Abstract
Aim
This study aimed to investigate the long-term effects of two exercise-based CR programs on physical activity (PA), sedentary behavior, physical fitness, quality of life (QoL), and mental health in coronary artery disease (CAD) patients.
Subject and methods
Seventy-two CAD participants were randomized (1:1:1) into HIIT, MICT, and control groups. Both training programs spanned 6 weeks with three supervised treadmill exercise sessions per week. MICT targeted ≈70–75% of peak heart rate (HR), while HIIT aimed for ≈85–95% of peak HR. The control group adhered to standard medical recommendations. Assessments at 6- and 12-months post-intervention included body composition, aerobic capacity, muscle strength, PA, SB, QoL, anxiety, and depression.
Results
Over the 6- and 12-month follow-up periods, both exercise groups maintained the levels of aerobic capacity (HIIT ≈ 19.6 ml kg−1 min−1, MICT ≈ 17.8 ml kg−1 min−1), QoL, and PA compared to baseline (p < .001). Symptoms of anxiety and depression remained lower than baseline (p < .001). The HIIT group demonstrated a significant decreasing trend in waist circumference (∆m3−m2% −2 cm, p = .033) compared to MICT (p = .016) and control (p = .001) at 6 months of follow-up. It was maintained at 12 months of follow-up with significant differences to MICT (p = .018) and control (p = .001). In contrast, the control group experienced deteriorations in body composition, SB, symptoms of anxiety, and depression, along with a decline in aerobic capacity over time.
Conclusion
Encouraging CAD patients to maintain elevated PA levels can promote cardiovascular, WC, and mental health. CR exercise programs can reduce cardiovascular risk factors and induce favorable lifestyle changes. Notably, HIIT demonstrated sustained improvements surpassing those of MICT. These findings underscore the importance of structured exercise-based CR programs in optimizing long-term outcomes for CAD patients.
Trial Registration
https://clinicaltrials.gov/ct2/show/NCT03538119 on May 25, 2018.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronary artery disease (CAD) is the main cause of death worldwide (Roth et al. 2020). Cardiac rehabilitation (CR) emerges as a pivotal constituent in the amelioration of morbidity and mortality rates associated with CAD (Stewart et al. 2017). The escalating incidence of cardiovascular diseases (CVD) and CR’s essential role in cardiac event convalescence contribute to an increasing demand for CR programs (Turk-Adawi et al. 2019). Regrettably, global CR participation remains notably suboptimal, primarily attributed to restricted accessibility issues (Mamataz et al. 2021). In Portugal, less than 8% of survivors of any CAD enrolled in CR programs, and adherence is relatively poor among patients who do enroll in CR settings (Andrade et al. 2018).
Physical inactivity represents an autonomous risk factor in individuals afflicted with CAD (Stewart et al. 2017). Consequently, CR programs advocate adherence to public health guidelines for physical activity (PA) to enhance health outcomes, specifically targeting the attainment of a minimum of 150 min of moderate-to-vigorous intensity physical activity (MVPA) per week (Woodruffe et al. 2015). The nexus between PA, sedentary behavior (SB), cardiovascular risk factors, and health-related quality of life (QoL) within the ambit of CR remains enigmatic. Scarce studies have explored the correlation between PA, SB, and cardiovascular risk factors among CAD patients engaged in CR. Researchers who have gauged SB and PA have discerned that elevated SB levels correlate with diminished high-density lipoprotein (HDL) levels, reduced exercise capacity, and augmented triglyceride levels, body mass index (BMI), waist circumference, and anxiety (Bäck et al. 2013; Piepoli et al. 2014). Conversely, heightened PA levels are associated with reductions in triglyceride levels, blood glucose, BMI, waist circumference, depression, anxiety, and increases in HDL levels and QoL (Bäck et al. 2013; Piepoli et al. 2014; Hurdus et al. 2020). In a recent systematic review appraising PA and SB in the secondary prevention of CAD, augmented PA levels resulted in improved 6-min walk test (6MWT) outcomes, enhanced QoL, and favorable blood glucose and lipid profiles (Vasankari et al. 2021). Moderate-intensity continuous training (MICT) has conventionally constituted the cornerstone of aerobic exercise prescription, targeting an intensity level within the range of 50–75% of heart rate (HR) (Piepoli et al. 2016). This approach yields both short- and long-term clinical benefits for CAD patients (Gonçalves et al. 2021). Nevertheless, approximately 30% of adults fail to comply due to time constraints, protracted duration and the intricacy of these exercise regimens contribute to patient attrition (Hallal et al. 2012). Nonetheless, high-intensity interval training (HIIT) has recently emerged as an alternative or adjunct strategy to MICT. HIIT involves repeated bouts of higher intensity exercise (85–100%) interspersed with periods of lower-intensity recovery (Ito 2019), and has been shown to be as effective, if not superior, to MICT in terms of enhancing clinical outcomes for CAD patients, encompassing improvements in body composition, VO2peak, HR response to exercise, and myocardial function (Gonçalves et al. 2021, 2023a; Taylor et al 2020). Importantly, HIIT also appears to offer a comparable level of safety to MICT, even for older participants in CR programs (Hannan et al. 2018; Rognmo et al 2012).
Despite the demonstrable favorable outcomes of both MICT and HIIT within community-based exercise CR programs, a considerable proportion of individuals fail to sustain their exercise regimens upon CR completion, with merely one-third of patients adhering to regular PA at the 6-month post-CR evaluation (Bock et al. 2003). Maintenance of PA is a critical facet that remains understudied, particularly in the prolonged context of CAD patients. It is plausible that any potential benefits accrued during CR participation might dissipate among patients who relinquish their established exercise routines. The current investigation was undertaken to scrutinize the effects of two distinct 6-week community-based exercise CR protocols, namely HIIT and MICT, on physical fitness, QoL, and psychological well-being. Additionally, this study aimed to juxtapose the exercise cohorts with a control group devoid of any interventions and to evaluate the long-term dynamics of lifestyle alterations and cardiovascular risk factors among patients.
Methods
This study is a single-blinded randomized controlled trial (RCT) and followed the CONSORT guidelines for RCTs (http://www.consort-statement.org).
Participants
Participants were recruited between March 2018 and November 2021 within the cardiology unit of the Hospital of Evora, Portugal. All patients who had undergone a coronary event and were referred by their cardiologist to the community-based exercise CR programs, 2 months after angioplasty, were evaluated for inclusion in this study. The inclusion criteria were age 18–80 years, left ventricular ejection fraction ≥ 45%, and New York Heart Association (NYHA) functional Class I or II. Patients were excluded from the study if the following criteria were met: severe exercise intolerance; uncontrolled arrhythmia; uncontrolled angina pectoris; severe kidney or lung diseases; musculoskeletal or neuromuscular conditions preventing exercise testing or training; and signs or symptoms of ischemia. Recruitment ended when the sample size for the primary outcome was attained. All participants completed a medical history and health questionnaire and provided written informed consent.
Randomization and masking
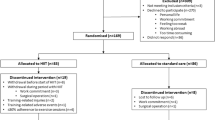
The sample size was calculated using the online G*Power software, considering an effect size of 0.3, a predefined sample power of 0.6, a predefined error probability defined as 0.05, and statistical power of 95%. Hence, a minimum sample size of 66 participants was determined (22 participants for each group) to identify significant changes. The number of participants was increased to cover an expectable dropout rate. After the baseline assessment and before the start of training protocols, the 72 participants were randomly assigned in a 1:1:1 allocation ratio to one of three groups: HIIT, MICT (traditional), and control (usual medical recommendations) (Fig. 1). To ensure allocation concealment, participants in each group were seen at a specific, prescheduled time, and appointments for each group did not coincide with appointments for any participants in either of the other groups. The three groups were similar regarding age, the extent of coronary artery disease, coronary risk factors, type of coronary event or left ventricular ejection fraction. Whereas patients and physicians allocated to the intervention group were aware of the allocated arm, outcome assessors and data analysts were kept blinded to the allocation.
Following health screening, 72 cardiac participants were enrolled in the study and allocated to one of three groups: (1) HIIT, (2) MICT, who did participate in formal exercise training, and (3) control, who did not participate in any exercise program training.
Outcome measures and assessments
Exercise testing
Initially, the participants were submitted to a clinical evaluation performed by a cardiologist. A supervised graded exercise test to record volitional fatigue, risks or symptoms of ischemia was performed on a treadmill with the Bruce protocol before the 6-week intervention period. The test was done in non-fasting conditions and under medication. Electrocardiography was recorded continuously, and blood pressure was measured with an arm cuff every 3 min. Functional capacity in metabolic equivalent value (METs) was calculated. As a high proportion of participants with CAD are prescribed beta-blocker therapy, this relative method of exercise intensity considers the likely lower HRpeak achieved by these participants during the exercise test. To ensure training exercise intensity was reflective of medication effects, all participants were instructed to take their usual medications before the maximal exercise test.
Biomarkers
Blood samples were drawn on the same day as exercise testing but were collected before exercise. All final blood samples were obtained 24–48 h after completion of the last exercise session. Levels of biomarkers: high-sensitive C-reactive protein (hsCRP), fasting blood glucose (FBG), hemoglobin A1c (HbA1c), total cholesterol, low- and high-density lipoprotein cholesterol (LDL-C and HDL-C), and triglycerides (TG) were collected. Blood samples were collected at baseline and at the end of the study.
Risk factor screening
On the second visit, the participants were submitted to a clinical evaluation of resting heart rate, blood pressure, medical history, body composition, aerobic capacity, muscle strength, PA and SB, QoL, anxiety and depression tests, performed by a physiologist at the laboratory of the University of Évora. Participants were asked to bring any medications that they were taking to the assessments. Initially, each participant completed a standardized questionnaire including demographic data, medical history, medication use, family history of CVD, and smoking status. They also completed the patient-reported QoL questionnaire and the Hospital Anxiety and Depression Scale (HADS). The QoL questionnaire consisted of the rating scale, Short Form 36 (SF-36; Quality Metric, Lincoln, Rhode Island, USA). As physical functioning, role functioning limitations due to physical problems, bodily pain, and general health domains of the SF-36 instrument are the most relevant for describing the health status of patients with cardiovascular disease, the present analysis was restricted to these four domains. For all reported QoL instruments, higher scores correspond to better QoL as perceived by the patient (Ware and Sherbourne 1992). The HADS questionnaire has been widely used to screen depression among cardiac patients in the hospitals. The HADS questionnaire has two subscales including anxiety and depression, each of which comprised items rated on 4-point Likert scales (Herrero et al. 2003). The total HADS score ranged between 0 and 42 with 0–14 being considered as low, 15–28 considered as moderate, and 29–42 being considered as high. For each subscale (anxiety and depression subscales), the scores ranged between 0 and 21, where 0–7 was considered low, 8–14 being moderate, and 15–21 was considered high. After completing the health questionnaires, the participant’s blood pressure, height, weight, and waist circumference were recorded.
The participants’ height (to nearest 0.5 cm) and weight (to nearest 0.1 kg) were measured. Body mass index (BMI) was calculated directly by the standard formula: weight(kg)/height(m)2. The waist circumference (WC) (to nearest 0.5 cm) was measured three times on the midpoint of the lowest rib and the iliac crest, and the mean of measurements was used in analyses (Liguori 2020). Body composition was then assessed by dual-energy X-ray absorptiometry (DXA). DXA scans were performed with QDR 2000 densitometers (DXA, Hologic QDR, Hologic, Inc., Bedford, MA, USA), using the array beam mode. The DXA scans were performed within 1 week before starting and 1 week after the completion of 18 community-based exercise CR sessions. Scans were used to measure total body mass, body fat mass, body lean mass, body fat percentage, and abdominal region fat percentage (defined as the area between the ribs and the pelvis by GE Healthcare systems). Percentages of the total were calculated accordingly. The scanner was calibrated daily against a manufacturer-supplied standard calibration block to control for possible baseline drift.
Aerobic capacity was represented as peak oxygen consumed (VO2peak, mL kg−1 min−1) that was calculated from the equation VO2peak = 4.9486 + 0.023 * walk distance (meters) that was determined via using 6-min walking test (6MWT) as described previously (American College of Sports 2013). The 6MWT was performed in a 50 m pre-marked University of Evora pavilion, and instructions and encouragements were given following the test’s guidelines (Guyatt et al. 1985). This test is well validated for CAD patients and has shown good reliability in this patient group (McDermott et al. 2014).
To measure the isokinetic muscle strength, we used the Isokinetic Dynamometer (Biodex®, System 3 Pro, Biodex Corp., Shirley, NY, USA). The protocol used was the concentric unilateral mode for the extensor and knee-dominant flexor muscles. Patients were tested in a seated position with hip flexion. Stabilization straps were applied to the trunk, waist, and thigh. Evaluations of peak torque (three repetitions) and fatigue resistance (20 repetitions) were carried out at angular velocities of 90°s−1 and 180°s−1 of the dominant knee. The peak torques of the knee extensor and flexor muscles were adjusted by body weight according to the following formula: strength (Nm) × 100/body weight (kg), since it is well known that the peak muscle power is closely associated with body weight (Maffiuletti et al. 2007).
After completing all clinical evaluations, patients were asked to wear a triaxial accelerometer (ActiGraph GT3X) on their hip placed anterior to the right iliac crest for seven consecutive days during waking and sleeping hours except when bathing or swimming. Acceleration data from the three planes were processed with ActiGraph software (ActiLife, version 6) using 15 s epochs (raw data recorded at 30 Hz) and the standard filter and were integrated into a vector magnitude count by taking the square root of the sum of squared axes (vertical, anterior–posterior, and medial–lateral). Daily averages (min/day) of accelerometer-measured PA were calculated for each patient and classified into five activity levels (sedentary time 1.00–1.99 MET, light PA 2.00–3.49 MET, and all activity ≥ 3.50 MET was classified as moderate-to-vigorous PA) using the limits set by the manufacturer. A valid day was defined as ≥ 10 h of wear time. All activity with intensities 1 MET (1 Met = 3.5 ml/kg/min) or higher was calculated on wear time. Patients with at least four valid days (3 weekdays and 1 weekend day) were included in the analyses (monitor wear time of ≥ 600 min/day) (Prince et al. 2015).
All measurements were taken at the beginning and completion of 18 sessions of community-based exercise CR programs. The protocols of pre- and post-intervention were the same for each patient. Compliance and adherence to exercise training was determined by recording the number of sessions attended.
Exercise training protocols
After hospital discharge, educational intervention, dietary advice, and psychological support were performed in all participants. The exercise programs consisted of 6 weeks of supervised treadmill exercise, three sessions per week (Fig. 2). If a session was missed, it was made up that week or the following week. Participants performed each exercise session in a group, including a maximum of three participants per session.
Training sessions were supervised by a physiologist. As training intensity increased, the participant’s heart rate, rate of perceived exertion (Borg scale), and cardiac symptoms were also taken into consideration. Heart rates were observed with Polar heart rate monitoring (Polar Electro Oy, Kempele, Finland), and blood pressure was measured at the commencement and the end of each session.
The 10-point Category-Ratio Borg Scale (Scherr et al. 2013), also commonly referred to as the Rating of Perceived Exertion, was used to assess participants’ perceived effort during exercise. The Borg Scale is a 10-point scale ranging from 0 to 10 with anchors ranging from “No exertion at all” (0) to “Maximal exertion” (10). Participants were asked to rate their exertion before (pre-exercise), immediately post minute to minute, and post-exercise. Buchheit and Laursen (2013) demonstrated that the RPE (Borg Scale) has shown a great correlation with HR, ventilation, and VO2 in individuals with and without CAD, and the correlation is not impacted by beta-blocker medication, a commonly used HR modulating medication by patients with CAD. Participants’ heart rate was recorded using Polar heart rate monitors minute to minute of exercise. The control group did not receive any additional follow-up regarding exercise beyond general advice on the importance of exercise and diet.
Each training session was initiated with a 5–10-min warm-up at 50–60% HRpeak and ended with 5 min of cool-down at 40% HRpeak. The HIIT group performed 4 × 4-min high-intensity intervals at 85–95% HRpeak followed by a 1-min recovery interval at 40% HRpeak, predicted with a supervised graded exercise test on a treadmill with the Bruce protocol (Bires et al. 2013). During the exercise, the participants were motivated to gradually increase their exercise intensity toward 6–9 (hard to very hard) on a 0 to 10 Borg scale. The MICT protocol (usual care) consisted of a continuous bout of moderate-intensity exercise to elicit 70–75% HRpeak, rating of perceived exertion 3 to 5 (fairly light to somewhat hard), for 27.5 min to equate the energy expenditure with the HIIT protocol (Fig. 3).
Ethical considerations
All work was conducted following the Declaration of Helsinki and registered at ClinicalTrials.gov (NCT03538119) on May 25, 2018. Ethics approval was obtained from the University of Evora Ethics Committee (reference number 17039). All participants signed a written informed consent before participating in this study.
Statistical analyses
According to the Shapiro–Wilk and the Levene test results, repeated measures ANOVA assumptions were not met. Thus, non-parametric statistics were performed. The Friedman test was used for within-group comparisons, and the Kruskal–Wallis test was used for between-group comparisons. Pairwise post hoc tests were also carried out when significant differences were found. Lastly, the Wilcoxon test was performed to compare paired fall data between the baseline and the post-intervention. The means and standard deviations were calculated for all variables. The variation value was calculated between the baseline, post-intervention, and follow-up evaluations as ∆: moment x – moment x−1. For significant differences between the evaluation moments, the respective delta percentage was also computed by the following formula: (∆%: [(moment x – moment x−1)/moment x−1] × 100). The effect size (ES) was calculated using Cohen’s method since the data were not normally distributed (Cohen 2013). The ES was computed and classified based on Cohen’s thresholds (small d = 0.10; medium d = 0.30; and large d ≥ 0.50) (Cohen 2013). Analyses were performed using the SPSS software package (version 24.0 for Windows, IMB Statistics). A value of p ≤ 0.05 was considered statistically significant for all analyses. A code was assigned to each participant to preserve their anonymity.
Based on guidelines, dyslipidemia was defined as an HDL-C level < 50 mg/dL in women or < 40 mg/dL in men and a TG level ≥ 150 mg/dL (Wilson et al. 2019). The cutoff for elevated hsCRP was ≥ 3.0 mg/L, according to national guidelines (Pearson et al. 2003). Criteria of diabetes mellitus diagnosis was defined according to the American Diabetic Association’s diagnostic criteria (Rey and Hawks 2022): pre-diabetic stage [HbA1c 5.7–6.4/impaired fasting blood glucose (100–125 mg/dL)] and diabetes mellitus (HbA1c ≥ 6.5 /fasting glucose ≥ 126 mg/dL). Impaired non-fasting glucose was defined as a glucose value ≥ 100 mg/dL, based on the American Diabetes Association (2003) expert recommendations. Overweight was defined as a BMI 25.0 to 29.9 kg/m2, and obesity was defined as a BMI ≥ 30 kg/m2 (Liguori and Medicine ACoS 2020). WC was measured by a trained examiner using a standard protocol. In this study, increased WC was defined as > 80 cm in women and > 94 cm in men (Sardinha et al. 2012).
Results
Baseline characteristics (Table 1) were not different for HIIT, MICT, and control groups: age (55 ± 9 vs. 55 ± 10 vs. 57 ± 11 years, respectively, p = 0.180), female (15% vs. 17% vs. 15%, p = 0.211), BMI (28.2 ± 4.5 vs. 29.4 ± 3.9 vs. 29.4 ± 4.3 kg/m2, p = 0.659), waist circumference (98.4 ± 14.5 vs. 101.1 ± 10.3 vs. 101.1 ± 10.8 cm, p = 0.218) and VO2max (34.7 ± 9.0 vs. 30.4 ± 6.3 vs. ± 23.5 ± 11.0 mL/kg/min p = 0.290). Comorbidities and medications were also not different between groups (p > 0.05).
Physical fitness (body composition, aerobic capacity, and muscle strength)
Longitudinal changes in physical fitness levels are shown in Fig. 4. At baseline, there were no differences across groups at the body composition measurements. Following 6 weeks of exercise, the results showed that the HIIT group demonstrated greater improvements compared to MICT in waist circumference (∆m2−m1% HIIT 4.1%, p = 0.002 vs. ∆m2−m1% MICT 2.5%, p = 0.002) and body fat mass (∆m2−m1% HIIT 4.5%, p < 0.001 vs. ∆m2−m1% MICT 3.2%, p < 0.001). The control group had no improvements; on the other hand, all values of body composition measurements increased over time. These results were only maintained at 6 months of follow-up in the HIIT group, which demonstrated a significant decreasing trend in waist circumference (∆m3−m2% −2 cm, p = 0.033) compared to MICT (p = 0.016) and Control (p = 0.001), and maintained at 12 months of follow-up with significant differences to MICT (p = 0.018) and control (p = 0.001). There were also significant differences in body fat at 6 months in the HIIT group compared to the MICT (p = 0.05) and control (p = 0.047) groups, as well as at 12 months compared to MICT (p = 0.048) and control (p = 0.028) groups. The respective ES from baseline to 6 weeks were small in the HIIT group in body weight (d = 0.20), abdominal fat percentage (d = 0.28) and BMI (d = 0.22), and medium in waist circumference (d = 0.34). Moreover, in the MICT group, the ES were small in body fat percentage (d = 0.22), total body fat mass (d = 0.22), and waist circumference (d = 0.22). The ES between post-intervention and the 6- and 12-months follow-up were small in HIIT in lean mass (d = 0.14 and d = 0.15, respectively), and in MICT in body fat mass (d = 0.11 and d = 0.10, respectively).
Impact of the community-based exercise CR programs on physical fitness indicators. Control = Control group; HIIT = high-intensity interval training; MICT = moderate-intensity continuous training; min = minutes; * p-value < .05, ** p-value < .01, *** p-value < .001; † significant differences between HIIT and Control, p < .05; ‡ significant differences between MICT and Control, p < .05; ‡ significant differences between HIIT and MICT, p < .05
Following the 6 weeks of supervised program, aerobic capacity significantly increased by 14% with HIIT (Δm2−m1 2.5 ± 1.5 ml kg−1 min−1, p < 0.001) and 9% with MICT (Δm2−m1 1.4 ± 1.2 ml kg−1 min−1, p < 0.001) (Fig. 4). There were significant differences between the HIIT group and the MICT group at the end of the intervention, at 6 months and 12 months of follow-up. Moreover, the control group decreased the VO2peak from the baseline to the end of the program (p = 0.003) and continued to decrease at 6 months (p = 0.008) and at 12 months (p = 0.016), with significant differences between the exercise groups at all evaluations (p < 0.001). The respective ES from baseline to 6 weeks were large in HIIT (d = 1.54) and MICT (d = 0.68), whereas those between post-intervention and the follow-up in HIIT were small at 6 months (d = 0.25) and medium at 12 months (d = 0.34).
The maximal strength of the knee extensors and flexors indicators can be seen in Fig. 4. Descriptive analysis demonstrates an increase of 13% at 6 weeks in the knee extension peak torque in the HIIT group (∆m2−m1 11.9 ± 27.6 N m, p = 0.007) and 10% in the MICT group (∆m2−m1 9.1 ± 22.8 N m, p = 0.061). These results were only not maintained at follow-up evaluations in the MICT group, which demonstrated a significant decrease trend at 6 months (∆m3−m2 −6.5 ± 17.8 N m, p = 0.022), and at 12 months (∆m4−m2 − 8.6 ± 12.9 N m, p = 0.002). The control group had a decrease of 0.4% (∆m2−m1 −3.0 ± 22.8 N m, p = 0.835) from the baseline to the end of the program and this trend was maintained throughout the follow-up evaluations. A positive increase between baseline and the 6 weeks was observed in the knee flexion peak torque of 15% in HIIT (∆m2−m1 7.2 ± 14.2 N·m, p = 0.002) and 14% in MICT (∆m2−m1 6.9 ± 16.0 N m, p = 0.022). These results were again not maintained at follow-up assessments in the MICT group, which demonstrated a significant downward trend at 6 and 12 months (∆m4−m2 −6.8 ± 11.0 N·m, p = 0.007) in this indicator. The control group decreased by a mean of 0.2% (∆m2−m1 − 0.3 ± 12.8 N m, p = 0.835) from the baseline to the end of the program and maintained the tendency of decreasing in the follow-up evaluations. Significant differences were observed between the HIIT group and the control group in the knee extension peak torque after the intervention (p = 0.025), and in the knee flexion peak torque after the intervention (p = 0.031) and at 12 months of follow-up (p = 0.028). The respective ES in the knee extension peak torque from baseline to 6 weeks were small in HIIT (d = 0.24) and MICT (d = 0.20), from post-intervention to 6-month follow-up the ES were small in HIIT (d = 0.21) and in MICT (d = 0.15), and from post-intervention to 12-month follow-up the ES were small in HIIT (d = 0.19) and in MICT (d = 0.20). The knee extension peak torque had a small ES from baseline to 6 weeks, small in HIIT (d = 0.29) and medium in MICT (d = 0.33), from post-intervention to 6-month follow-up the ES were small in HIIT (d = 0.18) and medium in MICT (d = 0.22), and from post-intervention to 12-month follow-up the ES were medium in MICT only (d = 0.35).
Physical activity and sedentary behavior
Figure 5 presents the PA and SB of exercise and control groups. Following the 6 weeks supervised program, HIIT decreased the sedentary time of 15% (Δm2−m1 −148.6 ± 106.1 min/day, p < 0.001), MICT decreased 10% (Δm2−m1 −105.5 ± 88.0 min/day, p < 0.001), and control decreased 0.1% (Δm2−m1 0.559 ± 73.8 min/day, p = 0.144). The control group spent 176 min more sedentary time than HIIT (p < 0.001) and 72 min more than MICT (p < 0.001). Regarding the PA, HIIT increased the daily step count of 33% (Δm2−m1 4162.3 ± 8339.7 step count, p < 0.001), MICT increased 10% (Δm2−m1 745.9 ± 1605.4 step count, p < 0.001), and control increased 6.5% (Δm2−m1 265.5 ± 1524.4 step count, p = 1.000). In LPA, HIIT increased 39% (Δm2−m1 80.1 ± 45.2 min/day, p < 0.001), MICT increased 30% (Δm2−m1 55.6 ± 60.3 min/day, p < 0.001), and control increased 9% (Δm2−m1 12.6 ± 65.5 daily step count, p = 0.532). In MVPA, HIIT improved significantly 54% (Δm2−m1 16.4 ± 14.4 min/day, p < 0.001), MICT improved 45% (Δm2−m1 13.4 ± 12.4 min/day, p < 0.001), and control improved 19% (Δm2−m1 4.5 ± 13.7 step count, p = 0.033). Although, the control group had the amount of LPA 72.6 min lower than HIIT (p = 0.003), and the amount of MVPA was 38 min lower than HIIT (p < 0.001) and 17 min lower than MICT (p < 0.001).
Impact of the community-based exercise CR programs on physical activity levels and sedentary behavior. Control = control group; HIIT = high-intensity interval training; MICT = moderate-intensity continuous training; min = minutes; * p-value < .05, ** p-value < .01, *** p-value < .001; † significant differences between HIIT and control, p < .05; ‡ significant differences between MICT and control, p < .05; significant differences between HIIT and MICT, p < .05
These results were not maintained at the follow-up evaluations in the exercise groups, which demonstrated a significant increasing trend in sedentary time at 6 months (∆m3−m2 HIIT 141.4 ± 127.9 min/day, p < 0.001 and ∆m3−m2 MICT 86.0 ± 75.6 min/day, p < 0.001) and also at 12 months (∆m4−m2 HIIT 144 ± 151.6 min/day, p < 0.001 and ∆m3−m2 MICT 50.0 ± 88.0 min/day, p < 0.001), but this results remains lower than prior to participating in community-based exercise CR programs. In PA levels at 6 and 12 months, MICT maintained the amount of LPA from post intervention, but MVPA decreased significantly. The same was observed in the HIIT group with a decreasing trend in LPA and MVPA. The control group had a decrease in all PA indicators at 6 and 12 months of follow-up evaluations (Fig. 5). Significant differences were observed between the exercise groups and the control group in sedentary time, MVPA and number of steps (p < 0.001) over time. The respective ES from baseline to 6 weeks in daily step count were small in HIIT (d = 0.26) and MICT (d = 0.27), in sedentary time were large in HIIT (d = 1.20) and MICT (d = 0.91), in LPA were large in HIIT (d = 1.01) and MICT (d = 0.67), and finally in MVPA were small in control (d = 0.20) and large in HIIT (d = 0.70) and MICT (d = 0.50).
Quality of life
The control group reported a lower QoL compared to both the HIIT and MICT groups at all assessed time points, except for the baseline measurement. Within the exercise cohorts, there was a statistically significant enhancement observed in seven out of the eight SF-36 dimensions following 6 weeks of engagement in the community-based exercise CR programs, as compared to the control group. These improved dimensions encompassed physical functioning, role-physical, role-emotional, mental health, vitality, and general health. Furthermore, individuals who participated in community-based exercise CR programs, particularly in the HIIT group, exhibited noteworthy temporal ameliorations in physical functioning (p = 0.022) when juxtaposed with their counterparts in the MICT group, as illustrated in Fig. 6.
Impact of the community-based exercise CR programs on quality of life indicators. Control = Control group; HIIT = high-intensity interval training; MICT = moderate-intensity continuous training; min = minutes; * p-value < .05, ** p-value < .01, *** p-value < .001; † significant differences between HIIT and control, p < .05; ‡ significant differences between MICT and control, p < .05; significant differences between HIIT and MICT, p < .05
Following 6 and 12 months of intervention, both exercise groups exhibited significant intragroup improvements. Specifically, in the MICT group, noteworthy enhancements were observed at the 6-month follow-up in social functioning, role-emotional, and mental health dimensions, as well as at the 12-month follow-up in physical functioning, social functioning, role-physical, and role-emotional dimensions. A parallel pattern was evident in the HIIT group, with significant within-group improvements seen in social functioning, role-physical, and mental health dimensions. Furthermore, at the 12-month follow-up, significant progress was observed in the role-physical dimension. In contrast, significant disparities were observed between the exercise groups and the control group across QoL indicators (p < 0.001), except for the bodily pain. Additionally, within the exercise groups, there were differences between the HIIT and MICT groups. These differences were evident in the “physical functioning” indicator after the intervention (p = 0.002), at 6 months (p = 0.023), and at 12 months of follow-up (p = 0.003). In the “role-physical” indicator, differences were observed after the intervention (p = 0.025) and at 12 months of follow-up (p = 0.017). Furthermore, distinctions were noted in the “role-emotional” indicator at the 12-month follow-up (p = 0.014). In the “social functioning” indicator disparities were evident at 6 months (p = 0.010) and at 12 months of follow-up (p = 0.004). For the “mental health” indicator, differences were observed after the intervention (p = 0.030), at 6 months (p = 0.015), and at 12 months of follow-up (p = 0.020). Lastly, differences were apparent in the “general health” indicator after the intervention (p = 0.016) and at 12 months of follow-up (p = 0.034). The ES from baseline to 6 weeks in the HIIT group were small in bodily pain (d = 0.21), medium in social functioning (d = 0.44), and large in physical functioning (d = 2.89), role-physical (d = 2.71), role-emotional (d = 1.57), mental health (d = 2.28), vitality (d = 1.93), and general health scores (d = 1.72). In the MICT group, the ES was small in bodily pain (d = 0.11), medium in social functioning (d = 0.33), and large in physical functioning (d = 2.50), role-physical (d = 1.71), role-emotional (d = 1.30), mental health (d = 1.84), vitality (d = 1.29), and general health scores (d = 1.03).
Anxiety and depression
At the outset of the study, anxiety scores were modestly elevated at baseline (mean HIIT = 7.5 ± 4.8, mean MICT = 7.7 ± 4.4, and mean control = 7.5 ± 4.6), decreasing after the community-based exercise CR program in the exercise groups (mean HIIT = 6.2 ± 4.6 and mean MICT = 6.5 ± 4.4). Similarly, the initial assessment of depression scores revealed a significant portion of patients with clinically elevated depression levels at baseline (mean HIIT = 4.5 ± 3.4, mean MICT = 4.6 ± 3.4, and mean control = 4.6 ± 3.6). Notably, upon completion of the 6-week community-based exercise program in the exercise groups, a reduction in the frequency of clinically elevated depression scores was observed, resulting in mean values of 4.0 ± 3.3 for HIIT and 4.2 ± 3.3 for MICT. Conversely, the control group witnessed an increase in depression scores, with a mean value of 4.7 ± 3.6, as depicted in Fig. 7.
The ES from baseline to 6 weeks in the HIIT group were medium in depression scores (d = 0.40) and large in anxiety scores (d = 1.00). In the MICT group, the ES were medium in depression scores (d = 0.40) and large in anxiety scores (d = 0.90).
Adherence and safety
Only one participant from each group discontinued the intervention, achieving 96% adherence in both exercise groups. There were no adverse events in either program (HIIT and MICT) during the exercise interventions. Thus, HIIT protocols proved to be a safe, effective, and pleasant tool for low-risk patients with CAD as well.
Discussion
This study represents a pioneering endeavor as the first RCT to systematically investigate the impact of community-based exercise CR programs on various health-related parameters, lifestyle modifications, and the evolution of cardiovascular risk factors at both the 6- and 12-month post-intervention time points. The study’s outcomes have revealed noteworthy insights. Low-risk CAD patients who engaged in HIIT and MICT interventions demonstrated substantial enhancements in a multitude of physical fitness parameters. These encompassed reductions in BMI, body fat percentage, total body fat mass, abdominal fat percentage, WC, and an elevation in VO2peak. Furthermore, the HIIT group displayed commendable improvements in knee extensor strength. Critically, these favorable changes exhibited a tendency to endure over the long term, underscoring the enduring advantages of exercise-based cardiac rehabilitation, especially concerning cardiovascular risk factors. Conversely, the control group, devoid of any exercise regimen, exhibited diminishing trends in physical fitness metrics. Regarding PA and SB, both exercise groups demonstrated heightened levels of physical activity compared to the control group. This was characterized by reductions in sedentary time and an increase in daily step count, LPA, and MVPA. Nevertheless, the preservation of these enhancements in physical activity presented a challenge during the 6- and 12-month follow-up assessments, with particular difficulty encountered in curtailing SB and augmenting MVPA. In the realm of QoL, both the HIIT and MICT programs engendered substantial ameliorations across various dimensions, encompassing physical functioning, emotional role, mental health, and general health when juxtaposed with the control group. Notably, these enhancements exhibited sustained continuity throughout the study, emphasizing the pivotal role of exercise-based cardiac rehabilitation in enhancing the QoL of CAD patients. Concerning anxiety and depression, both exercise cohorts experienced marked reductions in anxiety levels compared to the control group. In contrast, the control group displayed an exacerbation in depression scores following the intervention, signifying the potentially deleterious consequences of refraining from participation in cardiac rehabilitation post-cardiac event. Crucially, the exercise groups managed to preserve lower levels of anxiety and depression during the 6- and 12-month follow-up evaluations.
Among patients with low-risk CAD, both HIIT and MICT exercise programs led to significant enhancements in BMI, body fat percentage, total body fat mass, percentage of abdominal fat, WC, and VO2peak. The HIIT group additionally exhibited a substantial improvement in maximal knee extensor strength. Importantly, following the culmination of the community-based exercise CR program, these improvements were largely sustained over time, with only two significant variations observed: a reduction in WC after 6 months in the HIIT group and a significant decline in maximal knee flexor strength in the MICT group after 12 months. Conversely, the control group, devoid of any exercise program, experienced a decline in VO2peak, muscle strength, and PA, alongside unfavorable shifts in body composition and increased sedentary time from baseline to 6 weeks. This trend persisted at the 6- and 12-month follow-up assessments. These findings merit particular attention due to the well-established associations between body fat mass, abdominal fat, higher BMI, greater waist circumference, and waist–hip ratio with an elevated risk of cardiovascular events, all-cause mortality, and premature mortality (Di Angelantonio et al. 2016). It is noteworthy that exercise training disproportionately targets visceral fat reduction in comparison to overall body fat reserves, with exercise proving more effective than dietary interventions in inducing visceral fat loss (Pattyn et al. 2014). Within our RCT, both HIIT and MICT demonstrated substantial positive effects on body composition in CAD patients. In contrast, CAD patients who did not engage in any form of community-based exercise CR program following a cardiac event exhibited trends toward increased BMI, fat mass, and waist circumference. Notably, the HIIT intervention appeared to exert a more pronounced influence on body composition compared to MICT, a trend supported by prior research (Dun et al. 2019; Gonçalves et al. 2023b; Trapp et al. 2008; Zhang et al. 2017). For instance, Dun et al. (2019) included 120 patients who completed 36 CR sessions and compared HIIT (involving 4 to 8 alternating intervals of 30–60 s at a rating of 15–17 of RPE) and MICT (performed for 20 to 45 min at an RPE of 12 to 14), revealing that supervised HIIT led to significant reductions in total fat mass and abdominal fat percentage. Similarly, Trapp et al. (2008) compared HIIT and MICT, highlighting that the HIIT group experienced a more pronounced decrease in abdominal fat. In contrast, Zhang et al. (2017) demonstrated that both HIIT and MICT significantly reduced total and abdominal fat mass, with no discernible differences across groups.
Aerobic capacity (VO2peak) improved by 14%, equivalent to 2.5 mL kg−1 min−1 or nearly 1 MET in the HIIT group and 9% in the MICT group, and these results were maintained in the exercise groups at the follow-up evaluations. These results indicated that training intensity is essential in improving VO2peak in CAD patients since the improvements of HIIT were almost twice as good as in the MICT group. The mean difference of 0.9 mL kg−1 min−1 between HIIT and MICT holds clinical significance, as each 1 mL kg−1 min−1 enhancement in VO2peak during a CR program is associated with an approximately 8–17% reduction in all-cause and cardiovascular-related mortality (Davidson et al. 2018). These findings align with data from Keteyian et al. (2012), whose study involving 2812 cardiac patients demonstrated a 15% reduction in cardiovascular-specific mortality risk per 1 mL kg−1 min−1 VO2peak increment. Moreover, the superior efficacy of HIIT in elevating VO2peak compared to MICT during supervised training is similar to previous meta-analyses reporting group disparities of 1.5 to 1.6 mL kg−1 min−1 (Valkeinen et al. 2010; Pandey et al. 2015). Similarly, Rognmo et al. (2004) affirmed HIIT’s effectiveness in augmenting aerobic capacity in CAD patients. In addition, our prior systematic review with meta-analysis, encompassing 16 studies with 969 patients, revealed that both moderate-to-vigorous intensity (SMD = 1.84 mL kg−1 min−1; 95% CI [1.18, 2.50]) and vigorous-intensity (SMD = 1.80 mL kg−1 min−1; 95% CI [0.82, 2.78]) programs were linked to more substantial increases in relative VO2peak in contrast to moderate intensity interventions (SMD = 0.71 mL kg−1 min−1; 95% CI [0.27, 1.15]) (Gonçalves et al. 2021). Furthermore, Sandercock et al. (2013) reported greater improvements of 5.2 mL kg−1 min−1 (95% CI 4.1–6.4), while Uddin et al. (2016) presented enhancements of 3.3 mL kg−1 min−1 (95% CI 2.6–4.0). Nevertheless, in our investigation, the control group witnessed a decline in VO2peak from baseline, with this decline persisting at 6 and 12 months. This trend is disconcerting, as aerobic capacity is a robust predictor of both cardiovascular and all-cause mortality (Davidson et al. 2018). Martin et al. (2013) demonstrated that an increase in aerobic capacity post a 12-week exercise-based CR program correlated with a 13% reduction in overall mortality per metabolic equivalent elevation in VO2peak, with a 30% reduction among patients commencing the program with low fitness levels.
Muscle strength plays a crucial role in exercise capacity, and survival rates among CAD patients (Kamiya et al. 2014). At baseline, all groups exhibited low muscle strength levels, consistent with prior studies involving CAD patients pre-exercise programs (Marzolini et al. 2012; Fletcher et al. 2013). Following 6 weeks of intervention, our investigation revealed that both HIIT and MICT led to increased muscle strength in comparison to patients who did not participate in a community-based exercise CR program. Notably, HIIT induced more substantial gains in muscle strength compared to MICT, although these differences did not attain statistical significance, which aligns with our focus on aerobic training. Our study’s observed impact on muscle strength closely resembled the findings of Kida et al. (2008). In contrast, despite Yamamoto et al.’s report of increased muscle volume in CAD patients (Yamamoto et al. 2016), our study did not yield significant increases. Importantly, at follow-up assessments, the maintenance of these results was evident exclusively in the HIIT group.
The PA levels in the exercise groups significantly exceeded those in the control group, manifesting as a substantial reduction in sedentary time and increased daily step count, LPA, and MVPA. Notably, cardiac patients frequently exhibit low MVPA levels, which is a crucial component of CR programs. These programs emphasize reducing SB while promoting MVPA (Ambrosetti et al. 2021). Despite its significance, information concerning SB in patients with CVD patients remains scarce. Objective assessments of PA and SB have been scarcely explored (Bakker et al. 2021). Our results unveiled elevated SB levels across all three groups before enrollment, with daily routines predominantly characterized by LPA. This is concerning since SB independently heightens CVD risk (Stewart et al. 2017), our findings align with prior research indicating that CAD patients are predominantly sedentary (10.5–12 h/day), followed by extended periods of LPA (3.5 h/day) and minimal engagement in MVPA (20–65 min/day) (Prince et al. 2019; Biswas et al. 2018). As per PA guidelines, adults should accumulate 150 min per week in MVPA (Woodruffe et al. 2015); a criterion met by the exercise groups in our study. Six weeks post-intervention, we observed a significant surge in daily MVPA (+ 36 min/day, p < 0.001) in the HIIT group and (+ 23 min/day, p < 0.001) in the MICT group compared to the control. However, when comparing HIIT to MICT, similar daily LPA levels were noted. Previously, both LPA and MVPA have been associated with reduced CVD risk (LaMonte et al. 2017), aligning partially with our findings, where HIIT and MICT patients engaged slightly more in MVPA and exhibited reduced SB compared to previous studies (Diaz et al. 2017; Wennman et al. 2016; Vasankari et al. 2018). However, CR-induced increases in MVPA tend to be transient, with many patients reverting to inactive lifestyles within months (Bock et al. 2003). Our study confirmed this trend as, after the intervention, the exercise groups exhibited a significant trend toward increased SB and decreased MVPA at the 6- and 12-month follow-up assessments. This might be attributed to the brief intervention duration, particularly the intensive nature of HIIT, which led to a greater increase in SB. It aligns with the findings of Milkman et al. (2021), emphasizing the limited long-term behavior change associated with short-term, intensive health interventions. This observation is consistent with reports by Guiraud et al. (2012) and Dolansky et al. (2010), where only approximately 40% of patients sustained physical activity one year after CR, highlighting the challenge of maintaining long-term health behavior change.
Both the HIIT and MICT programs yielded significant enhancements in various aspects of QoL, including physical functioning, physical role, emotional role, mental health, vitality, and general health when compared to the control group. Notably, within the exercise groups, significant differences were observed only in the indicators of physical functioning and general health in patients undergoing the HIIT protocol compared to MICT. These improvements persisted through the 6- and 12-month follow-up assessments in both exercise groups. In contrast, the group that did not undergo any exercise intervention did not experience significant improvements in QoL. These results show that community-based exercise CR programs, even at moderate intensity, can play an important role in improving QoL. This is especially significant given the influence of CAD on an individual’s QoL, often associated with increased functional dependence (Cuerda et al. 2012). Our RCT aligns with existing literature supporting the notion that exercise-based CR programs consistently generate positive effects on QoL (Cuerda et al. 2012; Lovlien et al. 2017; Francis et al. 2019; Candelaria et al. 2020). For instance, Lovlien et al. (2017) observed QoL improvements in 142 women diagnosed with CAD following participation in exercise-based CR programs. A 2015 systematic review of RCTs comparing exercise-based CR programs to traditional care programs reported QoL enhancements in 14 out of 20 studies involving CAD patients (Anderson et al. 2016). Likewise, a 2018 meta-analysis encompassing 41 RCTs (N = 11.747) investigating CR measures and interventions revealed that exercise-based CR programs had a positive impact on QoL (Francis et al. 2019). A 2019 systematic review (14 RCTs, N = 1739) that documented clinically positive effects in two domains of the SF-36 at 6 months (physical role and general health) and one domain at 12 months (physical functioning) following exercise-based CR programs (Candelaria et al. 2020). Another study that closely aligns with our findings is that of Worcester et al. (1993), who observed that the QoL improvement provided by 11 weeks of MICT in 224 CAD patients was comparable to that achieved with HIIT. Additionally, in a RCT involving CAD patients, both HIIT training and traditional MICT were found to enhance QoL (Jaureguizar et al. 2016). Lastly, a cohort study involving 37 CAD patients who completed a 5-week exercise-based CR program revealed significant QoL improvements (Fallavollita et al. 2016).
Both exercise groups demonstrated significant reductions in anxiety levels when compared to the control group. The control group exhibited an increase in depression scores from baseline to post-intervention, underscoring the potential harm of not engaging in a community-based exercise CR program after a cardiac event. Furthermore, at the 6- and 12-month marks post-intervention, the HIIT and MICT groups continued to experience decreases in both anxiety and depression scores, while the control group maintained the previous upward trend. These findings hold substantial clinical relevance, as anxiety and depressive symptoms are associated with an elevated risk of subsequent cardiac events (Bakker et al. 2021; Celano et al. 2015; Emdin et al. 2016), and exercise-based CR programs have demonstrated their efficacy in reducing levels of anxiety and depression (Korzeniowska-Kubacka et al. 2017; Lavie and Milani 2004). Additionally, Bakker et al. (2021) identified an association between anxiety in CAD patients and higher levels of self-reported SB. Furthermore, our study revealed no significant differences in anxiety and depression reductions between the HIIT and MICT groups, suggesting that training intensity did not significantly impact these symptoms. These outcomes are consistent with those of several other authors, affirming the efficiency of exercise-based CR programs in mitigating symptoms of anxiety and depression (Lavie et al 2016; Zheng et al. 2019; Smith et al. 2017; Kachur et al. 2016). Lavie et al. (2016) demonstrated the enhancement of psychosocial functioning through exercise-based CR programs. Notably, a recent meta-analysis highlighted the effectiveness of exercise in reducing anxiety and depression in CAD patients (Zheng et al. 2019). Additionally, Smith et al. (2017) found that higher levels of PA following CR were associated with lower depressive and anxiety symptoms. Finally, Kachur et al. (2016) in a comprehensive meta-analysis and systematic review, studied 1150 CAD patients who completed a formal CR program, and they reported a low incidence of depression post-CR (6.8%), with a corresponding reduction in mortality (20.8%).
In summary, accumulating research indicates that HIIT has the potential, when compared to MICT, to induce changes in numerous physiologic and health-related markers, including greater improvement in body composition, aerobic capacity, PA, SB, quality of life, anxiety, and depression in CAD patients. Importantly, our study’s positive efficacy outcomes are promising, especially considering the relatively short intervention duration (6 weeks) and frequency (3 sessions per week, totaling 18 sessions per participant). Encouraging patients to maintain higher PA levels may contribute to long-term enhancements in both physical and mental health. Additionally, this study underscores the necessity for strategies aimed at promoting PA adherence following a cardiac event and sustaining PA after participation in a community-based CR exercise program. Perhaps a better understanding of physical and psychological obstacles hindering PA adherence could facilitate the maintenance of PA levels over time. We advocate for more vigilant post-CR exercise program monitoring of cardiac patients.
Study limitations
This study has some limitations that should be acknowledged. First, most of our participants were men, which is a frequently encountered referral bias in CRP (Cottin et al. 2004). Second, there was no specific control for habitual dietary intake, participants just followed the ideal dietary recommendations given by the medical specialist. Plus, when considering the results of this study, the possible confounding effects of concurrent medications should be considered although no change happened during the study period for doses of lipid-lowering and heart rate control medications. Additionally, the control group was not delivering diaries and we have no information about their PA habits during the intervention period from baseline to 6 weeks. A potential increase in PA could imply a reduced difference in effect between the groups. However, our study duration of 6 weeks was relatively short and with an extended duration of the intervention, one might expect an effect of clinical relevance.
Conclusions
This RCT demonstrates that participation in a community-based exercise CR program is strongly linked to improved aerobic capacity, PA, QoL, reduced WC, lower fat mass, decreased SB, and reduced anxiety and depressive symptoms among cardiac patients. Both exercise-based CR programs proved to be effective in mitigating cardiovascular risk factors and positively influencing these cardiac patients’ lifestyle. The HIIT group exhibited superior long-term improvements compared to the MICT group. Conversely, it is crucial to motivate patients to sustain higher levels of physical activity to enhance both cardiovascular and psychological well-being.
Data availability
The data that support the findings of this study are available from the corresponding author, C.G., upon reasonable request.
Abbreviations
- ACSM:
-
American College of Sports Medicine
- BMI:
-
Body mass index
- CAD:
-
Coronary artery disease
- CPET:
-
Cardiopulmonary exercise test
- CR:
-
Cardiac rehabilitation
- DBP:
-
Diastolic blood pressure
- DXA:
-
Dual-energy X-ray absorptiometry
- HDL-C:
-
High-density lipoprotein cholesterol
- HIIT:
-
High-intensity interval training
- HR:
-
Heart rate
- LDL-C:
-
Low-density lipoprotein cholesterol
- MET:
-
Metabolic equivalent
- MI:
-
Myocardial infarction
- MICT:
-
Moderate-intensity continuous training
- RPE:
-
Rating of perceived exertion
- SB:
-
Sedentary Behavior
- SBP:
-
Systolic blood pressure
References
Ambrosetti M, Abreu A, Corrà U, Davos CH, Hansen D, Frederix I et al (2021) Secondary prevention through comprehensive cardiovascular rehabilitation: from knowledge to implementation. 2020 update. A position paper from the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur J Prev Cardiol 28(5):460–495
American College of Sports Medicine (2013) ACSM’s guidelines for exercise testing and prescription. Lippincott Williams & Wilkins, Philadelphia
American Diabetes Association (2003) Standards of medical care for patients with diabetes mellitus. Diabetes Care 26(Suppl. 1):S33–S50
Anderson L, Oldridge N, Thompson DR et al (2016) Exercise-based cardiac rehabilitation for coronary heart disease. J Am Coll Cardiol 67:1–12
Andrade N, Alves E, Costa AR, Moura-Ferreira P, Azevedo A, Lunet N (2018) Knowledge about cardiovascular disease in Portugal. Rev Port Cardiol 37(8):669–677
Bäck M, Cider A, Gillström J, Herlitz J (2013) Physical activity in relation to cardiac risk markers in secondary prevention of coronary artery disease. Int J Cardiol 168:478–483
Bakker EA, van Bakel BM, Aengevaeren WR, Meindersma EP, Snoek JA, Waskowsky WM, van Kuijk AA, Jacobs MM, Hopman MT, Thijssen DH et al (2021) Sedentary behaviour in cardiovascular disease patients: Risk group identification and the impact of cardiac rehabilitation. Int J Cardiol 326:194–201
Bires AM, Lawson D, Wasser TE, Raber-Baer D (2013) Comparison of Bruce treadmill exercise test protocols: is ramped Bruce equal or superior to standard bruce in producing clinically valid studies for patients presenting for evaluation of cardiac ischemia or arrhythmia with body mass index equal to or greater than 30? J Nucl Med Technol 41(4):274–8
Biswas A, Oh PI, Faulkner GE, Alter DA (2018) A prospective study examining the influence of cardiac rehabilitation on the sedentary time of highly sedentary, physically inactive patients. Ann Phys Rehabil Med 61:207–214
Bock BC, Carmona-Barros RE, Esler JL, Tilkemeier PL (2003) Program participation and physical activity maintenance after cardiac rehabilitation. Behav Modif 27:37–53
Buchheit M, Laursen PB (2013) High-intensity interval training, solutions to the programming puzzle: part I: cardiopulmonary emphasis. Sports Med 43(5):313–338
Candelaria D, Randall S, Ladak L et al (2020) Health-related quality of life and exercise-based cardiac rehabilitation in contemporary acute coronary syndrome patients: a systematic review and meta-analysis. Qual Life Res 29:579–592
Celano CM, Millstein RA, Bedoya CA, Healy BC, Roest AM, Huffman JC (2015) Association between anxiety and mortality in patients with coronary artery disease: a meta- analysis. Am Heart J 170:1105–1115
Cohen J (2013) Statistical power analysis for the behavioral sciences. Routledge
Cottin Y, Cambou JP, Casillas JM, Ferrières J, Cantet C, Danchin N (2004) Specific profile and referral bias of rehabilitated patients after an acute coronary syndrome. J Cardiopulm Rehabil Prev 24(1):38–44
Cuerda RC, Diego IM, Martın JJ et al (2012) Cardiac rehabilitation programs and health-related quality of life. State of the art. Rev Esp Cardiol 65:72–79. https://doi.org/10.1016/j.recesp.2011.07.016
Davidson T et al (2018) Cardiorespiratory fitness versus physical activity as predictors of all-cause mortality in men. Am Heart J 196:156–162
Di Angelantonio E, Bhupathiraju SN, Wormser D, Gao P, Kaptoge S, De Gonzalez AB et al (2016) Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 388:776–786
Diaz KM, Howard VJ, Hutto B, Colabianchi N, Vena JE, Safford MM et al (2017) Patterns of sedentary behavior and mortality in U.S. middle-aged and older adults a national cohort study. Ann Intern Med 167(7):465–75
Dolansky RN, Mary A et al (2010) Women’s and men’s exercise adherence after a cardiac event: does age make a difference? Res Gerontol Nurs 3(1):30–38
Dun Y, Thomas RJ, Medina-Inojosa JR et al (2019) High-intensity interval training in cardiac rehabilitation: impact on fat mass in patients with myocardial infarction. Mayo Clin Proc 94(9):1718–1730
Emdin CA, Odutayo A, Wong CX, Tran J, Hsiao AJ, Hunn BHM (2016) Meta-analysis of anxiety as a risk factor for cardiovascular disease. Am J Cardiol 118:511–519
Fallavollita L, Marsili B, Castelli S, Cucchi F, Santillo E, Marini L et al (2016) Short-term results of a 5-week comprehensive cardiac rehabilitation program after first-time myocardial infarction. J Sports Med Phys Fitness 56:311–318
Fletcher GF, Ades PA, Kligfield P, Arena R, Balady GJ, Bittner GV, Coke LA, Fleg JL, Forman DE, Gerber TC, Gulati M, Madan K, Rhodes J, Thompson PD, Williams MA (2013) Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation 128:873–934
Francis T, Kabboul N, Rac V et al (2019) The effect of cardiac rehabilitation on health-related quality of life in patients with coronary artery disease: a meta-analysis. Can J Cardiol 35:352–364
Gan Y, Gong Y, Tong X, Sun H, Cong Y, Dong X et al (2014) Depression and the risk of coronary heart disease: a meta-analysis of prospective cohort studies. BMC Psychiat 14:371
Gonçalves C, Raimundo A, Abreu A, Bravo J (2021) Exercise intensity in patients with cardiovascular diseases: systematic review with meta-analysis. Int J Environ Res Public Health 18:3574
Gonçalves C, Parraca JA, Bravo J, Abreu A, Pais J, Raimundo A, Clemente-Suárez VJ (2023a) Influence of Two Exercise Programs on Heart Rate Variability, Body Temperature, Central Nervous System Fatigue, and Cortical Arousal after a Heart Attack. Int J Environ Res Public Health 20:199
Gonçalves C, Bravo J, Pais J, Abreu A, Raimundo A (2023b) Improving health outcomes in coronary artery disease patients with short-term Protocols of high-intensity interval training and moderate-intensity continuous training: a community-based randomized controlled trial. Cardiovasc Ther 2023:6297302
Guiraud T, Granger R, Gremeaux V, Bousquet M, Richard L, Soukarie L, Babin T, Labrunee M, Bosquet L, Pathak A (2012) Accelerometer as a tool to assess sedentarity and adherence to physical activity recommendations after cardiac rehabilitation program. Ann Phys Rehabil Med 55(5):312–21
Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, Berman LB (1985) The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J 132(8):919–923
Hallal PC, Andersen LB, Bull FC et al (2012) Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet 380(9838):247–257
Hannan AL, Hing W, Simas V et al (2018) High-intensity interval training versus moderate-intensity continuous training within cardiac rehabilitation: a systematic review and meta-analysis. Open Access J Sports Med 9(1):1–17
Herrero MJ, Blanch J, Peri JM, De Pablo J, Pintor L, Bulbena A (2003) A validation study of the hospital anxiety and depression scale (HADS) in a Spanish population. Gen Hosp Psychiatry 25:277–283
Hurdus B, Munyombwe T, Dondo TB et al (2020) Association of cardiac rehabilitation and health-related quality of life following acute myocardial infarction. Heart 106:1726–1731
Ito S (2019) High-intensity interval training for health benefits and care of cardiac diseases - The key to an efficient exercise protocol. World J Cardiol 11(7):171–188
Jaureguizar KV, Vicente-Campos D, Bautista LR et al (2016) Effect of high-intensity interval versus continuous exercise training on functional capacity and quality of life in patients with coronary artery disease: A randomized clinical trial. J Cardiopulm Rehabil Prev 36:96–105
Kachur S, Menezes AR, De Schutter A, Milani RV, Lavie CJ (2016) Significance of comorbid psychological stress and depression on outcomes after cardiac rehabilitation. Am J Med 129:1316–1321
Kamiya K, Mezzani A, Hotta K, Shimizu R, Kamekawa D, Noda C, Yamaoka-Tojo M, Matsunaga A, Masuda T (2014) Quadriceps isometric strength as a predictor of exercise capacity in coronary artery disease patients. Eur J Prev Cardiol 21:1285–1291
Keteyian SJ, Leifer ES, Houston-Miller N, Kraus WE, Brawner CA, O’Connor CM, Whellan DJ, Cooper LS, Fleg JL, Kitzman DW, Cohen-Solal A, Blumenthal JA, Rendall DS, Piña IL; HF-ACTION Investigators (2012) Relation between volume of exercise and clinical outcomes in patients with heart failure. J Am Coll Cardiol 60(19):1899–905
Kida K, Osada N, Akashi YJ, Sekizuka H, Omiya K, Miyake F (2008) The exercise training effects of skeletal muscle strength and muscle volume to improve functional capacity in patients with myocardial infarction. Int J Cardiol 129(2):180–186
Korzeniowska-Kubacka I, Bilińska M, Piotrowska D, Stepnowska M, Piotrowicz R (2017) The impact of exercise-onlybased rehabilitation on depression and anxiety in patients after myocardial infarction. Eur J Cardiovasc Nurs 16(5):390–396. https://doi.org/10.1177/1474515116682123
LaMonte MJ, Lewis CE, Buchner DM, Evenson KR, Rillamas-Sun E, Di C et al (2017) Both light intensity and moderate-to-vigorous physical activity measured by accelerometry are favorably associated with cardiometabolic risk factors in older women: The Objective Physical Activity and Cardiovascular Health (OPACH) study. J Am Heart Assoc 6(10):e007064
Lavie CJ, Milani R (2004) Benefits of cardiac rehabilitation in the elderly. Chest 126:1010–1012
Lavie CJ, Menezes AR, De Schutter A, Milani RV, Blumenthal JA (2016) Impact of cardiac rehabilitation and exercise training on psychological risk factors and subsequent prognosis in patients with cardiovascular disease. Can J Cardiol 32:S365–S373
Liguori G, Medicine ACoS (2020) ACSM’s Guidelines for Exercise Testing and Prescription. Lippincott Williams & Wilkins
Lovlien M, Mundal L, Hall-Lord ML (2017) Health-related quality of life, sense of coherence and leisure-time physical activity in women after an acute myocardial infarction. J Clin Nurs 26975–982. https://doi.org/10.1111/jocn.13411
Maffiuletti NA, Jubeau M, Munzinger U, Bizzini M, Agosti F, De Col A, Lafortuna CL, Sartorio A (2007) Differences in quadriceps muscle strength and fatigue between lean and obese subjects. Eur J Appl Physiol 101:51–59
Mamataz T, Uddin J, Ibn Alam S, Taylor RS, Pakosh M, Grace SL (2021) Effects of cardiac rehabilitation in low-and middle- income countries: a systematic review and meta-analysis of randomised controlled trials. Prog Cardiovasc Dis 70:119–174
Martin BJ, Arena R, Haykowsky M et al (2013) Cardiovascular fitness and mortality after contemporary cardiac rehabilitation. Mayo Clin Proc 88(5):455–463
Marzolini S, Oh PI, Brooks D (2012) Effect of combined aerobic and resistance training versus aerobic training alone in individuals with coronary artery disease: a meta-analysis. Eur J Prev Cardiol 19:81–94
McDermott MM, Guralnik JM, Criqui MH, Liu K, Kibbe MR, Ferrucci L (2014) Six-minute walk is a better outcome measure than treadmill walking tests in therapeutic trials of patients with peripheral artery disease. Circulation 130(1):61–68
Milkman KL, Gromet D, Ho H et al (2021) Mega studies improve the impact of applied behavioural science. Nature 600:478–483
Pandey A, Parashar A, Kumbhani D et al (2015) Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail 8(1):33–40
Pattyn N, Coeckelberghs E, Buys R, Cornelissen VA, Vanhees L (2014) Aerobic interval training vs. moderate continuous training in coronary artery disease patients: A systematic review and meta-analysis. Sports Med 44:687–700
Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO III, Criqui M et al (2003) Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 107(3):499
Piepoli MF, Corrà U, Adamopoulos S et al (2014) Secondary prevention in the clinical management of patients with cardiovascular diseases. Core components, standards and outcome measures for referral and delivery: a policy statement from the cardiac rehabilitation section of the European Association for Cardiovascular Prevention & Rehabilitation. Endorsed by the Committee for Practice Guidelines of the European Society of Cardiology. Eur J Prev Cardiol 21:664–681
Piepoli MF, Hoes AW, Agewall S et al (2016) European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 37(29):2315–2381
Prince SA, Reid RD, Reed JL (2019) Comparison of self-reported and objectively measured levels of sitting and physical activity and associations with markers of health in cardiac rehabilitation patients. Eur J Prev Cardiol 26:653–656
Prince SA, Reed JL, Mark AE et al (2015) A comparison of accelerometer cut-points among individuals with coronary artery disease PloS One 10
Rey JB, Hawks M (2022) Prevention or delay of type 2 diabetes mellitus: recommendations from the American Diabetes Association. Am Fam Physician 105(4):438–439
Rognmo Ø, Hetland E, Helgerud J, Hoff J, Slørdahl SA (2004) High intensity aerobic interval exercise is superior to moderate intensity exercise for increasing aerobic capacity in patients with coronary artery disease. Eur J Cardiovasc Prev Rehabil 11:216–222
Rognmo O, Moholdt T, Bakken H et al (2012) Cardiovascular risk of high- versus moderate-intensity aerobic exercise in coronary heart disease patients. Circulation 126(12):1436–1440
Roth GA, Mensah GA, Johnson CO et al (2020) Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol 76:2982–3021
Sandercock G, Hurtado V, Cardoso F (2013) Changes in cardiorespiratory fitness in cardiac rehabilitation patients: a meta-analysis. Int J Cardiol 167(3):894–902
Sardinha LB, Santos DA, Silva AM, Coelho-e-Silva MJ et al (2012) Prevalence of overweight, obesity, and abdominal obesity in a representative sample of Portuguese adults. PLoS ONE 7(10):e47883
Scherr J, Wolfarth B, Christle J, Pressler A, Wagenpfeil S, Halle M (2013) Associations between Borg’s rating of perceived exertion and physiological measures of exercise intensity. Eur J Appl Physiol 113:147–155
Smith PJ, Sherwood A, Mabe S, Watkins L, Hinderliter A, Blumenthal JA (2017) Physical activity and psychosocial function following cardiac rehabilitation: one-year follow-up of the ENHANCED study. Gen Hosp Psychiatry 49:32–36
Stewart RAH, Held C, Hadziosmanovic N et al (2017) Physical activity and mortality in patients with stable coronary heart disease. J Am Coll Cardiol 70:1689–1700
Taylor JL, Holland DJ, Mielke GI et al (2020) Effect of high-intensity interval training on visceral and liver fat in cardiac rehabilitation: a randomized controlled trial. Obesity (silver Spring) 28(7):1245–1253
Trapp EG, Chisholm DJ, Freund J, Boutcher SH (2008) The effects of high-intensity intermittent exercise training on fat loss and fasting insulin levels of young women. Int J Obes (lond) 32(4):684–691
Turk-Adawi K, Supervia M, Lopez-Jimenez F, Pesah E, Ding R, Britto RR, Bjarnason-Wehrens B, Derman W, Abreu A, Babu AS et al (2019) Cardiac rehabilitation availability and density around the globe. eClinicalMedicine 13:31–45
Uddin J, Zwisler AD, Lewinter C et al (2016) Predictors of exercise capacity following exercise-based rehabilitation in patients with coronary heart disease and heart failure: a meta-regression analysis. Eur J Prev Cardiol 23(7):683–693
Valkeinen H, Aaltonen S, Kujala UM (2010) Effects of exercise training on oxygen uptake in coronary heart disease: a systematic review and meta-analysis. Scand J Med Sci Sports 20(4):545–555
Vasankari V, Halonen J, Vasankari T et al (2021) Physical activity and sedentary behaviour in secondary prevention of coronary artery disease: a review. Am J Prev Cardiol 5:100146
Vasankari V, Husu P, Vähä-Ypyä H, Suni JH, Tokola K, Borodulin K et al (2018) Subjects with cardiovascular disease or high disease risk are more sedentary and less active than their healthy peers. BMJ Open Sport Exer Med 4(1)
Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36) (1992) I. Conceptual framework and item selection. Med Care 30:473–483
Wennman H, Vasankari T, Borodulin K (2016) Where to sit? Type of sitting matters for the Framingham cardiovascular risk score. AIMS Public Health 3(3):577–591
Wilson PWF, Polonsky TS, Miedema MD et al (2019) Systematic review for the 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 139:e1144–e1161
Woodruffe S, Neubeck L, Clark RA et al (2015) Australian Cardiovascular Health and Rehabilitation Association (ACRA) core components of cardiovascular disease secondary prevention and cardiac rehabilitation 2014. Heart Lung Circ 24:430–441
Worcester MC, Hare DL, Oliver RG, Reid MA, Goble AJ (1993) Early programmes of high and low intensity exercise and quality of life after acute myocardial infarction. BMJ 307:1244–1247
Yamamoto S, Hotta K, Ota E, Mori R, Matsunaga A (2016) Effects of resistance training on muscle strength, exercise capacity, and mobility in middle-aged and elderly patients with coronary artery disease: a meta-analysis. J Cardiol 68(2):125–134
Zhang H, Tong TK, Qiu W et al (2017) Comparable effects of high-intensity interval training and prolonged continuous exercise training on abdominal visceral fat reduction in obese young women. J Diabetes Res 2017:5071740
Zheng X, Zheng Y, Ma J et al (2019) Effect of exercise-based cardiac rehabilitation on anxiety and depression in patients with myocardial infarction: a systematic review and meta-analysis. Heart Lung 48:1–7
Acknowledgements
This work was supported by the Fundação para a Ciência e a Tecnologia (Portugal) and by the Comprehensive Health Research Centre (CHRC). We thank all authors of the original works cited in the present study, who readily assisted us by sharing their manuscripts for this Randomized Clinical Trial.
Funding
Open access funding provided by FCT|FCCN (b-on). This work is funded by national funds through the Foundation for Science and Technology, under the project UIDB/04923/2020, and by grant number SFRH/BD/138326/2018.
Author information
Authors and Affiliations
Contributions
Conceptualization, C.G., J.B., J.P., and A.R.; methodology, C.G. and J.P.; validation, C.G. and J.P.; formal analysis, C.G., J.B., and A.R.; investigation, C.G.; resources, C.G., J.B., J.P. and A.R.; data curation, C.G., J.B., and A.R.; writing—original draft preparation, C.G.; writing—review and editing, C.G., J.B., and A.R.; visualization, C.G., J.B., A.A., and A.R.; supervision, C.G., J.B., J.P., and A.R.; project administration, C.G., J.B. and A.R.; funding acquisition, C.G., J.B., and A.R. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the University of Evora research ethics system (ethical approval number 17039). All work was conducted following the Declaration of Helsinki and registered at ClinicalTrials.gov (NCT03538119) on May 25, 2018.
Institutional review board statement
Not applicable.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Conflicts of interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gonçalves, C., Bravo, J., Abreu, A. et al. Comparing high-intensity versus moderate-intensity exercise training in coronary artery disease patients: a randomized controlled trial with 6- and 12-month follow-up. J Public Health (Berl.) (2024). https://doi.org/10.1007/s10389-024-02224-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10389-024-02224-z