Abstract
Purpose
The purpose of this study was to evaluate and confirm the prognostic utility of comprehensive transthoracic echocardiography (TTE) using offline myocardial strain analyses in a Japanese coronavirus disease (COVID-19) cohort hospitalized in intensive care units.
Methods
We performed a retrospective analysis of 90 consecutive adult patients with COVID-19 who underwent clinically indicated standard two-dimensional TTE in intensive care wards. Patients on extracorporeal membrane oxygenation (ECMO) at the time of TTE were excluded. Biventricular strain assessments using vendor-independent offline speckle tracking analysis were performed. Patients with inadequate TTE image quality were also excluded.
Results
Among the 90 COVID-19 patients, 15 (17%) patients required venovenous or venoarterial ECMO. There were 25 (28%) in-hospital deaths. A composite event, defined as the combination of in-hospital mortality and subsequent initiation of ECMO, occurred in 32 patients. Multivariate logistic regression for composite events indicated that right ventricular free wall longitudinal strain (RV-FWLS) and mechanical ventilation at the time of TTE were independent risk factors for composite events (p = 0.01, odds ratio [OR] 1.09, 95% confidence interval [CI] 1.01–1.18; p = 0.04, OR 3.24, 95% CI 1.03–10.20). Cumulative survival probability plots generated using the Kaplan–Meier method for composite events with log-rank tests revealed a significant difference between subgroups divided by the cutoff value of RV-FWLS (p < 0.001).
Conclusion
Offline measurement of RV-FWLS may be a potent predictor of worse outcomes in COVID-19 requiring intensive care. Larger multicenter prospective studies are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the rapid international propagation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the coronavirus disease of 2019 (COVID-19) has become a pandemic. Angiotensin-converting enzyme-2 (ACE2), a receptor of SARS-CoV-2, has multiple functions in the renin-angiotensin system and amino acid transport. ACE2 is expressed in the vascular system, heart, kidneys, liver, retina, intestines, central nervous system, upper airway, and lungs [1, 2]. Therefore, although its clinical manifestations are respiratory symptoms, severe cardiovascular damage certainly occurs.
In COVID-19, heart involvement is a consequence of either hypoxemia from respiratory failure and hyperinflammation, pulmonary vessel injury, and direct or indirect myocardial distress, or all of them [3]. Transthoracic echocardiography (TTE) can help to elucidate the mechanism and establish the diagnosis of cardiac complications, which impacts patient management [4]. On the other hand, a comprehensive TTE data set may have another role as it has been utilized in common chronic or acute respiratory diseases [5, 6]. Since the earlier days of the COVID-19 pandemic, comprehensive TTE has been investigated to demonstrate its prognostic capability for COVID-19 by assessing the morphologic features and functions of the heart [7]. In addition, the prognostic value of more advanced ultrasound imaging technologies such as strain [8, 9] has been discussed but is still controversial. Thus, the purpose of this study was to further evaluate and confirm the prognostic utility of comprehensive TTE and biventricular longitudinal strains (LS) including right ventricular free wall longitudinal strain (RV-FWLS) patterns with the use of vender-independent offline speckle tracking analysis in a Japanese COVID-19 cohort who were hospitalized in intensive care units.
Materials and methods
We retrospectively analyzed consecutive patients with COVID-19, confirmed by real-time reverse transcriptase polymerase chain reaction assay or immunological antigen detection of nasal swabs and pharyngeal or pulmonary samples, who underwent clinically indicated echocardiographic studies in intensive care wards during their hospitalization at the Tokai University School of Medicine Hospital (Isehara, Kanagawa Prefecture, Japan) between February 2020 and November 2022. The exclusion criteria were: patients younger than 18 years of age, patients on extracorporeal membranous oxygenation (ECMO) at the time of TTE, and patients with poor TTE image quality or incomplete TTE visualization due to limited acoustic windows. The study site, which was established as a private educational hospital founded by the Tokai University School of Medicine, serves as a medical institution authorized to treat patients with Japanese national health insurance coverage for regional tertiary care and provides medical care for COVID-19 patients with respiratory failure requiring oxygen administration (oxygen saturation of peripheral artery ≤ 93% on room air) in accordance with the instructions and requests from the regional government, as previously described [10]. All the patients with COVID-19 were transferred or referred from other medical institutions under the medical control of the local authorities and hospitalized in dedicated intensive care wards. There were no general outpatient care services for COVID-19 patients. The Institutional Review Board for Clinical Research of Tokai University School of Medicine approved this observational study protocol. This study was conducted in accordance with the principles outlined in the Declaration of Helsinki. Written informed consent was obtained from all patients at admission.
Baseline clinical characteristics, laboratory tests, radiological examinations, and treatments were reviewed in digitalized medical records. The initial values of C-reactive protein, lactic dehydrogenase, white blood cell count, high-sensitivity troponin T, and D-dimer [11] levels on admission were collected. In most cases, D-dimer measurement was repeated every 2 days until its decline to obtain the peak values of D-dimer (D-dimer [max]) within 1 week after admission. Medical management of COVID-19 patients was provided in accordance with current Japanese COVID-19 clinical guidelines [12]. The endpoint of this study was defined as a composite of in-hospital mortality and subsequent use of ECMO after the index TTE.
As previously described [10], all standard TTEs were performed at the bedside with patients in the left lateral decubitus, supine, or sitting positions, in quarantined intensive care rooms for COVID-19 using a dedicated ultrasound machine (Aplio a/Verifia; CANON Medical Systems Corp., Ohtahara-Shi, Japan) by a single operator with full personal protective equipment, following the medical biosafety guidelines for TTE [13]. All images were then transferred via an institutional wireless network to the video server of the medical ultrasound laboratory (PrimeVitaPlus; NIHON KOHDEN Corp., Shinjuku-ku, Japan). Subsequently, a study review and comprehensive conventional measurements were performed in the medical ultrasound laboratory in accordance with the current TTE guidelines [14,15,16] and interpreted by staff cardiologists. Conventional parameters included right ventricular fractional area change, stroke volume index, maximum tricuspid regurgitation pressure gradient, and left-sided myocardial performance index (Tei index). The left ventricular end-diastolic volume, left ventricular end-systolic volume index, and left ventricular ejection fraction (LVEF) were assessed using the Simpson biplane method. Based on recent pulmonary hypertension (PH) clinical guidelines [17], PH was diagnosed with intermediate probability (possible PH) using TTE when the maximum tricuspid regurgitation (TR) velocity was faster than 2.8 m/s or either right ventricular (RV) dilatation (the basal RV diameter exceeded the left ventricular basal diameter in the apical four-chamber view) or flattening of the interventricular septum was present. Accordingly, PH was diagnosed with high probability (probable PH) when the maximum TR velocity was faster than 2.8 m/s with the presence of either RV dilatation or flattening of the interventricular septum or the maximum TR velocity was faster than 3.4 m/s. Swelling of the left ventricular wall was defined as average interventricular septum and posterior wall thicknesses > 12 mm.
Strain parameters of the left and right ventricles were measured by an experienced cardiologist who was blinded to the clinical outcomes using commercially available vendor-independent software for two-dimensional speckle tracking analysis (2D CPA; TomTec Imaging Systems, Unterschleissheim, Germany) according to the current guidelines [18,19,20,21]. The software algorithm automatically divided the left ventricle into six equidistant segments and tracked the speckle patterns on a frame-by-frame basis throughout the entire cardiac cycle in the apical four-chamber view, two-chamber view, and long-axis view. The endocardial and epicardial borders could be adjusted manually if necessary. The software automatically generated time-domain left ventricular (LV) curves for each of the six segments, from which the peak LS was measured. The LV-LS in each view was calculated as the average peak systolic LS of the six segments of the left ventricle. Finally, the left ventricular global longitudinal strain (LV-GLS) was calculated as the average LV-LS in three different views. The lower cutoff value for LV-GLS was − 15.0%, which was derived from a previous report [19]. As shown in Fig. 1, to obtain the right ventricular global longitudinal strain (RV-LS), the right ventricle in the apical four-chamber view was divided into six segments. If needed, an apical four-chamber RV-focused view was used for this purpose. The RV-LS was calculated as the average peak systolic LS of the six segments of the right ventricle. The RV-FWLS was calculated as the average peak systolic LS of the three segments of the RV free wall. RV dysfunction was defined as RV-FWLS ≥ − 19.0% [22].
Numerical data are presented as either the mean ± standard deviation or the median and range, and as a number (%) for categorical variables. Continuous variables were compared between the two groups using unpaired Student’s t-test or Wilcoxon rank-sum test depending on normality. The ratios of the category values between the two groups were analyzed using the Chi-squared test. For multivariate analysis, logistic regression analysis was performed. We considered all parameters that had a p-value < 0.05 as covariate candidates for the multivariate analysis, except for parameters with more than 10 instances of missing values. Cumulative survival probability plots were generated using the Kaplan–Meier method, and the log-rank test was performed to compare the curves. All statistical analyses were performed using JMP 14.0.0 (SAS Institute, Cary, NC, USA) software, and the significance level was set at p < 0.05.
Results
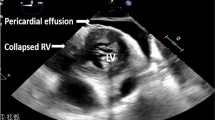
As shown in Fig. 2, there were 625 admissions and 613 hospitalizations in the intensive care wards for COVID-19 during the study period, of which 104 (17%) adult cases had a total of 133 TTE studies. Ten TTE studies (7.5%, 10/133) were not suitable for offline comprehensive two-dimensional and speckle tracking analyses for both ventricles due to poor echogenicity including lack of an adequate view of the RV free wall (n = 4) or limited echocardiographic windows (n = 6), and 29 follow-up studies and four TTE studies in patients on ECMO were excluded. Thus, the study group comprised 90 patients with COVID-19 (19 women; age, 68 ± 14 years). TTE studies were performed 1 day (median) after admission in intubated patients (n = 19) and 2 days (median) after admission in non-intubated patients (n = 71) (Table 1) by the referral of each treatment team’s physicians. The main reason for TTE referral was either “for the diagnosis of cardiovascular complications” (n = 9, 10%) or “for the evaluation of cardiac function and hemodynamic status” (n = 81, 90%). Speckle tracking analysis was performed on the apical four-chamber view in 75 patients (78%) and apical four-chamber RV-focused view in 15 patients (22%) when the visualization of the RV free wall was poor on the apical four-chamber view. The whole cohort was divided into two subgroups: the RV dysfunction subgroup (RV-FWLS ≥ − 19.0%, n = 42) and the normal RV function subgroup (RV-FWLS < − 19.0%, n = 48). The clinical characteristics of each subgroup were then compared (Table 1). Generally, in-hospital mortality, ECMO use, composite events, and TTE referral reasons were significantly different. As shown in Table 2, among the echocardiographic parameters, pericardial effusion was more frequent and RV-FWLS, as well as RV-LS, were significantly higher in the RV dysfunction subgroup.
There were 17 cases of cardiac complications—such as non-ST-elevation myocardial infarction (n = 1), ST-elevation myocardial infarction (n = 1), heart failure (n = 2), left ventricular thrombus (n = 2), myocarditis (n = 5), pulmonary embolism (n = 2), ventricular fibrillation/tachycardia (n = 2), cardiac tamponade (n = 1), and cardiogenic shock (n = 1)—while there were 16 cases with peripheral vascular complications—such as cerebral infarction (n = 4), cerebral hemorrhage (n = 3), transient ischemic attack (n = 1), and deep vein thrombosis (n = 8). Myocarditis was diagnosed by attending physicians only when the patient was presenting some combinations of the typical findings of the disease in terms of symptoms, electrocardiogram (ECG), echocardiogram, and laboratory findings. In-hospital mortality occurred in 25 patients (27%) among the whole cohort. Among the 90 patients without ECMO at the time of TTE, the composite events of in-hospital deaths (n = 25) and subsequent initiation of ECMO after TTE (n = 15) occurred in 32 patients (36%).
Table 3 indicates the results of logistic regression analysis for composite events. Four covariates, i.e., mechanical ventilation at the time of TTE, pericardial effusion, probable PH, and RV-FWLS, were listed with a p-value less than 0.05 according to univariate analysis. According to multivariate analysis, RV-FWLS and mechanical ventilation at the time of TTE were independent risk factors for composite events (p = 0.01, odds ratio [OR] 1.09, 95% confidence interval [CI] 1.01–1.18; p = 0.04, OR 3.24, 95% CI 1.03–10.20). Figure 3 shows the results of the cumulative survival probability plots using the Kaplan–Meier method for composite events. The log-rank tests revealed a significant difference in composite events (p < 0.001) between the subgroups divided by the presence of RV dysfunction.
Results of cumulative survival probability plots generated using the Kaplan–Meier method for a composite event. A composite event was defined as occurrence of in-hospital death or subsequent initiation of extracorporeal membrane oxygenation after the index transthoracic echocardiography. RV-FWLS right ventricular free wall longitudinal strain
Discussion
In this study, we retrospectively investigated the prognostic utility of comprehensive TTE parameters and biventricular LSs with the use of offline speckle tracking analysis in patients with COVID-19 requiring intensive medical care at a tertiary medical center. There are three main findings: (1) offline speckle tracking analysis for both ventricles was feasible in COVID-19 patients hospitalized in intensive care units (123/133, 92.5%); (2) among clinical factors that showed the relationship with composite events according to univariate analysis such as mechanical ventilation at the time of TTE, pericardial effusion, probable PH, and RV-FWLS, multivariate analysis selected mechanical ventilation at the time of TTE and RV-FWLS as independent risk factors; (3) cumulative survival probability plots generated using the Kaplan–Meier method revealed a poor outcome in patients with RV dysfunction assessed using RV-FWLS.
The clinical indications for TTE in hospitalized COVID-19 patients were mostly reported to be major acute cardiovascular events and deteriorating hemodynamic status of the patient [23]. However, another concern regarding the prognostic purpose of TTE has also been raised. It is well documented that an increase in serum biomarkers of myocardial injury has prognostic value in COVID-19 [12, 24]. Our study showed excellent feasibility of offline speckle tracking analysis for COVID-19 patients in intensive care units, which could assess both ventricular strains. This may further motivate TTE studies for prognostic purposes.
In this study, one of the possible risk factors for composite events according to univariate analysis was the existence of pericardial effusion. The presence of pericardial effusion and increased ventricular mass in COVID-19 (“a swollen heart”) may be a sign of cardiac impairment and lead to an increased risk of mortality [25, 26]. However, there were significant differences in the frequency of pericardial effusion between the patients with and without RV dysfunction assessed using RV-FWLS. Therefore, a possible confounding between pericardial effusion and RV dysfunction cannot be ruled out. In cases with pericardial effusion, myocardial injury caused by direct invasion of the pathogen may affect ventricular function not only on the left side but also on the right side. Thus, our opinion is that the presence of pericardial effusion may be related to RV dysfunction and contribute to developing a composite event.
In COVID-19, it is quite reasonable to deduce that TTE findings of PH or RV function are related to adverse events as the primary focus of pathogenic infiltration is commonly the respiratory system [7, 26,27,28,29,30]. Similar to previous studies, probable PH, which was a guideline-recommended TTE sign of PH, was one of the possible risk factors for composite events in our study. However, only 10% of patients had signs of “probable PH” versus higher in-hospital mortality (28%). We speculate that due to a large number of missing TRPG values, i.e., 44 cases (49%) in the cohort, the sensitivity of the current guideline-recommended TTE sign to detect pulmonary hypertension was relatively low in this study.
The direct evaluation of right heart overload or RV dysfunction might more accurately and sensitively predict patient outcomes [7, 27,28,29,30,31]. One of the recent developments in ultrasound imaging technology is myocardial strain measurement using two-dimensional speckle tracking. In several studies, both RV-LS [32, 33] and RV-FWLS [8, 9, 34, 35] seemed to have significant predictive value, while a systemic meta-analysis supported this concept [36]. Moreover, in patients with hemodynamic instability due to cardiopulmonary insufficiency, bedside measurement of RV-FWLS with TTE has largely been feasible and accurate [8, 9, 33]. However, a number of investigators still disagree [37, 38], and further confirmation based on multiple positive data is thus needed to establish the prognostic role of RV-FWLS in real-world COVID-19 clinical practice. In our study, multivariate logistic regression selected both RV-FWLS and mechanical ventilation at TTE as independent risk factors for composite events, while cumulative survival probability plots generated using the Kaplan–Meier method revealed poor outcomes in patients with RV dysfunction assessed using RV-FWLS. Thus, the results of this study support and re-confirm the potent prognostic capability of RV-FWLS in COVID-19.
There were some limitations associated with this study. We included patients receiving mechanical ventilation for RV strain assessments. This may invite some criticism of the accuracy of our RV functional assessments including RV strain patterns using TTE. However, multivariate logistic regression selected both mechanical ventilation at the time of TTE and RV-FWLs as independent risk factors for poor outcomes. We believe this result confirmed and ensured the significance of RV strain assessments in this study. As the study design was a retrospective observational study, TTE was ordered based on the attending medical care team’s decisions and performed in only 15% of all COVID-19 inpatients. This may have created significant selection bias. Thus, although the conclusions from this study cannot be generalized, our findings could provide guidance for future larger and/or multicenter prospective studies.
Conclusion
We investigated the prognostic utility of biventricular LS using offline speckle tracking analysis in patients with COVID-19 requiring intensive medical care. One of the TTE-derived LS assessments such as RV-FWLS may be a potent predictor of worse outcomes. Larger multicenter prospective studies are needed going forward.
Data availability
The datasets generated and analyzed in the current study are not publicly available because of patient privacy concerns.
References
Gheblawi M, Wang K, Viveiros A, et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126:1456–74.
Capotosto L, Nguyen BL, Ciardi MR, et al. Heart, COVID-19, and echocardiography. Echocardiography. 2020;37:1454–64.
Agricola E, Beneduce A, Esposito A, et al. Heart and lung multimodality imaging in COVID-19. JACC Cardiovasc Imaging. 2020;13:1792–808.
Szekely Y, Lichter Y, Taieb P, et al. Spectrum of cardiac manifestations in COVID-19: A systematic echocardiographic study. Circulation. 2020;142:342–53.
Fichet J, Moreau L, Genée O, et al. Feasibility of right ventricular longitudinal systolic function evaluation with transthoracic echocardiographic indices derived from tricuspid annular motion: a preliminary study in acute respiratory distress syndrome. Echocardiography. 2012;29:513–21.
Burgess MI, Mogulkoc N, Bright-Thomas RJ, et al. Comparison of echocardiographic markers of right ventricular function in determining prognosis in chronic pulmonary disease. J Am Soc Echocardiogr. 2002;15:633–9.
Shafiabadi Hassani N, Shojaee A, Khodaprast Z, et al. Echocardiographic features of cardiac injury related to COVID-19 and their prognostic value: a systematic review. J Intensive Care Med. 2021;36:500–8.
Bursi F, Santangelo G, Sansalone D, et al. Prognostic utility of quantitative offline 2D-echocardiography in hospitalized patients with COVID-19 disease. Echocardiography. 2020;37:2029–39.
Bieber S, Kraechan A, Hellmuth JC, et al. Left and right ventricular dysfunction in patients with COVID-19-associated myocardial injury. Infection. 2021;49:491–500.
Nagai T, Horinouchi H, Yoshioka K, et al. Value of standard echocardiography at the bedside for COVID-19 patients in intensive care units: a Japanese single-center analysis. J Med Ultrason. 2021;48:595–603.
Nie SF, Yu M, Xie T, et al. Cardiac troponin I is an independent predictor for mortality in hospitalized patients with COVID-19. Circulation. 2020;142:608–10.
Japan the Ministry of Health, Labour and Welfare and the National Institute of Infectious Diseases. Clinical management of patients with COVID-19: A guide for front-line healthcare workers. version 5.2 (in Japanese). https://www.mhlw.go.jp/content/000815065.pdf. Accessed Nov 1 2022
Kirkpatrick JN, Mitchell C, Taub C, et al. ASE statement on protection of patients and echocardiography service providers during the 2019 novel coronavirus outbreak: endorsed by the American college of cardiology. J Am Soc Echocardiogr. 2020;33:648–53.
Mitchell C, Rahko PS, Blauwet LA, et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American society of echocardiography. J Am Soc Echocardiogr. 2019;32:1–64.
Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2015;28:1-39.e14.
Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2016;29:277–314.
Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European society of cardiology (ESC) and the European respiratory society (ERS): endorsed by: association for European paediatric and congenital cardiology (AEPC), international society for heart and lung transplantation (ISHLT). Eur Heart J. 2016;37:67–119.
Mor-Avi V, Lang RM, Badano LP, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese society of echocardiography. J Am Soc Echocardiogr. 2011;24:277–313.
Badano LP, Kolias TJ, Muraru D, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/industry task force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2018;19:591–600.
Il’Giovine ZJ, Mulder H, Chiswell K, et al. Right ventricular longitudinal strain reproducibility using vendor-dependent and vendor-independent software. J Am Soc Echocardiogr. 2018;31:721-732.e5.
Nagata Y, Takeuchi M, Mizukoshi K, et al. Intervendor variability of two-dimensional strain using vendor-specific and vendor-independent software. J Am Soc Echocardiogr. 2015;28:630–41.
Morris DA, Krisper M, Nakatani S, et al. Normal range and usefulness of right ventricular systolic strain to detect subtle right ventricular systolic abnormalities in patients with heart failure: a multicentre study. Eur Heart J Cardiovasc Imaging. 2017;18:212–23.
Jain SS, Liu Q, Raikhelkar J, et al. Indications for and findings on transthoracic echocardiography in COVID-19. J Am Soc Echocardiogr. 2020;33:1278–84.
Xu H, Hou K, Xu R, et al. Clinical characteristics and risk factors of cardiac involvement in COVID-19. J Am Heart Assoc. 2020;9: e016807.
Duerr GD, Heine A, Hamiko M, et al. Parameters predicting COVID-19-induced myocardial injury and mortality. Life Sci. 2020;260: 118400.
Liu Y, Xie J, Gao P, et al. Swollen heart in COVID-19 patients who progress to critical illness: a perspective from echo-cardiologists. ESC Heart Fail. 2020;7:3621–32.
Doyen D, Dupland P, Morand L, et al. Characteristics of cardiac injury in critically ill patients with coronavirus disease 2019. Chest. 2021;159:1974–85.
Pagnesi M, Baldetti L, Beneduce A, et al. Pulmonary hypertension and right ventricular involvement in hospitalised patients with COVID-19. Heart. 2020;106:1324–31.
Mahmoud-Elsayed HM, Moody WE, Bradlow WM, et al. Echocardiographic findings in patients with COVID-19 pneumonia. Can J Cardiol. 2020;36:1203–7.
Rath D, Petersen-Uribe Á, Avdiu A, et al. Impaired cardiac function is associated with mortality in patients with acute COVID-19 infection. Clin Res Cardiol. 2020;109:1491–9.
Kim J, Volodarskiy A, Sultana R, et al. Prognostic utility of right ventricular remodeling over conventional risk stratification in patients with COVID-19. J Am Coll Cardiol. 2020;76:1965–77.
Li Y, Li H, Zhu S, et al. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC Cardiovasc Imaging. 2020;13:2287–99.
Lassen MCH, Skaarup KG, Lind JN, et al. Echocardiographic abnormalities, and predictors of mortality in hospitalized COVID-19 patients: the ECHOVID-19 study. ESC Heart Fail. 2020;7:4189–97.
Karagodin I, Carvalho Singulane C, Woodward GM, et al. Echocardiographic correlates of in-hospital death in patients with acute COVID-19 infection: the world alliance societies of echocardiography (WASE-COVID) study. J Am Soc Echocardiogr. 2021;34:819–30.
Sun W, Zhang Y, Wu C, et al. Incremental prognostic value of biventricular longitudinal strain and high-sensitivity troponin I in COVID-19 patients. Echocardiography. 2021;38:1272–81.
Wibowo A, Pranata R, Astuti A, et al. Left and right ventricular longitudinal strains are associated with poor outcome in COVID-19: a systematic review and meta-analysis. J Intensive Care. 2021;9:9.
Gibson LE, Fenza RD, Lang M, et al. Right ventricular strain is common in intubated COVID-19 patients and does not reflect the severity of respiratory illness. J Intensive Care Med. 2021;36:900–9.
Beyls C, Ghesquières T, Hermida A, et al. Feasibility, prediction and association of right ventricular free wall longitudinal strain with 30-day mortality in severe COVID-19 pneumonia: a prospective study. J Clin Med. 2022;11:3629.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare no conflicts of interest.
Ethical approval
The Institutional Review Board for Clinical Research of Tokai University School of Medicine approved the protocol. This study was conducted in accordance with the principles outlined in the Declaration of Helsinki. Written informed consent was obtained from all patients at admission.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Nagai, T., Horinouchi, H., Yoshioka, K. et al. Right ventricular free wall longitudinal strain assessment using offline speckle tracking in COVID-19 patients requiring intensive medical care. J Med Ultrasonics 50, 417–425 (2023). https://doi.org/10.1007/s10396-023-01305-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10396-023-01305-y