Abstract
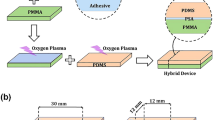
Reversible bonding that allows repeating assembly and disassembly of microfluidic devices is very useful for a number of applications such as surface functionalization, complex cell patterning, and other biological analysis. However, reversible microfluidic devices fabricated with the current standard procedures can only be used for low-pressure applications. In this paper, we describe and characterize a reliable, flexible, and reversible bonding technique of PDMS–PDMS (Poly-dimethyl siloxane) using an oxygen plasma treatment. Effects of control parameters, such as the thickness of the PDMS layer, the duration and power of the plasma treatment, the duration and temperature of the thermal treatment on the quality of the obtained devices are investigated. An optimal set of control parameters enabling the obtained devices to work at high flow rates and pressures (500 µL/min and 148 kPa) has been determined. Furthermore, the disassembly/assembly process of the device can be repeated up to four times.





Similar content being viewed by others
References
Anwar K, Han T, Kim SM (2011) Reversible sealing techniques for microdevice applications. Sens Actuators B Chem 153:301–311. doi:10.1016/j.snb.2010.11.002
Bhattacharya S, Datta A, Berg JM, Gangopadhyay S (2005) Studies on surface wettability of poly(dimethyl) siloxane (PDMS) and glass under oxygen-plasma treatment and correlation with bond strength. J Microelectromech Syst 14:590–597
Cai D, Neyer A (2010) Cost-effective and reliable sealing method for PDMS (PolyDiMethylSiloxane)-based microfluidic devices with various substrates. Microfluid Nanofluidics 9:855–864. doi:10.1007/s10404-010-0596-1
Chen X, Murawski A, Kuang G et al (2006) Sample preparation for MALDI mass spectrometry using an elastomeric device reversibly sealed on the MALDI target. Anal Chem 78:6160–6168. doi:10.1021/ac060286b
Chen Q, Li G, Nie Y et al (2013) Investigation and improvement of reversible microfluidic devices based on glass–PDMS–glass sandwich configuration. Microfluid Nanofluidics 16:83–90. doi:10.1007/s10404-013-1222-9
Chong SC, Xie L, Yobas L, et al (2005) Disposable polydimethylsioxane package for “bio-microfluidic system.” In: Electron components technol conference 2005 proceedings 55th pp 617–621. doi: 10.1109/ECTC.2005.1441333
Chung S, Park J, Chung C et al (2004) Multi-height micro structures in poly(dimethyl siloxane) lab-on-a-chip. Microsyst Technol 10:81–88. doi:10.1007/s00542-003-0301-3
Duffy DC, McDonald JC, Schueller OJ, Whitesides GM (1998) Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal Chem 70:4974–4984. doi:10.1021/ac980656z
Eddings MA, Johnson MA, Gale BK (2008) Determining the optimal PDMS–PDMS bonding technique for microfluidic devices. J Micromech Microeng 18:067001. doi:10.1088/0960-1317/18/6/067001
Fürjes P, Holczer EG, Tóth E et al (2014) PDMS microfluidics developed for polymer based photonic biosensors. Microsyst Technol. doi:10.1007/s00542-014-2130-y
Geng Z, Zhang L, Ju Y, et al (2011) Fabrication of reusable whole PDMS biochip for mesenchymal stem cell separation and enrichment. In: 2011 6th IEEE international conference nano/micro engineered and molecular systems IEEE, pp 5–8
Khademhosseini A, Yeh J, Eng G et al (2005) Cell docking inside microwells within reversibly sealed microfluidic channels for fabricating multiphenotype cell arrays. Lab Chip 5:1380–1386. doi:10.1039/b508096g
Le Berre M, Crozatier C, Velve Casquillas G, Chen Y (2006) Reversible assembling of microfluidic devices by aspiration. Microelectron Eng 83:1284–1287. doi:10.1016/j.mee.2006.01.257
McDonald JC, Whitesides GM (2002) Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Acc Chem Res 35:491–499
Mcdonald JC, Duffy DC, Anderson JR, Chiu DT (2000) Review general fabrication of microfluidic systems in poly (dimethylsiloxane). Electrophoresis 21:27–40
Neethirajan S, Kobayashi I, Nakajima M et al (2011) Microfluidics for food, agriculture and biosystems industries. Lab Chip 11:1574–1586. doi:10.1039/c0lc00230e
Oh KW, Lee K, Ahn B, Furlani EP (2012) Design of pressure-driven microfluidic networks using electric circuit analogy. Lab Chip 12:515–545. doi:10.1039/c2lc20799k
Rafat M, Raad DR, Rowat AC, Auguste DT (2009) Fabrication of reversibly adhesive fluidic devices using magnetism. Lab Chip 9:3016–3019. doi:10.1039/b907957b
Ramadan Q, Samper V, Poenar D, Yu C (2006) Magnetic-based microfluidic platform for biomolecular separation. Biomed Microdevices 8:151–158. doi:10.1007/s10544-006-7710-x
Rasponi M, Piraino F, Sadr N et al (2010) Reliable magnetic reversible assembly of complex microfluidic devices: fabrication, characterization, and biological validation. Microfluid Nanofluidics 10:1097–1107. doi:10.1007/s10404-010-0738-5
Stone HA, Stroock HD, Ajdari A (2004) Engineering flows in small devices. Annu Rev Fluid Mech 36:381–411. doi:10.1146/annurev.fluid.36.050802.122124
Sui G, Lee C-C, Kamei K-I et al (2007) A microfluidic platform for sequential ligand labeling and cell binding analysis. Biomed Microdevices 9:301–305. doi:10.1007/s10544-006-9033-3
Vézy C, Haddour N, Dempsey NM et al (2011) Simple method for reversible bonding of a polydimethylsiloxane microchannel to a variety of substrates. Micro Nano Lett 6:871. doi:10.1049/mnl.2011.0492
Wittig JH, Ryan AF, Asbeck PM (2005) A reusable microfluidic plate with alternate-choice architecture for assessing growth preference in tissue culture. J Neurosci Methods 144:79–89. doi:10.1016/j.jneumeth.2004.10.010
Acknowledgments
This work was partly supported by the French RENATECH network and Vietnamese Overseas Scholarship Program (Project-322). The authors wish to thank technicians of the clean room of the ‘Centrale de Technologie Universitaire (MINERVE-CTU)’ for their valuable help during the experimental tests. A special thank is also given to their colleague Mehdi Ammar for the AFM measurements.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dinh, T.H.N., Cao, H.H., Hamdi, F.S. et al. Development of reversible bonding for microfluidic applications. Microfluid Nanofluid 19, 751–756 (2015). https://doi.org/10.1007/s10404-015-1599-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10404-015-1599-8