References
Ali, S. Pressure drop correlations for flow through regular helical coil tubes. Fluid Dyn. Res. 28:295–310, 2001.
Bachler, P., N. Pinochet, J. Sotelo, G. Crelier, P. Irarrazaval, C. Tejos, and S. Uribe. Assessment of normal flow patterns in the pulmonary circulation by using 4D magnetic resonance velocity mapping. Magn. Reson. Imaging 31:178–188, 2013.
Barker A. J., P. van Ooij, K. Bandi, J. Garcia, M. Albaghdadi, P. McCarthy, R. O. Bonow, J. Carr, J. Collins, S. C. Malaisrie, and M. Markl. Viscous energy loss in the presence of abnormal aortic flow. Magn. Reson. Med. 2013.
Bechara, C. F. Comparing short and midterm Infrainguinal bypass patency rates between two ePTFE prosthetic grafts: spiral laminar flow and propaten. Vasc. Dis. Manag. 11:E54–E58, 2014.
Bogren, H. G., and M. H. Buonocore. 4D magnetic resonance velocity mapping of blood flow patterns in the aorta in young vs. elderly normal subjects. J. Magn. Reson. Imaging 10:861–869, 1999.
Burk, J., P. Blanke, Z. Stankovic, A. Barker, M. Russe, J. Geiger, A. Frydrychowicz, M. Langer, and M. Markl. Evaluation of 3D blood flow patterns and wall shear stress in the normal and dilated thoracic aorta using flow-sensitive 4D CMR. J. Cardiovasc. Magn. Reson. 14:84, 2012.
Caro, C. G., N. J. Cheshire, and N. Watkins. Preliminary comparative study of small amplitude helical and conventional ePTFE arteriovenous shunts in pigs. J. R. Soc. Interface 2:261–266, 2005.
Caro, C. G., D. J. Doorly, and M. Tarnawski. Non-planar curvature and branching of arteries and non-planar-type flow. Proc. R. Soc. Lond. A 452:185–197, 1996.
Caro, C. G., A. Seneviratne, K. B. Heraty, C. Monaco, M. G. Burke, R. Krams, C. C. Chang, P. Gilson, and G. Coppola. Intimal hyperplasia following implantation of helical-centreline and straight-centreline stents in common carotid arteries in healthy pigs: influence of intraluminal flow. J. R. Soc. Interface 11:20130578, 2014.
Chen, Z., F. Zhan, Y. Fan, and X. Deng. A novel way to reduce thrombus build-up in vena cava filters. Catheter. Cardiovasc. Interv. 78:792–798, 2011.
Chen, Z. S., Y. B. Fan, X. Y. Deng, and Z. P. Xu. Swirling flow can suppress flow disturbances in endovascular stents: a numerical study. ASAIO J. 55:543–549, 2009.
Chen, Z. S., X. W. Zhang, and X. Y. Deng. Swirling flow can suppress monocyte adhesion in the flow disturbance zones of the endovascular stent. Biorheology 49:341–352, 2012.
Chien, S. Molecular and mechanical bases of focal lipid accumulation in arterial wall. Prog. Biophys. Mol. Biol. 83:131–151, 2003.
Chiu, C. J., J. Terzis, and M. L. MacRae. Replacement of superior vena cava with the spiral composite vein graft. A versatile technique. Ann. Thorac. Surg. 17:555–560, 1974.
Chiu, J. J., and S. Chien. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol. Rev. 91:327–387, 2011.
Cookson, A. N., D. J. Doorly, and S. J. Sherwin. Mixing through stirring of steady flow in small amplitude helical tubes. Ann. Biomed. Eng. 37:710–721, 2009.
Coppola, G., and C. Caro. Oxygen mass transfer in a model three-dimensional artery. J. R. Soc. Interface 5:1067–1075, 2008.
Coppola, G., and C. Caro. Arterial geometry, flow pattern, wall shear and mass transport: potential physiological significance. J. R. Soc. Interface 6:519–528, 2009.
Deng, X., Y. Marois, T. How, Y. Merhi, M. King, R. Guidoin, and T. Karino. Luminal surface concentration of lipoprotein (LDL) and its effect on the wall uptake of cholesterol by canine carotid arteries. J. Vasc. Surg. 21:135–145, 1995.
Ding, Z., Y. Fan, X. Deng, F. Zhan, and H. Kang. Effect of swirling flow on the uptakes of native and oxidized LDLs in a straight segment of the rabbit thoracic aorta. Exp. Biol. Med. (Maywood) 235:506–513, 2010.
Doty, J. R., J. H. Flores, and D. B. Doty. Superior vena cava obstruction: bypass using spiral vein graft. Ann. Thorac. Surg. 67:1111–1116, 1999.
Duraiswamy, N., R. T. Schoephoerster, M. R. Moreno, and J. E. Moore. Stented artery flow patterns and their effects on the artery wall. Annu. Rev. Fluid Mech. 39:357–382, 2007.
Endo, S., Y. Sohara, and T. Karino. Flow patterns in dog aortic arch under a steady flow condition simulating mid-systole. Heart Vessels 11:180–191, 1996.
Ethier, C. R. Computational modeling of mass transfer and links to atherosclerosis. Ann. Biomed. Eng. 30:461–471, 2002.
Fan, Y. B., Z. P. Xu, W. T. Jiang, X. Y. Deng, K. Wang, and A. Q. Sun. An S-type bypass can improve the hemodynamics in the bypassed arteries and suppress intimal hyperplasia along the host artery floor. J. Biomech. 41:2498–2505, 2008.
Frazin, L. J., G. Lanza, M. Vonesh, F. Khasho, C. Spitzzeri, S. McGee, D. Mehlman, K. B. Chandran, J. Talano, and D. McPherson. Functional chiral asymmetry in descending thoracic aorta. Circulation 82:1985–1994, 1990.
Frazin, L. J., M. J. Vonesh, K. B. Chandran, T. Shipkowitz, A. S. Yaacoub, and D. D. McPherson. Confirmation and initial documentation of thoracic and abdominal aortic helical flow. An ultrasound study. ASAIO J. 42:951–956, 1996.
Frydrychowicz, A., C. J. Francois, and P. A. Turski. Four-dimensional phase contrast magnetic resonance angiography: potential clinical applications. Eur. J. Radiol. 80:24–35, 2011.
Frydrychowicz, A., M. Markl, D. Hirtler, A. Harloff, C. Schlensak, J. Geiger, B. Stiller, and R. Arnold. Aortic hemodynamics in patients with and without repair of aortic coarctation in vivo analysis by 4D flow-sensitive magnetic resonance imaging. Invest. Radiol. 46:317–325, 2011.
Frydrychowicz, A., A. F. Stalder, M. F. Russe, J. Bock, S. Bauer, A. Harloff, A. Berger, M. Langer, J. Hennig, and M. Markl. Three-dimensional analysis of segmental wall shear stress in the aorta by flow-sensitive four-dimensional-MRI. J. Magn. Reson. Imaging 30:77–84, 2009.
Frydrychowicz, A., J. T. Winterer, M. Zaitsev, B. Jung, J. Hennig, M. Langer, and M. Markl. Visualization of iliac and proximal femoral artery hemodynamics using time-resolved 3D phase contrast MRI at 3T. J. Magn. Reson. Imaging 25:1085–1092, 2007.
Gallo, D., G. De Santis, F. Negri, D. Tresoldi, R. Ponzini, D. Massai, M. A. Deriu, P. Segers, B. Verhegghe, G. Rizzo, and U. Morbiducci. On the use of in vivo measured flow rates as boundary conditions for image-based hemodynamic models of the human aorta: implications for indicators of abnormal flow. Ann. Biomed. Eng. 40:729–741, 2012.
Gallo, D., D. A. Steinman, P. B. Bijari, and U. Morbiducci. Helical flow in carotid bifurcation as surrogate marker of exposure to disturbed shear. J. Biomech. 45:2398–2404, 2012.
Grigioni, M., C. Daniele, U. Morbiducci, C. Del Gaudio, G. D’Avenio, A. Balducci, and V. Barbaro. A mathematical description of blood spiral flow in vessels: application to a numerical study of flow in arterial bending. J. Biomech. 38:1375–1386, 2005.
Gulan, U., B. Luthi, M. Holzner, A. Liberzon, A. Tsinober, and W. Kinzelbach. Experimental study of aortic flow in the ascending aorta via particle tracking velocimetry. Exp. Fluids 53:1469–1485, 2012.
Ha, H., and S. J. Lee. Effect of swirling inlet condition on the flow field in a stenosed arterial vessel model. Med. Eng. Phys. 36:119–128, 2014.
Hope, M., S. Wrenn, and P. Dyverfeldt. Clinical applications of aortic 4D flow imaging. Curr. Cardiovasc. Imaging Rep. 6:128–139, 2013.
Hope, M. D., T. A. Hope, S. E. Crook, K. G. Ordovas, T. H. Urbania, M. T. Alley, and C. B. Higgins. 4D flow CMR in assessment of valve-related ascending aortic disease. JACC Cardiovasc. Imaging 4:781–787, 2011.
Hope, M. D., T. A. Hope, A. K. Meadows, K. G. Ordovas, T. H. Urbania, M. T. Alley, and C. B. Higgins. Bicuspid aortic valve: four-dimensional MR evaluation of ascending aortic systolic flow patterns. Radiology 255:53–61, 2010.
Houston, J. G., S. J. Gandy, W. Milne, J. B. Dick, J. J. Belch, and P. A. Stonebridge. Spiral laminar flow in the abdominal aorta: a predictor of renal impairment deterioration in patients with renal artery stenosis? Nephrol. Dial. Transp. 19:1786–1791, 2004.
Houston, J. G., S. J. Gandy, D. G. Sheppard, J. B. Dick, J. J. Belch, and P. A. Stonebridge. Two-dimensional flow quantitative MRI of aortic arch blood flow patterns: effect of age, sex, and presence of carotid atheromatous disease on prevalence of spiral blood flow. J. Magn. Reson. Imaging 18:169–174, 2003.
Huijbregts, H. J., P. J. Blankestijn, C. G. Caro, N. J. Cheshire, M. T. Hoedt, and R. P. Tutein. Nolthenius, and F. L. Moll. A helical PTFE arteriovenous access graft to swirl flow across the distal anastomosis: results of a preliminary clinical study. Eur. J. Vasc. Endovasc. Surg. 33:472–475, 2007.
Jackson, S. P., W. S. Nesbitt, and E. Westein. Dynamics of platelet thrombus formation. J. Thromb. Haemost. 7(Suppl 1):17–20, 2009.
Jahrome, O. K., I. Hoefer, G. J. Houston, P. A. Stonebridge, P. J. Blankestijn, F. L. Moll, and G. J. de Borst. Hemodynamic effects of spiral ePTFE prosthesis compared with standard arteriovenous graft in a carotid to jugular vein porcine model. J. Vasc. Access 12:224–230, 2011.
Jin, S., J. Oshinski, and D. P. Giddens. Effects of wall motion and compliance on flow patterns in the ascending aorta. J. Biomech. Eng. 125:347–354, 2003.
Kaazempur-Mofrad, M. R., and C. R. Ethier. Mass transport in an anatomically realistic human right coronary artery. Ann. Biomed. Eng. 29:121–127, 2001.
Karino, T., H. L. Goldsmith, M. Motomiya, S. Mabuchi, and Y. Sohara. Flow patterns in vessels of simple and complex geometries. Ann. N. Y. Acad. Sci. 516:422–441, 1987.
Kilner, P. J., G. Z. Yang, R. H. Mohiaddin, D. N. Firmin, and D. B. Longmore. Helical and retrograde secondary flow patterns in the aortic arch studied by three-directional magnetic resonance velocity mapping. Circulation 88:2235–2247, 1993.
Knobloch, V., C. Binter, U. Gulan, A. Sigfridsson, M. Holzner, B. Luthi, and S. Kozerke. Mapping mean and fluctuating velocities by Bayesian multipoint MR velocity encoding-validation against 3D particle tracking velocimetry. Magn. Reson. Med. 71:1405–1415, 2014.
Koskinas, K. C., Y. S. Chatzizisis, A. P. Antoniadis, and G. D. Giannoglou. Role of endothelial shear stress in stent restenosis and thrombosis: pathophysiologic mechanisms and implications for clinical translation. J. Am. Coll. Cardiol. 59:1337–1349, 2012.
Ku, D. N., D. P. Giddens, C. K. Zarins, and S. Glagov. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis 5:293–302, 1985.
Lee, K. E., J. S. Lee, and J. Y. Yoo. A numerical study on steady flow in helically sinuous vascular prostheses. Med. Eng. Phys. 33:38–46, 2011.
Liu, X., Y. Fan, and X. Deng. Effect of spiral flow on the transport of oxygen in the aorta: a numerical study. Ann. Biomed. Eng. 38:917–926, 2010.
Liu, X., Y. Fan, X. Deng, and F. Zhan. Effect of non-Newtonian and pulsatile blood flow on mass transport in the human aorta. J. Biomech. 44:1123–1131, 2011.
Liu, X., Y. Fan, A. Sun, and X. Deng. Numerical simulation of nucleotide transport in the human thoracic aorta. J. Biomech. 46:819–827, 2013.
Liu, X., Y. Fan, X. Y. Xu, and X. Deng. Nitric oxide transport in an axisymmetric stenosis. J. R. Soc. Interface 9:2468–2478, 2012.
Liu, X., F. Pu, Y. Fan, X. Deng, D. Li, and S. Li. A numerical study on the flow of blood and the transport of LDL in the human aorta: the physiological significance of the helical flow in the aortic arch. Am. J. Physiol. Heart Circ. Physiol. 297:H163–H170, 2009.
Losi, P., S. Lombardi, E. Briganti, and G. Soldani. Luminal surface microgeometry affects platelet adhesion in small-diameter synthetic grafts. Biomaterials 25:4447–4455, 2004.
Loth, F., P. F. Fischer, and H. S. Bassiouny. Blood flow in end-to-side anastomoses. Annu. Rev. Fluid Mech. 40:367–393, 2008.
Lurie, F., and R. L. Kistner. On the existence of helical flow in veins of the lower extremities. J. Vasc. Surg.: Venous Lymphat. Disord. 1:134–138, 2013.
Mahadevia, R., A. J. Barker, S. Schnell, P. Entezari, P. Kansal, P. W. Fedak, S. C. Malaisrie, P. McCarthy, J. Collins, J. Carr, and M. Markl. Bicuspid aortic cusp fusion morphology alters aortic three-dimensional outflow patterns, wall shear stress, and expression of aortopathy. Circulation 129:673–682, 2014.
Malek, A. M., S. L. Alper, and S. Izumo. Hemodynamic shear stress and its role in atherosclerosis. JAMA 282:2035–2042, 1999.
Markl, M., M. T. Draney, M. D. Hope, J. M. Levin, F. P. Chan, M. T. Alley, N. J. Pelc, and R. J. Herfkens. Time-resolved 3-dimensional velocity mapping in the thoracic aorta: visualization of 3-directional blood flow patterns in healthy volunteers and patients. J. Comput. Assist. Tomogr. 28:459–468, 2004.
Markl, M., M. T. Draney, D. C. Miller, J. M. Levin, E. E. Williamson, N. J. Pelc, D. H. Liang, and R. J. Herfkens. Time-resolved three-dimensional magnetic resonance velocity mapping of aortic flow in healthy volunteers and patients after valve-sparing aortic root replacement. J. Thorac. Cardiovasc. Surg. 130:456–463, 2005.
Markl, M., A. Frydrychowicz, S. Kozerke, M. Hope, and O. Wieben. 4D flow MRI. J. Magn. Reson. Imaging 36:1015–1036, 2012.
Markl, M., P. J. Kilner, and T. Ebbers. Comprehensive 4D velocity mapping of the heart and great vessels by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 13:7, 2011.
Meierhofer, C., E. P. Schneider, C. Lyko, A. Hutter, S. Martinoff, M. Markl, A. Hager, J. Hess, H. Stern, and S. Fratz. Wall shear stress and flow patterns in the ascending aorta in patients with bicuspid aortic valves differ significantly from tricuspid aortic valves: a prospective study. Eur. Heart J. Cardiovasc. Imaging 14:797–804, 2013.
Morbiducci, U., D. Gallo, D. Massai, F. Consolo, R. Ponzini, L. Antiga, C. Bignardi, M. A. Deriu, and A. Redaelli. Outflow conditions for image-based hemodynamic models of the carotid bifurcation: implications for indicators of abnormal flow. J. Biomech. Eng. 132:091005, 2010.
Morbiducci, U., D. Gallo, R. Ponzini, D. Massai, L. Antiga, F. M. Montevecchi, and A. Redaelli. Quantitative analysis of bulk flow in image-based hemodynamic models of the carotid bifurcation: the influence of outflow conditions as test case. Ann. Biomed. Eng. 38:3688–3705, 2010.
Morbiducci, U., R. Ponzini, D. Gallo, C. Bignardi, and G. Rizzo. Inflow boundary conditions for image-based computational hemodynamics: impact of idealized versus measured velocity profiles in the human aorta. J. Biomech. 46:102–109, 2013.
Morbiducci, U., R. Ponzini, M. Grigioni, and A. Redaelli. Helical flow as fluid dynamic signature for atherogenesis risk in aortocoronary bypass: a numeric study. J. Biomech. 40:519–534, 2007.
Morbiducci, U., R. Ponzini, G. Rizzo, M. E. Biancolini, F. Iannaccone, D. Gallo, and A. Redaelli. Synthetic dataset generation for the analysis and the evaluation of image-based hemodynamics of the human aorta. Med. Biol. Eng. Comput. 50:145–154, 2012.
Morbiducci, U., R. Ponzini, G. Rizzo, M. Cadioli, A. Esposito, F. De Cobelli, A. Del Maschio, F. M. Montevecchi, and A. Redaelli. In vivo quantification of helical blood flow in human aorta by time-resolved three-dimensional cine phase contrast magnetic resonance imaging. Ann. Biomed. Eng. 37:516–531, 2009.
Morbiducci, U., R. Ponzini, G. Rizzo, M. Cadioli, A. Esposito, F. M. Montevecchi, and A. Redaelli. Mechanistic insight into the physiological relevance of helical blood flow in the human aorta: an in vivo study. Biomech. Model. Mechanobiol. 10:339–355, 2011.
Naphon, P., and S. Wongwises. A review of flow and heat transfer characteristics in curved tubes. Renew. Sustain. Energy Rev. 10:463–490, 2006.
Nesbitt, W. S., E. Westein, F. J. Tovar-Lopez, E. Tolouei, A. Mitchell, J. Fu, J. Carberry, A. Fouras, and S. P. Jackson. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat. Med. 15:665–673, 2009.
Papaharilaou, Y., D. J. Doorly, and S. J. Sherwin. The influence of out-of-plane geometry on pulsatile flow within a distal end-to-side anastomosis. J. Biomech. 35:1225–1239, 2002.
Paul, M. C., and A. Larman. Investigation of spiral blood flow in a model of arterial stenosis. Med. Eng. Phys. 31:1195–1203, 2009.
Sauvage, L. R., M. W. Walker, K. Berger, S. B. Robel, M. M. Lischko, S. G. Yates, and G. A. Logan. Current arterial prostheses. Experimental evaluation by implantation in the carotid and circumflex coronary arteries of the dog. Arch. Surg. 114:687–691, 1979.
Scanlon, V. C., and T. Sanders. Essentials of Anatomy and Physiology, 5th edition. Philadelphia: F. A. Davis Company, 2007, pp. 297–297.
Seed, W. A., and N. B. Wood. Velocity patterns in the aorta. Cardiovasc. Res. 5:319–330, 1971.
Segadal, L., and K. Matre. Blood velocity distribution in the human ascending aorta. Circulation 76:90–100, 1987.
Sherwin, S. J., O. Shah, D. J. Doorly, J. Peiro, Y. Papaharilaou, N. Watkins, C. G. Caro, and C. L. Dumoulin. The influence of out-of-plane geometry on the flow within a distal end-to-side anastomosis. J. Biomech. Eng.-T ASME 122:86–95, 2000.
Stankovic, Z., B. D. Allen, J. Garcia, K. B. Jarvis, and M. Markl. 4D flow imaging with MRI. Cardiovasc. Diagn. Ther. 4:173–192, 2014.
Stary, H. C., A. B. Chandler, S. Glagov, J. R. Guyton, W. Insull, Jr., M. E. Rosenfeld, S. A. Schaffer, C. J. Schwartz, W. D. Wagner, and R. W. Wissler. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 89:2462–2478, 1994.
Stonebridge, P. A., and C. M. Brophy. Spiral laminar flow in arteries? Lancet 338:1360–1361, 1991.
Stonebridge, P. A., P. R. Hoskins, P. L. Allan, and J. F. Belch. Spiral laminar flow in vivo. Clin. Sci. (Lond). 91:17–21, 1996.
Stonebridge, P. A., F. Vermassen, J. Dick, J. J. Belch, and G. Houston. Spiral laminar flow prosthetic bypass graft: medium-term results from a first-in-man structured registry study. Ann. Vasc. Surg. 26:1093–1099, 2012.
Sun, A., Y. Fan, and X. Deng. Numerical investigation of blood flow in the distal end of an axis-deviated arterial bypass model. Biorheology. 46:83–92, 2009.
Sun, A. Q., Y. B. Fan, and X. Y. Deng. Numerical comparative study on the hemodynamic performance of a new helical graft with noncircular cross section and SwirlGraft. Artif. Organs. 34:22–27, 2010.
Sun, A. Q., Y. B. Fan, and X. Y. Deng. Intentionally induced swirling flow may improve the hemodynamic performance of coronary bifurcation stenting. Catheter. Cardiovasc. Interv. 79:371–377, 2012.
Tarbell, J. M. Mass transport in arteries and the localization of atherosclerosis. Annu. Rev. Biomed. Eng. 5:79–118, 2003.
Uchida, Y., T. Tomaru, F. Nakamura, A. Furuse, Y. Fujimori, and K. Hasegawa. Percutaneous coronary angioscopy in patients with ischemic heart disease. Am. Heart J. 114:1216–1222, 1987.
Van Canneyt, K., U. Morbiducci, S. Eloot, G. De Santis, P. Segers, and P. Verdonck. A computational exploration of helical arterio-venous graft designs. J. Biomech. 46:345–353, 2013.
Van Langenhove, G., J. J. Wentzel, R. Krams, C. J. Slager, J. N. Hamburger, and P. W. Serruys. Helical velocity patterns in a human coronary artery: a three-dimensional computational fluid dynamic reconstruction showing the relation with local wall thickness. Circulation 102:E22–E24, 2000.
Wen, J., T. H. Zheng, W. T. Jiang, X. Y. Deng, and Y. B. Fan. A comparative study of helical-type and traditional-type artery nypass grafts: numerical simulation. ASAIO J. 57:399–406, 2011.
Wetzel, S., S. Meckel, A. Frydrychowicz, L. Bonati, E. W. Radue, K. Scheffler, J. Hennig, and M. Markl. In vivo assessment and visualization of intracranial arterial hemodynamics with flow-sensitized 4D MR imaging at 3T. AJNR Am. J. Neuroradiol. 28:433–438, 2007.
Wootton, D. M., and D. N. Ku. Fluid mechanics of vascular systems, diseases, and thrombosis. Annu. Rev. Biomed. Eng. 1:299–329, 1999.
Yamamoto, K., A. Aribowo, Y. Hayamizu, T. Hirose, and K. Kawahara. Visualization of the flow in a helical pipe. Fluid Dyn. Res. 30:251–267, 2002.
Yashiro, K., H. Shiratori, and H. Hamada. Haemodynamics determined by a genetic programme govern asymmetric development of the aortic arch. Nature 450:285–288, 2007.
Zabielski, L., and A. J. Mestel. Steady flow in a helically symmetric pipe. J. Fluid Mech. 370:297–320, 1998.
Zabielski, L., and A. J. Mestel. Unsteady blood flow in a helically symmetric pipe. J. Fluid Mech. 370:321–345, 1998.
Zabielski, L., and A. J. Mestel. Helical flow around arterial bends for varying body mass. J. Biomech. Eng. 122:135–142, 2000.
Zhan, F., Y. Fan, and X. Deng. Swirling flow created in a glass tube suppressed platelet adhesion to the surface of the tube: its implication in the design of small-caliber arterial grafts. Thromb. Res. 125:413–418, 2010.
Zhan, F., Y. B. Fan, and X. Y. Deng. Effect of swirling flow on platelet concentration distribution in small-caliber artificial grafts and end-to-end anastomoses. Acta Mech Sinica. 27:833–839, 2011.
Zhan, F., Y. B. Fan, X. Y. Deng, and Z. P. Xu. The beneficial effect of swirling flow on platelet adhesion to the surface of a sudden tubular expansion tube: its potential application in end-to-end arterial anastomosis. ASAIO J. 56:172–179, 2010.
Zhang, Z., Y. Fan, X. Deng, G. Wang, H. Zhang, and R. Guidoin. Simulation of blood flow in a small-diameter vascular graft model with a swirl (spiral) flow guider. Sci. China C Life Sci. 51:913–921, 2008.
Zheng, T., J. Wen, W. Jiang, X. Deng, and Y. Fan. Numerical investigation of oxygen mass transfer in a helical-type artery bypass graft. Comput. Methods Biomech. Biomed. Eng. 17:549–559, 2014.
Zheng, T. H., Y. B. Fan, Y. Xiong, W. T. Jiang, and X. Y. Deng. Hemodynamic performance study on small diameter helical grafts. ASAIO J. 55:192–199, 2009.
Acknowledgments
This work is supported by Grants-in-Aid from the National Natural Science Research Foundation of China (No. 11332003, 31200703, 11102014, 11202016, 11421202), Specialized Research Fund for the Doctoral Program of Higher Education (20121102120038), Special Fund for Excellent Doctor Degree Dissertation of Beijing (20131000601) and the 111 Project (B13003).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Associate Editor Andreas Anayiotos oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Liu, X., Sun, A., Fan, Y. et al. Physiological Significance of Helical Flow in the Arterial System and its Potential Clinical Applications. Ann Biomed Eng 43, 3–15 (2015). https://doi.org/10.1007/s10439-014-1097-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-014-1097-2