Abstract
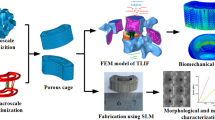
Interbody fusion cages are frequently used clinically for the treatment of infectious and degenerative diseases of the spine, however, further clinical applications are often plagued by some drawbacks, such as stress shielding and cage subsidence. This study aims to propose a new concept of personalized design of spinal cages: a multi-scale joint design approach from microscopic to macroscopic to create a spinal cage that matches the elastic modulus of the lumbar bony endplate and the surgical procedure. Specifically, a triple periodic minimal surface (TPMS) is introduced to simulate the porous structure of polyetheretherketone (PEEK) material cage, and the pore parameters are adjusted to achieve customized macroscopic mechanical properties and permeability of the pore structure. Then, multi-objective (flexion-extension, lateral bending, and axial torsion) topology optimization was performed for percutaneous endoscopic posterior lumbar interbody fusion (PE-PLIF) surgery to obtain a new porous topology cage of secondary design. Finally, a comparative biomechanical analysis based on the finite element method was performed on the commonly used clinical titanium cage, PEEK cage, and the new porous topology cage designed in this paper. The results showed that the new porous topology cage had lower bone-cage interface stress and higher internal bone graft stress and strain energy density (SED) compared to the other two cages, demonstrating a lower cage subsidence rate and risk of stress shielding. In conclusion, the new porous topology cage is an ideal choice for interbody fusion due to its good biomechanical properties. The cage design ideas and concepts presented in this paper can also be used to guide other different intervertebral fusion procedures.

摘要
椎间融合器在临床上经常用于脊柱感染性和退行性疾病的治疗, 然而, 进一步的临床应用通常受到一些缺陷的困扰, 如应力遮 挡和融合器沉降. 本研究旨在提出一种新的脊柱融合器个性化设计理念: 从微观到宏观的多尺度联合设计方法, 创建与腰椎骨终板弹 性模量和手术形式皆匹配的脊柱融合器. 具体而言, 引入三周期极小曲面来模拟聚醚醚酮(PEEK)融合器的多孔结构, 通过调整孔隙参 数以实现定制的宏观力学性能和孔隙结构的渗透性能. 接着, 针对经皮内镜下腰椎后路椎间融合术(PE-PLIF)进行多目标(屈伸、弯曲 和扭转)拓扑优化, 得到二次设计的新型多孔拓扑融合器. 最后, 将临床常用的钛融合器、PEEK融合器和本文设计的新型融合器进行 基于有限元方法的生物力学对比分析. 结果表明, 与其他两种融合器相比, 新型多孔拓扑融合器有着较低的骨-融合器界面应力和较高 的内部移植骨应力和应变能密度, 证明其有着较低的融合器沉降率和应力屏蔽的风险. 总之, 新型多孔拓扑融合器由于其良好的生物 力学性能成为椎间融合术的理想选择. 本文所提出的融合器设计思路和理念也可用于指导其他不同椎间融合术.
Similar content being viewed by others
References
D. Shedid, and E. C. Benzel, Decision making process, Neurosurgery 60, S1 (2007).
A. Abbushi, M. Čabraja, U. W. Thomale, C. Woiciechowsky, and S. N. Kroppenstedt, The influence of cage positioning and cage type on cage migration and fusion rates in patients with monosegmental posterior lumbar interbody fusion and posterior fixation, Eur. Spine J. 18, 1621 (2009).
H. Huang, J. Liu, L. Wang, and Y. Fan, A critical review on the biomechanical study of cervical interbody fusion cage, Med. Novel Tech. Devices 11, 100070 (2021).
E. Zhou, H. Huang, Y. Zhao, L. Wang, and Y. Fan, The effects of titanium mesh cage size on the biomechanical responses of cervical spine after anterior cervical corpectomy and fusion: A finite element study, Clin. Biomech. 91, 105547 (2022).
N. Liu, T. Lu, Y. Wang, Z. Sun, J. Li, and X. He, Effects of new cage profiles on the improvement in biomechanical performance of multilevel anterior cervical corpectomy and fusion: A finite element analysis, World Neurosurg. 129, e87 (2019).
Y. Yao, L. Wang, J. Li, S. Tian, M. Zhang, and Y. Fan, A novel auxetic structure based bone screw design: Tensile mechanical characterization and pullout fixation strength evaluation, Mater. Des. 188, 108424 (2020).
Y. Yao, H. Yuan, H. Huang, J. Liu, L. Wang, and Y. Fan, Biomechanical design and analysis of auxetic pedicle screw to resist loosening, Comput. Biol. Med. 133, 104386 (2021).
H. J. Choi, J. J. Lee, Y. J. Park, J. W. Shin, H. J. Sung, J. W. Shin, Y. Wu, and J. K. Kim, MG-63 osteoblast-like cell proliferation on auxetic PLGA scaffold with mechanical stimulation for bone tissue regeneration, Biomater. Res. 20, 33 (2016).
V. A. Lvov, F. S. Senatov, A. S. Shinkaryov, S. V. Chernyshikhin, A. A. Gromov, and V. A. Sheremetyev, Experimental 3D printed reentrant auxetic and honeycomb spinal cages based on Ti-6Al-4V: Computer-aided design concept and mechanical characterization, Compos. Struct. 310, 116766 (2023).
K. Ou, Q. Liu, X. Liu, Q. Fu, J. Fan, and Y. Sun, Self-exothermic esterification-crosslinking of bio-polymer/graphene composite for application in interbody fusion cage, MRS Commun. 13, 8 (2023).
S. M. Kurtz, and J. N. Devine, PEEK biomaterials in trauma, orthopedic, and spinal implants, Biomaterials 28, 4845 (2007).
C. Caparrós, J. Guillem-Martí, M. Molmeneu, M. Punset, J. A. Calero, and F. J. Gil, Mechanical properties and in vitro biological response to porous titanium alloys prepared for use in intervertebral implants, J. Mech. Behav. Biomed. Mater. 39, 79 (2014).
Y. N. Chen, and C. W. Chang, Computational comparison of three different cage porosities in posterior lumbar interbody fusion with porous cage, Comput. Biol. Med. 139, 105036 (2021).
O. Nemoto, T. Asazuma, Y. Yato, H. Imabayashi, H. Yasuoka, and A. Fujikawa, Comparison of fusion rates following transforaminal lumbar interbody fusion using polyetheretherketone cages or titanium cages with transpedicular instrumentation, Eur. Spine J. 23, 2150 (2014).
Y. Assem, R. J. Mobbs, M. H. Pelletier, K. Phan, and W. R. Walsh, Radiological and clinical outcomes of novel Ti/PEEK combined spinal fusion cages: A systematic review and preclinical evaluation, Eur. Spine J. 26, 593 (2017).
N. Z. Zhang, Q. S. Xiong, J. Yao, B. L. Liu, M. Zhang, and C. K. Cheng, Biomechanical changes at the adjacent segments induced by a lordotic porous interbody fusion cage, Comput. Biol. Med. 143, 105320 (2022).
H. Wang, Y. Wan, Q. Li, Y. Xia, X. Liu, Z. Liu, and X. Li, Porous fusion cage design via integrated global-local topology optimization and biomechanical analysis of performance, J. Mech. Behav. Biomed. Mater. 112, 103982 (2020).
S. Rastegar, P. J. Arnoux, X. Wang, and C. É. Aubin, Biomechanical analysis of segmental lumbar lordosis and risk of cage subsidence with different cage heights and alternative placements in transforaminal lumbar interbody fusion, Comput. Methods Biomech. Biomed. Eng. 23, 456 (2020).
L. He, Q. Xiang, Y. Yang, T. Y. Tsai, Y. Yu, and L. Cheng, The anterior and traverse cage can provide optimal biomechanical performance for both traditional and percutaneous endoscopic transforaminal lumbar interbody fusion, Comput. Biol. Med. 131, 104291 (2021).
A. Calvo-Echenique, J. Cegoñino, R. Chueca, and A. Pérez-del Palomar, Stand-alone lumbar cage subsidence: A biomechanical sensitivity study of cage design and placement, Comput. Methods Programs Biomed. 162, 211 (2018).
M. Wohlgemuth, N. Yufa, J. Hoffman, and E. L. Thomas, Triply periodic bicontinuous cubic microdomain morphologies by symmetries, Macromolecules 34, 6083 (2001).
X. Li, C. Wang, C. Tian, S. Fu, Y. Rong, and L. Wang, Digital design and performance evaluation of porous metal-bonded grinding wheels based on minimal surface and 3D printing, Mater. Des. 203, 109556 (2021).
B. Jetté, V. Brailovski, M. Dumas, C. Simoneau, and P. Terriault, Femoral stem incorporating a diamond cubic lattice structure: Design, manufacture and testing, J. Mech. Behav. Biomed. Mater. 77, 58 (2018).
C. Simoneau, P. Terriault, B. Jetté, M. Dumas, and V. Brailovski, Development of a porous metallic femoral stem: Design, manufacturing, simulation and mechanical testing, Mater. Des. 114, 546 (2017).
L. Li, J. Shi, K. Zhang, L. Yang, F. Yu, L. Zhu, H. Liang, X. Wang, and Q. Jiang, Early osteointegration evaluation of porous Ti6Al4V scaffolds designed based on triply periodic minimal surface models, J. Orthopaedic Translation 19, 94 (2019).
P. H. Warnke, T. Douglas, P. Wollny, E. Sherry, M. Steiner, S. Galonska, S. T. Becker, I. N. Springer, J. Wiltfang, and S. Sivananthan, Rapid prototyping: Porous titanium alloy scaffolds produced by selective laser melting for bone tissue engineering, Tissue Eng. Part C-Methods 15, 115 (2008).
X. P. Tan, Y. J. Tan, C. S. L. Chow, S. B. Tor, and W. Y. Yeong, Metallic powder-bed based 3D printing of cellular scaffolds for orthopaedic implants: A state-of-the-art review on manufacturing, topological design, mechanical properties and biocompatibility, Mater. Sci. Eng.-C 76, 1328 (2017).
N. Taniguchi, S. Fujibayashi, M. Takemoto, K. Sasaki, B. Otsuki, T. Nakamura, T. Matsushita, T. Kokubo, and S. Matsuda, Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment, Mater. Sci. Eng.-C 59, 690 (2016).
M. Belwanshi, P. Jayaswal, and A. Aherwar, Mechanical behaviour investigation of PEEK coated titanium alloys for hip arthroplasty using finite element analysis, Mater. Today-Proc. 56, 2808 (2022).
S. P. Singh, M. Shukla, and R. K. Srivastava, Lattice modeling and CFD simulation for prediction of permeability in porous scaffolds, Mater. Today-Proc. 5, 18879 (2018).
H. Chen, Y. Liu, C. Wang, A. Zhang, B. Chen, Q. Han, and J. Wang, Design and properties of biomimetic irregular scaffolds for bone tissue engineering, Comput. Biol. Med. 130, 104241 (2021).
Y. Yan, J. Li, J. Yu, Y. Wang, H. Dong, Y. Sun, X. Wu, L. He, W. Chen, and H. Feng, Biomechanical evaluation of two fusion techniques based on finite element analysis: Percutaneous endoscopic and minimally invasive transforaminal lumbar interbody fusion, Med. Novel Tech. Devices 16, 100138 (2022).
X. Zhao, L. Du, Y. Xie, and J. Zhao, Effect of lumbar lordosis on the adjacent segment in transforaminal lumbar interbody fusion: A finite element analysis, World Neurosurg. 114, e114 (2018).
L. X. Guo, and W. J. Li, A biomechanical investigation of thoracolumbar burst fracture under vertical impact loads using finite element method, Clin. Biomech. 68, 29 (2019).
A. Kahaer, Z. Zhou, J. Maitirouzi, S. Wang, W. Shi, N. Abuduwaili, X. Maimaiti, D. Liu, W. Sheng, and P. Rexiti, Biomechanical investigation of the posterior pedicle screw fixation system at level L4–L5 lumbar segment with traditional and cortical trajectories: A finite element study, J. Healthcare Eng. 2022, 4826507 (2022).
B. Eidel, A. Gote, C. P. Fritzen, A. Ohrndorf, and H. J. Christ, Tibial implant fixation in TKA worth a revision?—how to avoid stress-shielding even for stiff metallic implants, Comput. Methods Biomech. Biomed. Eng. 24, 320 (2021).
L. Wang, X. Ding, W. Feng, Y. Gao, S. Zhao, and Y. Fan, Biomechanical study on implantable and interventional medical devices, Acta Mech. Sin. 37, 875 (2021).
S. J. Hollister, Scaffold design and manufacturing: From concept to clinic, Adv. Mater. 21, 3330 (2009).
S. Gómez, M. D. Vlad, J. López, and E. Fernández, Design and properties of 3D scaffolds for bone tissue engineering, Acta Biomater. 42, 341 (2016).
B. M. Ferguson, A. Entezari, J. Fang, and Q. Li, Optimal placement of fixation system for scaffold-based mandibular reconstruction, J. Mech. Behav. Biomed. Mater. 126, 104855 (2022).
W. A. Chapkin, D. L. Simone, G. J. Frank, and J. W. Baur, Mechanical behavior and energy dissipation of infilled, composite Ti-6Al-4V trusses, Mater. Des. 203, 109602 (2021).
M. van Dijk, T. H. Smit, S. Sugihara, E. H. Burger, and P. I. Wuisman, The effect of cage stiffness on the rate of lumbar interbody fusion, Spine 27, 682 (2002).
Y. Wu, J. Loaiza, R. Banerji, O. Blouin, and E. Morgan, Structure-function relationships of the human vertebral endplate, JOR Spine 4, e1170 (2021).
P. J. Ehrlich, and L. E. Lanyon, Mechanical strain and bone cell function: A review, Osteoporos. Int. 13, 688 (2002).
D. Ambrosi, G. A. Ateshian, E. M. Arruda, S. C. Cowin, J. Dumais, A. Goriely, G. A. Holzapfel, J. D. Humphrey, R. Kemkemer, E. Kuhl, J. E. Olberding, L. A. Taber, and K. Garikipati, Perspectives on biological growth and remodeling, J. Mech. Phys. Solids 59, 863 (2011).
T. Adachi, Y. Osako, M. Tanaka, M. Hojo, and S. J. Hollister, Framework for optimal design of porous scaffold microstructure by computational simulation of bone regeneration, Biomaterials 27, 3964 (2006).
H. Wang, Y. Wan, Q. Li, X. Liu, M. Yu, X. Zhang, Y. Xia, Q. Sun, and Z. Liu, Multiscale design and biomechanical evaluation of porous spinal fusion cage to realize specified mechanical properties, Bio-des. Manuf. 5, 277 (2022).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 11972242 and 12272250), China Postdoctoral Science Foundation (Grant No. 2020M680913), Shanxi Scholarship Council of China and Shanxi Postgraduate Innovation Project, and Shanxi Huajin Orthopaedic Public Foundation.
Author information
Authors and Affiliations
Contributions
Yang Yan conducted a literature search and wrote the first draft of the article. Jianhao Yu, Yan Wang, and Hao Dong drew the figures. Yanqin Wang commented on previous versions of the manuscript and made changes to the manuscript. Liming He provided the model parameters. Yanru Xue and Xiaogang Wu revised and edited the final version. Haoyu Feng and Weiyi Chen are responsible for overseeing and leading the planning and execution of research activities, including mentorship external to the core team.
Corresponding authors
Additional information
Conflict of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Yan, Y., Yu, J., Wang, Y. et al. A newly designed personalized interbody fusion cage and its biomechanical analysis. Acta Mech. Sin. 39, 623047 (2023). https://doi.org/10.1007/s10409-023-23047-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10409-023-23047-x