Abstract
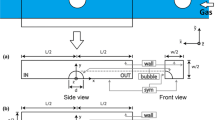
A model is proposed to describe the dissolution of a chain of spherical pure gas bubbles into a nonvolatile liquid, along square and circular microchannels. The gas–liquid interface is considered stress-free or rigid. This model enables predicting, in each considered case, the evolution along the microchannel of the bubble diameter, the bubble velocity, the separation distance between two successive bubbles, the liquid fraction, the pressure in both the liquid and the gas phases, the concentration of the dissolved gas in the liquid phase and the mass transfer coefficient between the bubble and the liquid phase. The influence on the gas dissolution of the interfacial boundary condition, the microchannel type, the operating conditions and the physicochemical properties of the liquid and gas is analyzed. Existing experimental data for a nearly square microchannel are convincingly reproduced using the model. The validity and the different applications of the model are also discussed.





Similar content being viewed by others
References
Abolhasani M, Gunther A, Kumacheva E (2014) Microfluidic studies of carbon dioxide. Angew Chem Int Ed 53:2–13
Bruus H (2008) Theoretical microfluidics, vol 18. Oxford University Press, Oxford, pp 41–50, 75
Clift R, Grace JR, Weber ME (1978) Bubbles, drops and particles. Academic Press, New York
Cubaud T, Ho CM (2004) Transport of bubbles in square microchannels. Phys Fluids 16:4575
Cubaud T, Sauzade M, Sun R (2012) \({\text{CO}}_2\) dissolution in water using long serpentine microchannels. Biomicrofluidics 6:022002
Haberman WL, Morton RK (1953) An experimental investigation of the drag and shape of air bubbles rising in various liquids. David Taylor Model Basin, Report 802
Kashid MN, Renken A, Kiwi-Minsker L (2011) Gas–liquid and liquid–liquid mass transfer in microstructured reactors. Chem Eng Sci 66(17):3876–3897
Kim N, Evans ET, Park DS, Soper SA, Murphy MC, Nikitopoulos DE (2011) Gas–liquid two-phase flows in rectangular polymer micro-channels. Exp Fluids 51(2):373–393
Leitner W (2002) Supercritical carbon dioxide as a green reaction medium for catalysis. Acc Chem Res 35:746–756
Mikaelian D, Haut B, Scheid B (2015) Bubbly flow and gas–liquid mass transfer in square and circular microchannels for stress-free and rigid interfaces. CFD analysis. Microfluid Nanofluid. doi:10.1007/s10404-015-1578-0
Park JI, Nie Z, Kumachev A, Abdelrahman AI, Binks BP, Stone HA, Kumacheva E (2009) A microfluidic approach to chemically driven assembly of colloidal particles at gas–liquid interfaces. Angew Chem Int Ed 48:5300–5304
Shim S, Wan J, Hilgenfeldt S, Panchal PD, Stone HA (2014) Dissolution without disappearing: multicomponent gas exchange for \({\text{CO}}_2\) bubbles in a microfluidic channel. Lab Chip 14(14):2428–2436
Song H, Chen DL, Ismagilov RF (2006) Reactions in droplets in microfluidic channels. Angew Chem Int Ed 45(44):7336–7356
Stan CA, Guglielmini L, Ellerbee AK, Caviezel D, Stone HA, Whitesides GM (2011) Sheathless hydrodynamic positioning of buoyant drops and bubbles inside microchannels. Phys Rev E 84:036302
Sun R, Cubaud T (2011) Dissolution of carbon dioxide bubbles and microfluidic multiphase flows. Lab Chip 11:2924–2928
Terwagne D, Brojan M, Reis PM (2014) Smart morphable surfaces for aerodynamic drag control. Adv Mater 26:6608
Thiele J, Abate AR, Shum HC, Bachtler S, Frster S, Weitz DA (2010) Fabrication of polymersomes using double-emulsion templates in glass-coated stamped microfluidic devices. Small 6:1723–1727
Trana V-T, Benot J-P, Venier-Julienne M-C (2011) Why and how to prepare biodegradable, monodispersed, polymeric microparticles in the field of pharmacy? Int J Pharm 407:1–11
Triplett KA, Ghiaasiaan SM, Abdel-Khalik SI, Sadowski DL (1999a) Gas–liquid two-phase flow in microchannels, Part I: two-phase flow patterns. Int J Multiph Flow 25(3):377–394
Triplett KA, Ghiaasiaan SM, Abdel-Khalik SI, LeMouel A, McCord BN (1999b) Gas–liquid two-phase flow in microchannels, Part II: void fraction and pressure drop. Int J Multiph Flow 25(3):395–410
Voicu D, Abolhasani M, Choueiri R, Lestari G, Seiler C, Menard G, Greener J, Guenther A, Stephan DW, Kumacheva E (2014) Microfluidic studies of \({\text{CO}}_2\) sequestration by frustrated Lewis pairs. J Am Chem Soc 136:3875–3880
Yue J, Chen G, Yuan Q, Luo L, Gonthier Y (2007) Hydrodynamics and mass transfer characteristics in gas–liquid flow through a rectangular microchannel. Chem Eng Sci 62(7):2096–2108
Ziemecka I, van Steijn V, Koper GJM, Kreutzer MT, van Esch JH (2011a) All-aqueous core–shell droplets produced in a microfluidic device. Soft Matter 7:9878–9880
Ziemecka I, van Steijn V, Koper GJM, Rosso M, Brizard AM, van Esch JH, Kreutzer MT (2011b) Monodisperse hydrogel microspheres by forced droplet formation in aqueous two-phase systems. Lab Chip 11:620–624
Acknowledgments
The authors thank Sam Dehaeck for the useful comment about the possible volatility of the liquid. D.M. and B.S. acknowledge the Fonds de la Recherche Scientifique (F.R.S.-F.N.R.S.) for their financial contribution. This research has been performed under the umbrella of the COST action MP1106 and also takes part in the Inter-university Attraction Pole Programme (IAP 7/38 MicroMAST) initiated by the Belgian Science Policy Office.
Author information
Authors and Affiliations
Corresponding author
Appendix: Supplementary materials for the model construction
Appendix: Supplementary materials for the model construction
1.1 Separation distance between two successive bubbles along the microchannel
For \(\Delta x \rightarrow 0\), it can be considered that a bubble moves from x to \(x+\Delta x\) at a velocity \(V_B(x)\). The time for this bubble to travel the distance \(\Delta x\) is equal to \(\Delta x/V_B(x)\). During this time, the preceding bubble moves on a distance \(\Delta x - \ell (x+\Delta x)+\ell (x)\) at a velocity \(V_B(x- \ell (x))\):
or, after rearrangement,
For \(\Delta x \rightarrow 0\), Eq. 18 leads to:
where the prime denotes the derivative with respect to x. As \(\ell (x)\ll x\), except of course in the vicinity of the inlet, \(V_B(x)-V_B(x-\ell (x))\) is approximated by the first order of its Taylor expansion, i.e., \(V_B'(x) \ell (x)\). Equation 19 then simplifies into Eq. 11.
1.2 Concentration of the dissolved gas in the liquid phase along the microchannel
For a control segment of the microchannel of length \(\Delta x\) (see Fig. 6), the following mass balance can be written for the transferred species between the gas phase and the liquid phase, using the ideal gas law:
Q L is taken independent of x thanks to the fact that the gas density is much lower than the liquid density, such that the amount of gas dissolved in the liquid does not significantly affect its flow rate.
Using Eq. 9, Eq. 20 can be rearranged as
For \(\Delta x \rightarrow 0\), Eq. 21 becomes
or equivalently recasts into Eq. 15.
1.3 Bubble diameter along the microchannel
If a bubble is at the coordinate x at time t and at the coordinate \(x+\Delta x\) at time \(t+\Delta t\) and if \(\Delta t \rightarrow 0\) is considered, \(\Delta t\) can be replaced by \(\Delta x/V_B(x)\) and the following mass balance can be written for the transferred species between the gas phase and the liquid phase, using the ideal gas law:
For \(\Delta x \rightarrow 0\), Eq. 23 becomes
or equivalently recasts into Eq. 16.
Rights and permissions
About this article
Cite this article
Mikaelian, D., Haut, B. & Scheid, B. Bubbly flow and gas–liquid mass transfer in square and circular microchannels for stress-free and rigid interfaces: dissolution model. Microfluid Nanofluid 19, 899–911 (2015). https://doi.org/10.1007/s10404-015-1619-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-015-1619-8