Abstract
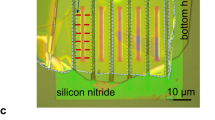
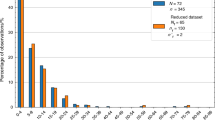
We present an experimental study that focuses on pressure-driven flow of distilled water through γ alumina membranes with 5, 10 and 20 nm pore radii. The nanopore geometry, pore size and porosity are characterized using scanning electron microscopy images taken pre- and post-flow experiments. Comparisons of these images have shown reduction in the pore size, which is attributed to precipitation of hydroxyl groups on alumina surfaces. Measured flowrates compared with the Hagen–Poiseuille flow relations consistently predict 2.2 nm reductions in the pore size for three different membranes. This behavior can be explained by the formation of a thick stick layer of water molecules over hydroxylated alumina surfaces, evidenced by water droplet contact angle measurements that exhibit increased hydrophilicity of alumina surfaces. Other possible effects of the mismatch between theory and experiments such as unaccounted pressure losses in the system or the streaming potential effects were also considered, but shown to be negligible for current experimental conditions.





Similar content being viewed by others
References
Abràmoff MD, Magalhães PJ, Ram SJ (2004) Image processing with ImageJ. Biophoton Int 11:36–42
Ai Y, Yalcin SE, Gu D, Baysal O, Baumgart H, Qian S, Beskok A (2010) A low-voltage nano-porous electroosmotic pump. J Colloid Interface Sci 350:465–470
Alexander M, Thompson G, Beamson G (2000) Characterization of the oxide/hydroxide surface of aluminium using x-ray photoelectron spectroscopy: a procedure for curve fitting the O 1 s core level. Surf Interface Anal 29:468–477
Alwitt RS (1976) Aluminium–water system oxides and oxide films, vol 4. Marcel Dekker, Inc, New York, pp 169–254
Belaud V, Valette S, Stremsdoerfer G, Bigerelle M, Benayoun S (2015) Wettability versus roughness: multi-scales approach. Tribol Int 82:343–349
Bowers A, Huang C (1985) Adsorption characteristics of polyacetic amino acids onto hydrous γ-Al2O3. J Colloid Interface Sci 105:197–215
Bowers AR, Huang C (1986) Adsorption characteristics of metal-EDTA complexes onto hydrous oxides. J Colloid Interface Sci 110:575–590
Burgreen D, Nakache F (1964) Electrokinetic flow in ultrafine capillary slits1. J Phys Chem 68:1084–1091
Cooper C, Burch R (1999) An investigation of catalytic ozonation for the oxidation of halocarbons in drinking water preparation. Water Res 33:3695–3700
Daiguji H, Yang P, Majumdar A (2004) Ion transport in nanofluidic channels. Nano Lett 4:137–142
Das S, Guha A, Mitra SK (2013) Exploring new scaling regimes for streaming potential and electroviscous effects in a nanocapillary with overlapping Electric Double Layers. Anal Chim Acta 804:159–166
De Gennes P-G (1985) Wetting: statics and dynamics. Rev Mod Phys 57:827
Digne M, Sautet P, Raybaud P, Euzen P, Toulhoat H (2004) Use of DFT to achieve a rational understanding of acid–basic properties of γ-alumina surfaces. J Catal 226:54–68
Duran C, Sato K, Hotta Y, Watari K (2007) Covalently connected particles in green bodies fabricated by tape casting. J Am Ceram Soc 90:279–282
Dutta P, Beskok A (2001) Analytical solution of combined electroosmotic/pressure driven flows in two-dimensional straight channels: finite Debye layer effects. Anal Chem 73:1979–1986
Ernst M, Lurot F, Schrotter J-C (2004) Catalytic ozonation of refractory organic model compounds in aqueous solution by aluminum oxide. Appl Catal B Environ 47:15–25
Falk K, Sedlmeier F, Joly L, Netz RR, Bocquet L (2010) Molecular origin of fast water transport in carbon nanotube membranes: superlubricity versus curvature dependent friction. Nano Lett 10:4067–4073
Fan R, Karnik R, Yue M, Li D, Majumdar A, Yang P (2005) DNA translocation in inorganic nanotubes. Nano Lett 5:1633–1637
Faust SD, Aly OM (1998) Chemistry of water treatment. CRC Press, Boca Raton
Figeys D, Aebersold R (1998) Nanoflow solvent gradient delivery from a microfabricated device for protein identifications by electrospray ionization mass spectrometry. Anal Chem 70:3721–3727
Fournier-Bidoz S, Kitaev V, Routkevitch D, Manners I, Ozin GA (2004) Highly ordered nanosphere imprinted nanochannel alumina (NINA). Adv Mater 16:2193–2196
Fung Y-LE, Wang H (2013) Investigation of reinforcement of porous alumina by nickel aluminate spinel for its use as ceramic membrane. J Membr Sci 444:252–258
Ghorbanian J, Beskok A (2016) Scale effects in nano-channel liquid flows. Microfluid Nanofluidics 20:121
Ghorbanian J, Celebi AT, Beskok A (2016) A phenomenological continuum model for force-driven nano-channel liquid flows. J Chem Phys 145:184109
Greenlee LF, Lawler DF, Freeman BD, Marrot B, Moulin P (2009) Reverse osmosis desalination: water sources, technology, and today’s challenges. Water Res 43:2317–2348
Gruener S, Hofmann T, Wallacher D, Kityk AV, Huber P (2009) Capillary rise of water in hydrophilic nanopores. Phys Rev E 79:067301
Gruener S, Wallacher D, Greulich S, Busch M, Huber P (2016) Hydraulic transport across hydrophilic and hydrophobic nanopores: flow experiments with water and n-hexane. Phys Rev E 93:013102
James RO, Parks GA (1982) Characterization of aqueous colloids by their electrical double-layer and intrinsic surface chemical properties. In: Surface and colloid science. Springer, pp 119–216
Jansons KM (1988) Determination of the macroscopic (partial) slip boundary condition for a viscous flow over a randomly rough surface with a perfect slip microscopic boundary condition. Phys Fluids 31:15–17
Jingxian Z, Dongliang J, Weisensel L, Greil P (2004) Deflocculants for tape casting of TiO 2 slurries. J Eur Ceram Soc 24:2259–2265
Joseph S, Aluru N (2008) Why are carbon nanotubes fast transporters of water? Nano Lett 8:452–458
Kane PF, Larrabee GB (2013) Characterization of solid surfaces. Springer, Berlin
Kannam SK, Todd B, Hansen JS, Daivis PJ (2011) Slip flow in graphene nanochannels. J Chem Phys 135:016313
Karniadakis G, Beskok A, Gad-el-Hak M (2002) Micro flows: fundamentals and simulation. Appl Mech Rev 55:B76
Karniadakis GE, Beskok A, Aluru N (2006) Microflows and nanoflows: fundamentals and simulation, vol 29. Springer, Berlin
Kasprzyk-Hordern B (2004) Chemistry of alumina, reactions in aqueous solution and its application in water treatment. Adv Colloid Interface Sci 110:19–48
Koplik J, Banavar JR, Willemsen JF (1989) Molecular dynamics of fluid flow at solid surfaces. Phys Fluids A Fluid Dyn 1:781–794
Kosmulski M (2001) Chemical properties of material surfaces, vol 102. CRC Press, Boca Raton
Kumar SM, Roy S (2008) Filtration characteristics in dead-end microfiltration of living Saccharomyces cerevisiae cells by alumina membranes. Desalination 229:348–361
Legube B, Leitner NKV (1999) Catalytic ozonation: a promising advanced oxidation technology for water treatment. Catal Today 53:61–72
Lei L, Hu X, Chu H, Chen G, Yue P (1997) Catalytic wet air oxidation of dyeing and printing wastewater. Water Sci Technol 35:311–319
Li L, Mo J, Li Z (2014) Flow and slip transition in nanochannels. Phys Rev E 90:033003
Light TS (1984) Temperature dependence and measurement of resistivity of pure water. Anal Chem 56:1138–1142
Linsen BG (1970) Physical and chemical aspects of adsorbents and catalysts. Academic Press, London
Liu Z-H, Kanjo Y, Mizutani S (2009) Removal mechanisms for endocrine disrupting compounds (EDCs) in wastewater treatment—physical means, biodegradation, and chemical advanced oxidation: a review. Sci Total Environ 407:731–748
Morterra C, Magnacca G (1996) A case study: surface chemistry and surface structure of catalytic aluminas, as studied by vibrational spectroscopy of adsorbed species. Catal Today 27:497–532
Natsume T, Yamauchi Y, Nakayama H, Shinkawa T, Yanagida M, Takahashi N, Isobe T (2002) A direct nanoflow liquid chromatography-tandem mass spectrometry system for interaction proteomics. Anal Chem 74:4725–4733
Neto C, Evans DR, Bonaccurso E, Butt H-J, Craig VS (2005) Boundary slip in Newtonian liquids: a review of experimental studies. Rep Prog Phys 68:2859
Nguyen CT, Kim B (2016) Stress and surface tension analyses of water on graphene-coated copper surfaces. Int J Precis Eng Manuf 17:503–510
Nowack B, Lützenkirchen J, Behra P, Sigg L (1996) Modeling the adsorption of metal-EDTA complexes onto oxides. Environ Sci Technol 30:2397–2405
Park J, Regalbuto JR (1995) A simple, accurate determination of oxide PZC and the strong buffering effect of oxide surfaces at incipient wetness. J Colloid Interface Sci 175:239–252
Patel F, Baig MA, Laoui T (2011) Processing of porous alumina substrate for multilayered ceramic filter. Desalination Water Treat 35:33–38
Ponomarev I, Meyerovich A (2003) Surface roughness and effective stick-slip motion. Phys Rev E 67:026302
Ran C, Ding G, Liu W, Deng Y, Hou W (2008) Wetting on nanoporous alumina surface: transition between Wenzel and Cassie states controlled by surface structure. Langmuir 24:9952–9955
Regalbuto J, Navada A, Shadid S, Bricker M, Chen Q (1999) An experimental verification of the physical nature of Pt adsorption onto alumina. J Catal 184:335–348
Reymond J, Kolenda F (1999) Estimation of the point of zero charge of simple and mixed oxides by mass titration. Powder Technol 103:30–36
Rice C, Whitehead R (1965) Electrokinetic flow in a narrow cylindrical capillary. J Phys Chem 69:4017–4024
Richardson S (1973) On the no-slip boundary condition. J Fluid Mech 59:707–719
Routkevitch D, Chan J, Xu J, Moskovits M (1997) Porous anodic alumina templates for advanced nanofabrication. In: Proceedings of the international symposium on pits and pores: formation, properties, and significance for advanced luminescent materials, vol 7. pp 350–357
Sposito G (1995) The environmental chemistry of aluminum. CRC Press, Boca Raton
Sprycha R (1989a) Electrical double layer at alumina/electrolyte interface: I. Surface charge and zeta potential. J Colloid Interface Sci 127:1–11
Sprycha R (1989b) Electrical double layer at alumina/electrolyte interface: II. Adsorption of supporting electrolyte ions. J Colloid Interface Sci 127:12–25
Sung J, Zhang L, Tian C, Shen YR, Waychunas GA (2011) Effect of pH on the water/α-Al2O3 (1102) interface structure studied by sum-frequency vibrational spectroscopy. J Phys Chem C 115:13887–13893
Tang C-M, Li X-L (2013) Separative capability of γ-Al2O3 porous ceramic membrane modified by ZIF-8. Korean J Chem Eng 30:1119–1124
Thomas F, Schouller E, Bottero J (1995) Adsorption of salicylate and polyacrylate on mesoporous aluminas. Colloids Surf A Physicochem Eng Asp 95:271–279
Tufenkji N, Elimelech M (2004) Deviation from the classical colloid filtration theory in the presence of repulsive DLVO interactions. Langmuir 20:10818–10828
Vajandar SK, Xu D, Markov DA, Wikswo JP, Hofmeister W, Li D (2007) SiO2-coated porous anodic alumina membranes for high flow rate electroosmotic pumping. Nanotechnology 18:275705
van der Heyden FH, Bonthuis DJ, Stein D, Meyer C, Dekker C (2007) Power generation by pressure-driven transport of ions in nanofluidic channels. Nano Lett 7:1022–1025
Van Heetvelde P et al (2013) A new method to graft titania using Grignard reagents. Chem Commun 49:6998–7000
Vincent O, Szenicer A, Stroock AD (2016) Capillarity-driven flows at the continuum limit. Soft Matter 12:6656–6661
Voronov RS, Papavassiliou DV, Lee LL (2006) Boundary slip and wetting properties of interfaces: correlation of the contact angle with the slip length. J Chem Phys 124:204701
Wei CC, Chen OY, Liu Y, Li K (2008) Ceramic asymmetric hollow fibre membranes—one step fabrication process. J Membr Sci 320:191–197
Werder T, Walther JH, Jaffe R, Halicioglu T, Koumoutsakos P (2003) On the water–carbon interaction for use in molecular dynamics simulations of graphite and carbon nanotubes. J Phys Chem B 107:1345–1352
Wijnja H, Schulthess C (1999) ATR–FTIR and DRIFT spectroscopy of carbonate species at the aged γ-Al2O3/water interface. Spectrochim Acta A Mol Biomol Spectrosc 55:861–872
Ye J, Yin Q, Zhou Y (2009) Superhydrophilicity of anodic aluminum oxide films: from “honeycomb” to “bird’s nest”. Thin Solid Films 517:6012–6015
Yopps J, Fuerstenau D (1964) The zero point of charge of alpha-alumina. J Colloid Sci 19:61–71
Acknowledgements
The authors would like to acknowledge valuable discussions with Prof. Michael Lattman of SMU Chemistry Department and assistance of Ms. Lael Irani and Mr. Vahid Jabbari for the zeta-potential measurements. This research was supported by Lyle School of Engineering Interdisciplinary Seed Funding.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Koklu, A., Li, J., Sengor, S. et al. Pressure-driven water flow through hydrophilic alumina nanomembranes. Microfluid Nanofluid 21, 124 (2017). https://doi.org/10.1007/s10404-017-1960-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-017-1960-1