Abstract
Aim
Musculoskeletal disorders are a major public health problem in most developed countries. As a main cause of chronic pain, they have resulted in an increasing prescription of opioids worldwide. With regard to the situation in Germany, this study aimed at estimating the prevalence of musculoskeletal diseases such as chronic low back pain (CLBP) and hip/knee osteoarthritis (OA) and at depicting the applied treatment patterns.
Subject and methods
German claims data from the InGef Research Database were analyzed over a 6-year period (2011–2016). The dataset contains over 4 million people, enrolled in German statutory health insurances. Inpatient and outpatient diagnoses were considered for case identification of hip/knee OA and CLBP. The World Health Organization (WHO) analgesic ladder was applied to categorize patients according to their pain management interventions. Information on demographics, comorbidities, and adjuvant medication was collected.
Results
In 2016, n = 2,693,481 individuals (50.5% female, 49.5% male) were assigned to the study population; 62.5% of them were aged 18–60 years. In 2016, n = 146,443 patients (5.4%) with CLBP and n = 307,256 patients (11.4%) with hip/knee OA were identified. Of those with pre-specified pain management interventions (CLBP: 66.3%; hip/knee OA: 65.1%), most patients received WHO I class drugs (CLBP: 73.6%; hip/knee OA: 68.7%) as the highest level.
Conclusion
This study provides indications that CLBP and hip/knee OA are common chronic pain conditions in Germany, which are often subjected to pharmacological pain management. Compared to non-opioid analgesic prescriptions of the WHO I class, the dispensation of WHO class II and III opioids was markedly lower, though present to a considerable extent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The majority of the chronic pain burden worldwide results from musculoskeletal disorders (Croft et al. 2010). Thus, musculoskeletal disorders are emerging to be a major public health problem in most developed countries. In the older population, the overall chronic musculoskeletal pain prevalence is estimated as high as 35.7% (Cimas et al. 2018). The most prevalent musculoskeletal diseases are low back pain and osteoarthritis (OA), which are major contributors to impairment in individuals over 50 years of age (Palazzo et al. 2014). The effects of these conditions on the individuals and the health care system are substantial, and include decreased health-related quality of life, reduced social participation, work absenteeism and significant direct and indirect health costs (Duenas et al. 2016; Bunzli et al. 2013: Langley et al. 2010; Juniper et al. 2009; Merx et al. 2007).
Worldwide, an increase in the use and misuse of opioids as well as associated deaths has been observed. The Global Burden of Disease Study showed a significant 22.3% increase in disability-adjusted life years (DALYs) due to opioid use between 2005 and 2015 (GBD 2015 DALYs and HALE collaborators 2015). For a long time, available data on opioid use in Germany were scarce, outdated, and showed very heterogeneous results (Kraus et al. 2019). In the meantime, the data situation has improved considerably. A recent review showed heterogeneous results but also points towards an increasing proportion of opioid users as well as an increasing amount of opioid use per patient (Rosner et al. 2019). At the same time, the authors concluded that there is no evidence of an opioid epidemic in Germany. Furthermore, another study points out that nearly all individuals who are addicted to opioids receive professional support (Kraus et al. 2019). So, although these studies do not point towards an opioid epidemic in Germany, pain management in routine care should be subject to real-world data studies.
Back and joint pain seem to be common reasons for opioid use in Germany (Just et al. 2019; Häuser et al. 2020). Since there is currently little evidence available with regard to the frequency and patterns of pain management/opioid use in patients with chronic low back pain (CLBP) and hip/knee OA, the aim of this study was to describe current routine pain treatment in these populations. Furthermore, we aimed at estimating the prevalence of CLBP and hip/knee OA and characterizing affected patients.
Patients and methods
Data source
This study was based on data from the Institute for Applied Health Research Berlin (InGef) Research Database. The InGef Research Database is an anonymized healthcare claims database with longitudinal data over a look-back period of up to 6 years. The data originate from approximately 6.7 million Germans insured in one of more than 60 out of the existing 103 German Statutory Health Insurance (SHI) funds currently contributing data to the database (mainly company or guild health insurance funds). Claims data are transferred directly from the healthcare providers to a specialized data centre owned and safeguarded by the SHIs themselves, which covers data warehousing and information technology services. In the data centre, all patient-level and provider-level data are anonymized with respect to the individual insurant, healthcare provider (e.g., physician, practice, hospital, and pharmacy) and each SHI, before entering the InGef Research Database, to comply with German data protection regulations and German federal law. For the purpose of this analysis, a sample of approximately 4 million individuals was drawn, which is considered representative of the German population with regard to age, sex, morbidity, mortality, and prescription drug use (Andersohn and Walker 2016). The database offers information on outpatient physician visits and drug dispensations as well as hospitalizations. The hospital data inform about diagnostic and therapeutic procedures with the exact date, the dates of admission and discharge, the reason for discharge, and diagnoses subdivided into hospital main discharge diagnoses and secondary diagnoses. The outpatient data contain information on diagnostic and therapeutic procedures including the date. Outpatient diagnoses are classified as confirmed diagnoses, suspected diagnoses, status post diagnoses, and diagnoses ruled out. All diagnoses are documented according to the German Modification of the International Classification of Diseases 10th Revision (ICD-10-GM). Data on outpatient prescriptions of reimbursed drugs do not make it possible to identify the reason for the medication, i.e., the indication for the drug prescription. However, this can be deduced with some probability via the coincidence of various characteristics: the presence of diagnoses from inpatient and outpatient care, documentation of medical procedures, and/or prescriptions for remedies and aids. On the other hand, the data comprise information on the pharmaceutical reference number, the date of prescription, and the prescription itself. For each dispensed drug, the packaging size, the strength and formulation of the drug, the defined daily dose (DDD), and the anatomical–therapeutic–chemical code (ATC code) can be attached based on a pharmaceutical reference database (Andersohn and Walker 2016).
Since the German SHI data cover such a large portion of the population (appr. 85%) and contain all reimbursement-related information on a wide variety of health care modalities, these health insurance data are considered to be highly valid when it comes to depicting medical care under real-world conditions (Swart et al. 2015).
Study design
The study was conducted in a retrospective, observational design. Data from 2011 until 2016 were covered in this analysis. The study was descriptive; thus, no hypotheses were pre-specified.
The use of specific health services was reported for different observation periods or points in time. First, the highest-level pain management intervention in 2016 was described. This indicator refers to the health service received data at a specific point in 2016 (index date). Second, the use of pain management interventions and adjuvant treatment options in the calendar year 2016 was described. This indicator refers to the calendar year 2016 as observation period. Third, patterns of pain management in the 5 years prior to the index date were described.
The study was conducted in accordance with legal and regulatory requirements, as well as with scientific purpose, value, and rigor. It also complies with the applicable reporting standards (Swart et al. 2016). The implementation follows generally accepted research practices described in guidelines for good pharmacoepidemiology practices (GPP) issued by the Inter-national Society for Pharmacoepidemiology (ISPE), good epidemiological practice (GEP) issued by the German Society for Epidemiology (DGEpi), and the Good Practice in Secondary Data Analysis (GPS) issued by the Working Group for the Survey and Utilization of Secondary Data (AGENS) of the German Society for Social Medicine and Prevention (DGSMP) and the German Society for Epidemiology (DGEpi). With reference to the ethical recommendations given in the latter guideline, “secondary data analyses must be conducted in accordance with ethical principles and respect human dignity as well as human rights.” It is, however, not mandatory to consult with an ethics committee, “if all the data protection provisions on pseudo-anonymization of all personal data are fulfilled … and no link to primary data is intended.” Since the study at hand respects all these conditions, a vote by an ethics committee has not been requested (Swart et al. 2015).
Inclusion and exclusion criteria of the study population
Patients were included in the study population if they had continuous insurance coverage in 2016 or continuous insurance coverage until the date of death in 2016. For assessment of patterns of pain management, a continuous insurance period between 2011 and 2015 was also required. Missing information on sex or year of birth as well as an age below 18 years led to an exclusion from the study. Analyses on patterns of pain management and characteristics were carried out in the subpopulations of patients fulfilling the identification criteria for hip/knee OA or CLBP.
Identification of patients
German ICD-10 diagnosis codes were used to identify patients with hip OA (M16.* unilateral and bilateral primary and secondary coxarthrosis; unilateral and bilateral coxarthrosis resulting from hip dysplasia; unilateral and bilateral post-traumatic coxarthrosis; unspecified coxarthrosis) and knee OA (M17.* unilateral and bilateral primary and secondary gonarthrosis; unilateral and bilateral post-traumatic gonarthrosis; unspecified gonarthrosis) as well as CLBP (M54.5 low back pain and M47.86 other spondylosis: lumbar region), with inpatient and outpatient diagnoses being considered. For confirmation of a chronic condition, patients were identified, if the inpatient diagnosis was the main discharge diagnosis in one quarter or if the outpatient diagnosis was documented in at least two consecutive quarters. The first quarter of 2017 was additionally considered for confirmation of an outpatient diagnosis in the last quarter of 2016.
Assignment to subpopulation with medication according to WHO analgesic ladder
Based on the highest level of the pain management interventions in 2016, identified patients were assigned to categories according to the World Health Organization (WHO) analgesic ladder, a scheme for the use of drugs in pain management (WHO 1996): beyond WHO scheme (at least one surgical or minimal invasive interventions or inpatient/day-clinic interdisciplinary multimodal pain therapy program of at least 7 days duration; for complete list, see supplementary Table S1), WHO III (strong opioids), WHO II (weak opioids), WHO I (non-opioid analgesics). Patients receiving no reimbursed pain management intervention were assigned to the category “no prescribed treatment”. The index date was defined as the last date of the pain management intervention in 2016 with the highest therapeutic intensity. Patients fulfilling the criteria for both the CLBP population and the hip/knee OA population were considered for both disease groups. Tables 1, 2, and 3 summarize frequency, distribution, and patient characteristics of pain management interventions in 2016.
Patient comorbidity
The 365 days preceding the index date in 2016 were used to determine comorbidities. Hospital main or second discharge diagnoses and confirmed outpatient diagnoses from office-based physicians were considered to describe the frequency of pre-specified diseases in patients with hip/knee OA and CLBP (operationalization, see supplementary Table S2). One documented diagnosis was sufficient to be identified as having the respective comorbidity.
Drug use
Use of specific medications/comedications was determined based on the calendar year 2016 and during the 5-year period preceding the index date, separately. At least one dispensation of the respective drug or drug class was sufficient to meet the requirements for the presence of a medication/comedication. In addition, the sum of dispensed DDD during the 5-year period preceding the index date was obtained for WHO I, WHO II and WHO III class drugs.
Use of adjuvant interventions
Use of adjuvant interventions was determined based on the calendar year 2016. Adjuvant interventions were pre-specified (for definition, see supplementary Table S3). At least one intervention was sufficient to meet the requirement for the presence of an adjuvant intervention.
Statistical analyses
Only descriptive statistical analyses were conducted. All indicators were analyzed for each treated subpopulation (WHO I, WHO II, WHO III, and beyond WHO) separately. Absolute and relative frequencies were calculated and tabulated for the drug prescriptions (Tables 4 and 5). In the text of the results chapter, the ranks of the relative frequencies are presented. For prescription quantities, mean values of defined daily doses (DDD) were reported (supplementary Table S4 and Table S5). For the remaining cardinally scaled data, mean and standard deviation were calculated. Data management and data analyses were performed with SAS Enterprise Guide 4.3.
Results
Population sizes
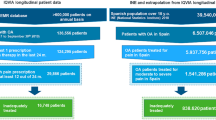
The total number of insured persons in the InGef Research Database in 2016 was n = 4,347,960. Of these, continuously observable in 2016 (or until their death in the same year) were n = 3,899,618 insured persons. Of these, at least 18 years old were n = 3,330,699. Of these, again continuously observable over the 6-year study period (2011–2016) were n = 2,693,481 individuals were included in the study population in 2016. Of those, 50.5% (n = 1,353,886) were female and 49.5% (n = 1,334,595) were male. Patients aged 18–60 years accounted for 62.5% of the study population. Overall, patients’ age ranged from 18 to over 90 years.
Within the study population, 146,443 individuals (5.4%) met the criteria for CLBP in the calendar year 2016, which was considered as the administrative CLBP treatment prevalence within the study population. The age-specific prevalence of CLBP for both sexes in the calendar year 2016 is displayed in Fig. 1. In the CLBP population, 55.1% and 44.9% of patients suffering from CLBP were female and male respectively. The simultaneous presence of hip/knee OA was observed in 39.1% of CLBP patients.
In 2016, 307,256 patients (11.4%) with hip/knee OA were identified. The age-specific prevalence of hip/knee OA for both sexes is shown in Fig. 2. In the hip/knee OA population, 55.9% of the patients were female and 44.1% male. Of those included in the hip/knee OA population, 19.1% also had CLBP in 2016.
Highest level of pain management intervention in 2016
In the CLBP population, 97,122 (66.3%) received at least one of the pre-specified pain management interventions according to the WHO scheme or beyond the WHO scheme in the calendar year 2016, while there were 199,969 (65.1%) in the hip/knee OA population. Of those treated, the majority (CLBP: 73.6%; hip/knee OA: 68.7%) received WHO I drugs as the highest-level intervention at index date, followed by WHO II drugs (CLBP: 16.1%; hip/knee OA: 13.3%). While WHO III drugs accounted for 7.8% in both populations, beyond WHO interventions were less common in CLBP patients (2.5%) than in hip/knee OA patients (10.1%) (Table 1).
In CLBP patients, inpatient/day-clinic interdisciplinary multimodal pain therapy programs of at least 7 days duration were by far the most frequent beyond WHO intervention (45.3%) followed by spinal fusion (27.2%), electrothermal procedures (20.1%) and facet denervations (10.2%). In hip/knee OA patients, joint replacement accounted for the majority of beyond WHO interventions (80.3%), whereas surgical interventions on the meniscus or connective tissue were the second-most frequent (9.8%) intervention. Other pre-specified interventions were performed less frequently in hip/knee OA patients at index date in 2016 (inpatient/day-clinic interdisciplinary multimodal pain therapy programs of at least 7 days duration: 5.0%, radiosynoviorthesis: 4.0%, lavage: 1.7%, osteotomy: 0.9%, arthrodesis: 0.1%).Footnote 1
Characteristics of treated patients
Women were overrepresented in each pain management intervention subgroup in both pain populations, especially in the WHO III drug subgroup. Furthermore, patients with hip/knee OA had a higher mean age than CLBP patients. In patients with hip/knee OA and in patients with CLBP, higher levels of pain management interventions seem to be associated with a higher mean age. This holds true for every pain management intervention subgroup, except for the beyond WHO intervention group (Tables 2 and 3).
The analysis of pre-specified comorbidities revealed heterogeneous results with regard to differences between the four pain management intervention subgroups (Tables 2 and 3). In general, prevalence of pre-specified comorbidities increased with higher levels of pain management interventions. Most frequent comorbid conditions were arterial hypertension, diabetes mellitus, and depression in both patients with hip/knee OA and CLBP. However, the beyond WHO intervention subgroup often had a lower comorbidity burden, except for comorbidities like depression, anxiety, and insomnia in the CLBP population. Comorbidity burden in the beyond WHO intervention subgroup was more comparable to that of WHO I subgroup patients (in CLBP patients) or WHO II subgroup patients (in hip/knee OA patients).
Use of drugs
In both patient groups, the prevalence of opioid use is approximately one quarter of patients (CLBP: 25.8%; hip/knee OA: 24.8%). Non-steroidal anti-inflammatory drugs (NSAIDs) were prescribed most often in the WHO I intervention subgroups (CLBP: 83.3%; hip/knee OA: 80.8%) and the least in the WHO III intervention subgroups (CLBP: 56.2%; hip/knee OA: 48.1%). The majority of the NSAID dispensations consisted of ibuprofen and diclofenac (supplementary Table S6). The most prescribed non-opioid analgesic outside the NSAID class was metamizole, prescription prevalence varied from 39.7% (WHO I subgroup) to 65.7% (beyond WHO subgroup) in CLBP patients and 40.1% (WHO I subgroup) to 67.6% (WHO III subgroup) in hip/knee OA patients respectively (Table S6 and Table S7). Beside the WHO II intervention subgroups (100% by definition), weak opioids were prescribed to 31.0% of the WHO III, to 42.9% of the beyond WHO intervention subgroups of the CLBP patients, and to 27.2% of the WHO III and 24.8% of the beyond WHO intervention subgroups of the hip/knee OA patients in 2016. The most often prescribed weak opioid was tilidine plus naloxone, followed by tramadol. Beside the WHO III intervention groups (100% by definition), strong opioids were prescribed to 35.8% (CLBP) and 11.6% (hip/knee OA) of the beyond WHO intervention subgroups. The most prescribed strong opioid in the WHO III and beyond WHO intervention subgroups of the CLBP patients was oxycodone. The most prescribed strong opioid in the WHO III subgroup of the hip/knee OA patients was fentanyl (27.6%) and in the beyond WHO intervention subgroup oxycodone (4.1%).
When analyzing the use of comedication across the pain management intervention subgroups in 2016, proton pump inhibitors were by far the most often used drug class in both CLBP and hip/knee OA patients. Depending on the pain management intervention subgroup, dispensations of proton pump inhibitors varied from 43.4% (WHO I subgroup) to 73.0% (WHO III subgroup) of the CLBP patients and from 44.9% (WHO I subgroup) to 73.3% (WHO III subgroup) of hip/knee OA patients. Antidepressants and antithrombotics were also used comparatively often in all four pain management intervention subgroups in CLBP (range for antidepressants: 16.7%–42.7%; range for antithrombotics: 18.8%-41.5%) and hip/knee OA patients (range for antidepressants: 15.3%–39.4%; range for antithrombotics: 25.5%–57.9%). In general, the WHO III intervention subgroup showed the highest prescription prevalence of the pre-specified non-pain medications followed by the beyond WHO pain management intervention subgroup in the CLBP population and followed by WHO II group patients in the hip/knee OA population (Tables 4 and 5).
For a detailed overview of medication use at ATC-7-digit level of both pain populations, see supplementary Table S6 and Table S7.
Use of adjuvant treatment options
In 2016, physiotherapy was the most often-used adjuvant treatment in all four pain management intervention subgroups in CLBP and hip/knee OA patients. Depending on pain management intervention subgroups, 52.9% and 43.7% (WHO I subgroups) up to 75.2% and 82.2% (beyond WHO intervention subgroups) of the CLBP and hip/knee OA patients received physiotherapy in 2016 respectively. The use of acupuncture differed slightly between all four pain management intervention subgroups in both CLBP and hip/knee OA patients. In CLBP, transcutaneous electronic nerve stimulations (TENS) and therapeutic injections were more frequently used in patients with a higher-level pain management intervention. In the hip/knee OA population, all other adjuvant therapies were only observed for a small proportion of patients (range for TENS: 2.6%–3.8%, range for therapeutic injections: 0.2%–1.6%).
Patterns of pain management in the 5 years prior to the index date
Considering pain treatments over the 5-year period prior to the index date in 2016, pain management patterns were very heterogeneous. Nevertheless, almost all CLBP and hip/knee OA patients had prescriptions of WHO I drugs during that period (supplementary Table S4 and Table S5). Furthermore, 34.1% of all treated CLBP patients had been prescribed WHO II class drugs, and 10.1% WHO III drugs. Similar trends were seen with the hip/knee OA patients. Hence, patients who received only WHO II or WHO III class drugs were rarely observed and accounted for less than 2 % (data not shown). At the same time, pain treatments were infrequently conducted strictly according to the WHO analgesic ladder escalation scheme (data not shown). For example, only 4.2% of treated hip/knee OA patients and 4.7% of CLBP patients who received WHO III drugs as the highest-level intervention in 2016 have received analgesics (WHO I and WHO II class drugs; WHO I and WHO II and WHO III class drugs) according to the stepwise escalation scheme in the previous 5 years (data not shown). However, most of the WHO III intervention subgroup members (CLBP: 60.2%; hip/knee OA: 57.2%) received the respective drugs in the 5 years before the index date, but not according to the stepwise escalation scheme. This pattern was not followed in the beyond WHO intervention subgroups, where only 26.3% (CLBP) and 8.8% (hip/knee OA) of the patients were prescribed all drugs of the escalation scheme, but in the “wrong” order. A stepwise escalation of analgesic drugs according to the WHO scheme was only observed in 1.1% (CLBP) and 0.2% (hip/knee OA) of all treated patients.
In addition, it was observed that those patients who received WHO III drugs as the highest-level intervention in 2016 received a higher amount (mean DDD) of WHO III class drugs during the 5-year period (CLBP: mean DDD = 644.1; hip/knee OA: mean DDD = 660.8) compared to the amount of WHO II class drugs received by patients who received WHO II drugs as the highest-level intervention in 2016 (CLBP: mean DDD = 442.4; hip/knee OA: mean DDD = 496.3) (supplementary Table S4 and Table S5). The amount of WHO I drug class use during the 5-year period was high in every pain management intervention subgroup, and was approximately a quarter higher than the amount of WHO II drug class use in the WHO II pain management intervention subgroup and WHO III drug class use in the WHO III pain management intervention subgroup.
Discussion
Opioids attain their importance due to the fact that they are among the most effective analgesic drug classes in pharmacotherapy. As a consequence of their therapeutic potency, the amount of opioid prescriptions as well as the number of people receiving opioid treatment have increased during the last decades (OECD 2019). The rapid growth of the opioid consumption goes along with circumscribed changes in prescription patterns, pointing towards stronger opioids. Increasing opioid prescription in CLBP and hip/knee OA has contributed to the opioid epidemic in the United States (Lee et al. 2019). In Europe, Germany has become the second largest market for opioid pain relievers behind the United Kingdom and ahead of Spain (Hider-Mlynarz et al. 2018). This development is also attributed to the increasing prevalence of musculoskeletal diseases such as CLBP and hip/knee OA. Despite the risk of misuse, abuse, overdose, and addiction, a large-scale evaluation of epidemiologic data for opioid analgesics was still lacking among patients with CLBP and hip/knee OA. This study aimed to investigate the pain management interventions in a large cohort of patients with CLBP and knee/hip OA using a representative German claims dataset.
In our study, a 1-year prevalence of 11.4% and 5.4% for patients suffering from hip/knee OA and CLBP respectively was observed. Based on extrapolation of our data, estimates of the prevalent population in Germany were 6.9 million patients with knee/hip OA and 3.5 million with CLBP. Pre-specified pain management interventions were observed in two-thirds of the observed population, with WHO I drugs being the most frequently used drugs in CLBP patients (73.6%) and hip/knee OA patients (68.7%). In contrast to this, every fourth CLBP patient (23.9%) and every fifth OA patient (21.1%) received some opioids of WHO class II or III over the study period.
The prevalence estimates for hip/knee OA observed in our study were in good accordance with those observed in another German claims data-based study. In this study from 2018 (Postler et al. 2018), the hip/knee OA burden based on data of one large health insurance company (BARMER) was analyzed, with a restriction to patients aged 60 years or older. A prevalence of 17.5% in the age group 60–69 years (15.3% in our study) was revealed, which increased to 31.0% in the age group 80–89 years (31.4% in our study). The prevalence estimates for hip/knee OA obtained from our study may therefore be judged similar as in other German claims data-based studies. However, our results on the prevalence of CLBP are substantially lower than those of another study (Kuntz et al. 2017), which reports a 1-year prevalence of CLBP of 17.1% in men (4.9% in our study) and 24.4% in women (5.9% in our study). The large difference between the two studies may be explained by two factors. Firstly, different definitions of the back pain location (comparable study: back pain; our study: inpatient/outpatient diagnoses for lower back condition) and, secondly, by the fact that the mentioned study (Kuntz et al. 2017) used data obtained from a large survey, based on computer-assisted telephone interviews of patients. Such interviews allow the identification of conditions reported by the patients, although they may have not been diagnosed by a physician yet. Hence, they would not appear in claims data yet, as used in our study.
The difference in prevalence estimates for CLBP between our study and other studies may also have an impact on the extent of pain management attributed to this condition — in the way that, if there is a corresponding number of unreported cases, there may also be a corresponding amount of unreported analgesic drug consumption, which could lead to an underestimation of applied pain management strategies overall.
When analyzing the characteristics of the four pain management intervention subgroups, we observed that the mean age and the comorbidity burden increased in higher-level pain management intervention subgroups. However, patients receiving a beyond WHO intervention had a comparably low comorbidity burden. Indeed, beyond WHO intervention patients seemed to be more similar to the WHO I pain management intervention subgroup (in CLBP patients) or the WHO II pain management intervention subgroup (in hip/knee OA patients). The beyond WHO pain management intervention subgroup also had a lower mean age than the WHO II and WHO III subgroups, as shown in Tables 2 and 3. This might point towards the assumption that such interventions (e.g., surgical or minimal invasive interventions, inpatient/day-clinic interdisciplinary multimodal pain therapy programs of at least 7 days duration) may be more frequently offered to patients of the WHO I or WHO II intervention subgroups. However, 35.8% (CLBP) and 11.6% (hip/knee OA) of the beyond WHO intervention subgroup received WHO III medication in the year 2016 anyway.
In accordance with the previously mentioned study conducted by Postler and colleagues in 2018 (Postler et al. 2018), the most frequent comorbidities were atrial hypertension, diabetes mellitus and depression in the treated hip/knee OA patient population as well as in the CLBP group. Differences regarding the occurrence of comorbid conditions such as hypertension, diabetes mellitus, cancer, and ischemic heart disease between the four pain management intervention subgroups may be explained by the differences in age, since most of those diseases are age-associated and the mean age increased in higher pain management intervention subgroups (Jaul and Barron 2017). This may also explain the comparatively low comorbidity burden in patients undergoing beyond WHO interventions. However, a different trend was observed for anxiety, insomnia, and depression in CLBP patients. The rates for these comorbidities increased in the beyond WHO intervention subgroup. This differing trend might be plausible, since anxiety and depression are known to be common comorbid conditions in CLBP patients and risk factors (“yellow flags”) for the chronification of pain (BÄK et al. 2017). Due to their psychological strain, patients with these comorbidities might be enriched in the highest-level intervention subgroup, which includes intensified therapy regimes such as inpatient or day-clinic interdisciplinary multimodal pain therapy programs of at least 7 days duration (45.3%).
The results on comedication may reflect already discussed differences between the four pain management intervention subgroups due to the different mean ages (e.g., ischemic heart diseases/antithrombotics) and risk factors (e.g., depression/antidepressants). Use of laxatives and antiemetics might have been higher in the WHO III intervention subgroups, as constipation and nausea are common side-effects of opioids (Benyamin et al. 2008).
The principle of the WHO analgesic ladder is the incremental escalation of pain medication by adjusting it to the patients’ pain level (Ballantyne et al. 2016). Our results suggest a general orientation of treatment regimen in German routine care towards this principle by showing a higher frequency of WHO I drugs, then WHO II and WHO III drugs as the highest-level pain management interventions in both, treated patients with CLBP and hip/knee OA in the year 2016. With back pain and osteoarthritis being one of the most common reasons for opioid prescription (Werber et al. 2015), we found that within those treated every fifth patient received weak opioids and every tenth patient received strong opioids during 2016 (cf. Tables 4 and 5: weak opioids: 19.6% in CLBP, 18,0% in hip/knee OA; strong opioids: 8.7% in CLBP, 9.0% in hip/knee OA). Also, every third patient had been prescribed weak opioids and every tenth patient received strong opioids in the previous 5-year period. This trend is in line with German drug sales reports of 2015, indicating higher prescription rates for weak than for strong opioids across all indications (Hider-Mlynarz et al. 2018). Compared to the proportion of patients prescribed with NSAIDs, which come with significant risks and limitations as well (Cooper et al. 2019), the proportion of patients receiving opioids could be considered of smaller amount, though markedly present. Summarizing our findings, in comparison to current literature (Hider-Mlynarz et al. 2018; Postler et al. 2018; Häuser et al. 2017), we can conclude that the patterns of opioid prescription in Germany follow similar trends as in other developed countries.
Although strong opioids were used in a smaller proportion of patients with CLBP and hip/knee OA compared to weak opioids, we observed a higher amount of dispensed strong opioids in these patients. The higher amount of dispensed strong opioids could point towards an either more frequent, more intense, or longer therapy compared to weak opioids. Drugs of the WHO I class were used by a large proportion of patients and in comparatively large amounts suggesting that, irrespective of the usage of weak or strong opioids, drugs of the WHO I class are an essential part of the pain therapy in the observed patient populations.
Strengths and limitations
The primary strength of the present study is the large, unselected, and nationwide sample, leading to accurate estimates of the population sizes, characteristics, and drug use of patients suffering from CLBP or hip/knee OA. Furthermore, methodological challenges in health services research such as recall and selection bias could be mitigated by conducting the study on claims data.
The InGef Research Database originates from approximately 6.7 million Germans insured in one of more than 60 out of the existing 103 German Statutory Health Insurance (SHI) funds currently contributing data to the database (mainly company or guild health insurance funds). It is known that socio-demographic information as well as information on morbidity varies between the different types of health insurance companies in Germany (Hoffmann and Icks 2012). Thus, if these differences between health insurance companies are correlated with indicators assessed in this study and if these factors are systematically distorted in the analytic sample (e.g., over-representation of persons with higher socio-economic status), biased estimates may be the result. However, recent publications (Andersohn and Walker 2016) point towards a high external validity of the InGef Health Research Database in terms of measures of morbidity, mortality and drug usage. Thus, biased results are highly unlikely.
Potential limitations lie in the fact that German claims data do not make it possible to obtain information on the reason for drug prescriptions (i.e., the indication). Thus, the use of analgesic drugs observed in a cohort of patients with CLBP and hip/knee OA might also have been due to other indications. The overall picture of therapeutic care, however, is composed of various characteristics: the presence of diagnoses from inpatient and outpatient care, documentation of medical procedures, and/or prescriptions for remedies and aids, so there is some likelihood that analgesic drug use is attributable to CLBP or OA.
Due to the challenge of combining data from various origins and uniting them in the routine database of the statutory health insurance companies, there is, on the one hand, a delay of at least 9 months in Germany until the data are available at all. Until the approval for data use has been obtained and the claims data have been converted into an operational data set that enables scientific analyses, further months will be allocated, which — including all the plausibility and validation steps during the data analyses — may also become years. Such secondary data analyses may therefore, as in the present case, include observational data that, at first sight, seem to be “outdated”,
Another potential limitation results from the billing context of the data: since intra-articular injections are not reimbursed separately in the German healthcare system, the utilization of corticosteroid and hyaluronic acid injections may be underestimated by this study.
Several WHO I drugs are classified as over the counter (OTC) drugs in Germany. Although these drugs can be reimbursed by SHIs, costs for the patient might be higher compared to buying the respective drug as an OTC drug. This is due to a sometimes-higher co-payment for a reimbursed drug compared to the OTC price. Therefore, underestimation of WHO I class drugs, particularly when used as monotherapy (not as adjuvant therapy with WHO II class drugs), is likely, and must be considered when interpreting the results.
Conclusion
Based on a large and representative sample, this study provides indications that CLBP and hip/knee OA are frequent chronic pain conditions in Germany, which are associated with a high comorbidity burden. Analgesic drugs are an essential treatment component for patients with CLBP and hip/knee OA. In this study, which examined the routine care of patients with such diseases in Germany using a statutory health insurance sample, WHO I drugs (non-opioid analgesics) were prescribed most often and in combination with all pharmacological and non-pharmacological treatment options. Germany is the second highest prescribing country of the G4 (France, Italy, United Kingdom, Germany) of opioid analgesics (Hider-Mlynarz et al. 2018). Compared to non-opioid analgesic prescriptions of the WHO I class for CLBP (73.6%) and hip/knee OA (68.7%), the dispensation of WHO class II and III opioids was markedly lower (23.9% and 21.1% respectively), though present to a considerable extent.
Notes
The numbers add up to > 100%, since few patients may have received more than one beyond WHO intervention on the index date.
References
Andersohn F, Walker J (2016) Characteristics and external validity of the German Health Risk Institute (HRI) database. Pharmacoepidemiol Drug Saf 25(1):106–109
Ballantyne JC, Kalso E, Stannard C (2016) WHO analgesic ladder: a good concept gone astray. BMJ 352:i20
Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R (2008) opioid complications and side effects. Pain Physician 11(2 Suppl):S105–S120
Bundesärztekammer (BÄK), Kassenärztliche Bundesvereinigung (KBV), Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF) (2017) Nationale VersorgungsLeitlinie nicht-spezifischer Kreuzschmerz — Langfassung, 2nd edn, version 1. BÄK, KBV, AWMF, Berlin
Bunzli S, Watkins R, Smith A, Schutze R, O'Sullivan P (2013) Lives on hold: a qualitative synthesis exploring the experience of chronic low-back pain. Clin J Pain 29(10):907–916
Cimas M, Ayala A, Sanz B, Agullo-Tomas MS, Escobar A, Forjaz MJ (2018) Chronic musculoskeletal pain in European older adults: cross-national and gender differences. Eur J Pain 22(2):333–345
Cooper C, Chapurlat R, Al-Daghri N, Herrero-Beaumont G, Bruyere O, Rannou F, Roth R, Uebelhart D, Reginster JY (2019) Safety of oral non-selective non-steroidal anti-inflammatory drugs in osteoarthritis: what does the literature say? Drugs Aging 36(Suppl 1):15–24
Croft P, Blyth F, van der Windt D (2010) The global occurrence of chronic pain: an introduction. In: Croft P, Blyth F, van der Windt D (eds) Chronic pain epidemiology from aetiology to public health. Oxford University Press, Oxford, pp 9–18
Duenas M, Ojeda B, Salazar A, Mico JA, Failde I (2016) A review of chronic pain impact on patients, their social environment and the health care system. J Pain Res 9:457–467
GBD 2015 DALYs and HALE collaborators (2015) Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet 388(10053):1603–1658
Häuser W, Schug S, Furlan AD (2017) The opioid epidemic and national guidelines for opioid therapy for chronic noncancer pain: a perspective from different continents. Pain Rep 2(3):e599
Häuser W, Bock F, Hüppe M, Nothacker M, Norda H, Radbruch L, Schiltenwolf M, Schuler M, Tölle T, Viniol A, Petzke F; Koautoren für die Konsensusgruppe der 2. Aktualisierung der S3-Leitlinie LONTS (2020) Recommendations of the second update of the LONTS guidelines — long-term opioid therapy for chronic noncancer pain. Schmerz 34:204–244 [in German]
Hider-Mlynarz K, Cavalie P, Maison P (2018) Trends in analgesic consumption in France over the last 10 years and comparison of patterns across Europe. Br J Clin Pharmacol 84(6):1324–1334
Hoffmann F, Icks A (2012) Structural differences between health insurance funds and their impact on health services research: results from the Bertelsmann Health-Care Monitor. Gesundheitswesen 74(5):291–297 [in German]
Jaul E, Barron J (2017) Age-related diseases and clinical and public health implications for the 85 years old and over population. Front Public Health 5:335
Juniper M, Le TK, Mladsi D (2009) The epidemiology, economic burden, and pharmacological treatment of chronic low back pain in France, Germany, Italy, Spain and the UK: a literature-based review. Expert Opin Pharmacother 10(16):2581–2592
Just JM, Schwerbrock F, Bleckwenn M, Schnakenberg R, Weckbecker K (2019) Opioid use disorder in chronic non-cancer pain in Germany: a cross sectional study. BMJ Open 9(4):e026871
Kraus L, Seitz NN, Schulte B, Cremer-Schaeffer P, Braun B, Verthein U, Pfeiffer-Gerschel T (2019) Estimation of the number of people with opioid addiction in Germany. Dtsch Arztebl Int 116(9):137–143
Kuntz B, Hoebel J, Fuchs J, Neuhauser H, Lampert T (2017) Soziale Ungleichheit und chronische Rückenschmerzen bei Erwachsenen in Deutschland. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 60:783–791
Langley P, Muller-Schwefe G, Nicolaou A, Liedgens H, Pergolizzi J, Varrassi G (2010) The societal impact of pain in the European Union: health-related quality of life and healthcare resource utilization. J Med Econ 13(3):571–581
Lee SW, Patel J, Kim SY, Miranda-Comas G, Herrera J, Bartels MN (2019) Use of opioid analgesics in patients with chronic low back pain and knee osteoarthritis. Am J Phys Med Rehabil 98(8):e97–e98
Merx H, Dreinhöfer KE, Gunther KP (2007) Socioeconomic relevance of osteoarthritis in Germany. Z Orthop Unfall 145(4):421–429 [in German]
OECD (2019) Addressing problematic opioid use in OECD countries. OECD, Paris
Palazzo C, Ravaud JF, Papelard A, Ravaud P, Poiraudeau S (2014) The burden of musculoskeletal conditions. PLoS One 9(3):e90633
Postler A, Ramos AL, Goronzy J, Gunther KP, Lange T, Schmitt J, Zink A, Hoffmann F (2018) Prevalence and treatment of hip and knee osteoarthritis in people aged 60 years or older in Germany: an analysis based on health insurance claims data. Clin Interv Aging 13:2339–2349
Rosner B, Neicun J, Yang JC, Roman-Urrestarazu A (2019) Opioid prescription patterns in Germany and the global opioid epidemic: systematic review of available evidence. PLoS One 14(8):e0221153
Swart E, Gothe H, Geyer S, Jaunzeme J, Maier B, Grobe TG, Ihle P; On behalf of the German Society for Social Medicine and Prevention (DGSMP) and the German Society for Epidemiology (DGEpi) (2015) Good practice of secondary data analysis (GPS): guidelines and recommendations. Gesundheitswesen 77:120-126 (English version available at https://www.dgepi.de/assets/Leitlinien-und-Empfehlungen/Practice-in-Secondary-Data-Analysis.pdf)
Swart E, Bitzer EM, Gothe H, Harling M, Hoffmann F, Horenkamp-Sonntag D, Maier B, March S, Petzold T, Röhrig R, Rommel A, Schink T, Wagner C, Wobbe S, Schmitt J (2016) A consensus German reporting standard for secondary data analyses, version 2 (STROSA-STandardisierte BerichtsROutine für SekundärdatenAnalysen). Gesundheitswesen 78(S 01):e145–e160
Werber A, Marschall U, L'Hoest H, Hauser W, Moradi B, Schiltenwolf M (2015) Opioid therapy in the treatment of chronic pain conditions in Germany. Pain Physician 18(3):E323–E331
World Health Organization (1996) Cancer pain relief: with a guide to opioid availability, 2nd edn. World Health Organization, Geneva
Acknowledgements
We thank Katharina Wiebe for assistance with designing the study. We thank Valeria Weber and Denise Tschechowski for the critical review of the manuscript. We thank Ariane Höer for providing her pharmacological expertise.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded by Pfizer Pharma GmbH.
Author information
Authors and Affiliations
Contributions
CO and HG were involved in the design of the study, the analysis and interpretation of data, and in writing the manuscript. EH and DK were involved in the design of the study, in the interpretation of data, and in writing the manuscript. TJ and MS were involved in the interpretation of data, and in writing the manuscript. CB was involved in writing the manuscript. WG and NS were involved in the analysis of the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical statement
In accordance with the Good Practice in Secondary Data Analysis (GPS), “Guideline 1: Ethics” (English version available at https://www.dgepi.de/assets/Leitlinien-und-Empfehlungen/Practice-in-Secondary-Data-Analysis.pdf) “secondary data analyses must be conducted in accordance with ethical principles and respect human dignity as well as human rights.” According to this guideline, it is not mandatory to consult with an ethics committee “if all the data protection provisions on de-identification of all personal data are fulfilled … and no link to primary data is intended” (Swart et al. 2015). All patient-level data in the InGef research database are de-identified to comply with German data protection regulations. Use of the study database for health services research is therefore fully compliant with German federal law and, accordingly, institutional review board/ethical approval and informed consent of the patient have not been requested.
Conflict of interest
This study was funded by Pfizer Pharma GmbH. The authors had complete autonomy for the process of establishing the protocol, carrying out the analyses and interpreting the results. This also includes the full right to publish the results without limitation.
EH is a paid employee of Pfizer. MS was a paid employee of Pfizer at the time the study was conducted.
HG is a senior research affiliate of both the Medical Faculty of TU Dresden and UMIT – Private University for Health Sciences, Medical Informatics and Technology. He is also a paid employee of the vendor IGES GmbH. CO and CB were paid employees of IGES GmbH at the time the study was conducted. IGES GmbH is a paid consultant to Pfizer and Eli Lilly for data analysis and development of the manuscript.
WG and NS were paid employees of the vendor InGef, at the time the study was conducted. InGef was contracted by the IGES GmbH. The costs for data analysis carried out by InGef were included in the costs paid to vendor IGES GmbH by Pfizer.
DK and TJ received honoraria from Pfizer related to their advisory services during the study protocol development and data analysis. DK and TJ did not receive honoraria from Pfizer related to the development of this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hradetzky, E., Ohlmeier, C., Brinkmann, C. et al. Epidemiology and routine care treatment of patients with hip or knee osteoarthritis and chronic lower back pain: real-world evidence from Germany. J Public Health (Berl.) 30, 2855–2867 (2022). https://doi.org/10.1007/s10389-022-01700-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10389-022-01700-8