Abstract
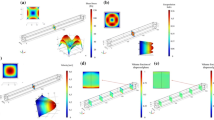
An investigation of red blood cells (RBCs) margination’s dependence on channel cross-section shape is presented. The irregularity of the vascular cross-section has been proved to satisfy the condition for stiffened RBCs to perform margination in vivo, while the effect of channel geometry along channel width on cell margination has not been revealed. To illustrate this problem, RBCs’ flowing behaviors in three different microchannels with cross-section of circular, rectangular and irregular are investigated, the forces acted on normal and stiffened RBCs are analyzed and calculated, the motions of RBCs are simulated, and the combined effect of channel geometry and fluid property is demonstrated. The stiffened RBCs are found to perform margination in rectangular and irregular channel with viscoelastic fluid, while not in circular channel under the same fluid condition. Furthermore, the importance of fluid viscoelasticity to cell margination is demonstrated in different microchannel. Our findings might offer some new insights to design microfluidic devices for cell-sorting technology and drug delivery system with high efficiency.





Similar content being viewed by others
References
Abbitt KB, Nash GB (2001) Characteristics of leucocyte adhesion directly observed in flowing whole blood in vitro. Brit J Haematol 112:55–63
Abkarian M, Viallat A (2005) Dynamics of vesicles in a wall-bounded shear flow. Biophys J 89:1055–1066
Abkarian M, Lartigue C, Viallat A (2002) Tank treading and unbinding of deformable vesicles in shear flow: determination of the lift force. Phys Rev Lett 88:068103
Alapan Y, Little JA, Gurkan UA (2014) Heterogeneous red blood cell adhesion and deformability in sickle cell disease. Sci Rep 4:7173
Alapan Y, Kim C, Adhikari A, Gray KE, Gurkan-Cavusoglu E, Little JA, Gurkan UA (2016a) Sickle cell disease biochip: a functional red blood cell adhesion assay for monitoring sickle cell disease. Transl Res 173:74–91 (e78)
Alapan Y, Matsuyama Y, Little JA, Gurkan UA (2016b) Dynamic deformability of sickle red blood cells in microphysiological flow. Technology (Singap World Sci) 4:71–79
Barr JD, Chauhan AK, Schaeffer GV et al (2013) Red blood cells mediate the onset of thrombosis in the ferric chloride murine model. Blood 121(18):3733–3741
Byrnes JR, Wolberg AS (2017) Red blood cells in thrombosis. Blood 130(16):1795
Callens N, Minetti C, Coupier G, Mader MA, Dubois F, Misbah C, Podgorski T (2008) Hydrodynamic lift of vesicles under shear flow in microgravity. EPL 83:24002
Chen X, Cui DF, Chen J (2009) Design, fabrication and characterization of nano-filters in silicon microfluidic channels based on MEMS technology. Electrophoresis 30:3168–3173
Chen Y, Li D, Li Y, Wan J, Li J, Chen H (2017) Margination of stiffened red blood cells regulated by vessel geometry. Sci Rep 7:15253
D'Apolito R, Tomaiuolo G, Taraballi F et al (2015) Red blood cells affect the margination of microparticles in synthetic microcapillaries and intravital microcirculation as a function of their size and shape. J Controlled Release 217(8 Supplement):263–272
D'Avino G, Greco F, Maffettone PL (2017) Particle migration due to viscoelasticity of the suspending liquid, and Its relevance in microfluidic devices. Annu Rev Fluid Mech 49(1):341–360
Dong-Yeon L, Dong-Min K, Dae-Gab G, Jinwon P (2007) A calibrated atomic force microscope using an orthogonal scanner and a calibrated laser interferometer. Appl Surf Sci 253(8):3945–3951
Doshi N, Prabhakarpandian B, Rea-Ramsey A, Pant K, Sundaram S, Mitragotri S (2010) Flow and adhesion of drug carriers in blood vessels depend on their shape: a study using model synthetic microvascular networks. J Control Release 146:196–200
Fiebig E, Ley K, Arfors KE (1991) Rapid leukocyte accumulation by "spontaneous" rolling and adhesion in the exteriorized rabbit mesentery. Int J Microcirc Clin Exp 10(2):127
Freund JB (2007) Leukocyte margination in a model microvessel. Phys Fluids 19(2):023301
Gentile F, Chiappini C, Fine D et al (2008) The effect of shape on the margination dynamics of non-neutrally buoyant particles in two-dimensional shear flows. J Biomech 41(10):2312–2318
Glenister FK, Coppel RL, Cowman AF, Mohandas N, Cooke BM (2002) Contribution of parasite proteins to altered mechanical properties of malaria-infected red blood cells. Blood 99:1060–1063
Guo Q, Duffy SP, Matthews K, Deng XY, Santoso AT, Islamzada E, Ma HS (2016) Deformability based sorting of red blood cells improves diagnostic sensitivity for malaria caused by Plasmodium falciparum. Lab Chip 16:645–654
Hach T, Johansson BL, Ekberg K, Forst T, Kunt T, Wahren J (2002) C-peptide and its fragments increase red blood cell deformability in type 1 diabetes. Diabetes 51:A621–A621
Hou HW, Bhagat AA, Chong AG, Mao P, Tan KS, Han J, Lim CT (2010) Deformability based cell margination—a simple microfluidic design for malaria-infected erythrocyte separation. Lab Chip 10:2605–2613
Hur SC, Henderson-MacLennan NK, McCabe ERB, Di Carlo D (2011) Deformability-based cell classification and enrichment using inertial microfluidics. Lab Chip 11:912–920
Jain A, Munn LL (2009) Determinants of leukocyte margination in rectangular microchannels. Plos One 4:e7104
Katanov D, Gompper G, Fedosov DA (2015) Microvascular blood flow resistance: role of red blood cell migration and dispersion. Microvasc Res 99:57–66
Kim S, Ong PK, Yalcin O, Intaglietta M, Johnson PC (2009) The cell-free layer in microvascular blood flow. Biorheology 46:181–189
Kumar A, Graham MD (2012) Margination and segregation in confined flows of blood and other multicomponent suspensions. Soft Matter 8:10536–10548
Leal LG (1980) Particle Motions in a Viscous Fluid. Fluid Mech 12:435–476
Mcdonald JC, Duffy DC, Anderson JR et al (2000) Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 2000:27–40
Nam J, Shin Y, Tan JKS, Lim YB, Lim CT, Kim S (2016) High-throughput malaria parasite separation using a viscoelastic fluid for ultrasensitive PCR detection. Lab on a Chip 16:2086–2092
Nix S, Imai Y, Ishikawa T (2016) Lateral migration of a capsule in a parabolic flow. J Biomech 49:2249–2254
Piagnerelli M, Boudjeltia KZ, Vanhaeverbeek M, Vincent JL (2003) Red blood cell rheology in sepsis. Intens Care Med 29:1052–1061
Ruano-Lopez JM, Aguirregabiria M, Tijero M, Arroyo MT, Elizalde J, Berganzo J, Aranburu I, Blanco FJ, Mayora K (2006) A new SU-8 process to integrate buried waveguides and sealed microchannels for a Lab-on-a-Chip. Sensor Actuat B-Chem 114:542–551
Shen SF, Ma C, Zhao L, Wang YL, Wang JC, Xu J, Li TB, Pang L, Wang JY (2014) High-throughput rare cell separation from blood samples using steric hindrance and inertial microfluidics. Lab Chip 14:2525–2538
Takeishi N, Imai Y, Nakaaki K et al (2014) Leukocyte margination at arteriole shear rate. Physiol Rep 2(6):e12037
Tanner RI, Huang X, Xue SC, Phan-Thien N (1998) Finite volume computational methods for polymer processing. In: Simulation of materials processing: theory, methods and applications, pp 3–9
Tehrani MA (1996) An experimental study of particle migration in pipe flow of viscoelastic fluids. J Rheol 40:1057–1077
Thompson AJ, Mastria EM, Eniola-Adefeso O (2013) The margination propensity of ellipsoidal micro/nanoparticles to the endothelium in human blood flow. Biomaterials 34(23):5863–5871
Toy R, Hayden E, Shoup C et al (2011) The effects of particle size, density and shape on margination of nanoparticles in microcirculation. Nanotechnology 22(11):115101
Tsukada K, Sekizuka E, Oshio C, Minamitani H (2001) Direct measurement of erythrocyte deformability in diabetes mellitus with a transparent microchannel capillary model and high-speed video camera system. Microvasc Res 61:231–239
Vahidkhah K, Bagchi P (2015) Microparticle shape effects on margination, near-wall dynamics and adhesion in a three-dimensional simulation of red blood cell suspension. Soft Matter 11(11):2097–2109
Vlahovska PM, Podgorski T, Misbah C (2009) Vesicles and red blood cells in flow: from individual dynamics to rheology. C R Phys 10:775–789
Wang Y, Mannino RG, Myers DR, Li W, Joiner CH, Lam WA (2015) Vessel geometry interacts with red blood cell stiffness to promote endothelial dysfunction in sickle cell disease. Blood 126(23):965
Yang S, Lee SS, Ahn SW, Kang K, Shim W, Lee G, Hyun K, Kim JM (2012) Deformability-selective particle entrainment and separation in a rectangular microchannel using medium viscoelasticity. Soft Matter 8:5011
Zhao H, Shaqfeh ESG, Narsimhan V (2012) Shear-induced particle migration and margination in a cellular suspension. Phys Fluids 24(1):011902
Acknowledgements
This work is supported by the National Natural Science Foundation of China (Grants no. 51322501 and no. 51420105006), and the Beijing NSFC project (no. 3172018). We also thank the support from “The Joint Foundation of Advance Research and the Ministry of Education, China (Research on Innovative Technology of Cold Atmospheric Plasma for Rapid Sterilization, Hemostasis and Healing)”. We thank Bing Dong for his support on this work, and thank Chi Zhang for his help on the numerical simulation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the topical collection “2018 International Conference of Microfluidics, Nanofluidics and Lab-on-a-Chip, Beijing, China” guest edited by Guoqing Hu, Ting Si and Zhaomiao Liu.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, Y., Li, Y., Li, D. et al. Margination mechanism of stiffened red blood cell in microchannel with different cross-section shapes. Microfluid Nanofluid 23, 25 (2019). https://doi.org/10.1007/s10404-019-2190-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-019-2190-5