Abstract
Background
Salvage concurrent chemoradiotherapy is effective against locoregional recurrence after curative resection of esophageal squamous cell carcinoma. However, there is no consensus on its application. We investigated the outcomes of salvage concurrent chemoradiotherapy (60 Gy in 30 fractions) with three-dimensional conformal radiotherapy and 5-fluorouracil/platinum-based chemotherapy.
Methods
We retrospectively investigated the outcomes and prognostic factors in 51 patients with esophageal squamous cell carcinoma treated with salvage concurrent chemoradiotherapy.
Results
The median follow-up was 17.5 (range, 2.8–116.1) months. The overall response, complete response, and partial response rates were 74.5%, 49.0%, and 25.5%, respectively. The median progression-free survival was 8.2 months; the 3-year progression-free survival rate was 22.9%. The median overall survival was 23.1 months; the 3-year overall survival rate was 40.7%. Overall survival was significantly longer in patients with a complete response than in those without (median overall survival: not reached vs. 15.3 months); 3-year overall survival rate: 62.5% vs. 20.3% (hazard ratio: 0.222; P < 0.001). Multivariate analysis showed that the independent prognostic factor for overall survival was < 25 mm longest diameter of metastatic lymph nodes (hazard ratio: 3.71).
Conclusions
Salvage concurrent chemoradiotherapy (60 Gy in 30 fractions) with three-dimensional conformal radiotherapy and 5-fluorouracil/platinum-based chemotherapy was an effective and safe treatment for locoregional recurrence after curative resection of esophageal squamous cell carcinoma, especially in those approaching a complete response. Additionally, a shorter longest diameter of metastatic lymph nodes may be associated with better long-term survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophageal cancer is the sixth leading cause of cancer-related deaths worldwide [1] and is histologically divided into squamous cell carcinoma and adenocarcinoma. Esophageal squamous cell carcinoma (ESCC) is common in East Asia and Africa and is more prevalent than esophageal adenocarcinoma in Europe and less prevalent than esophageal adenocarcinoma in North America [2]. Although survival outcomes in patients with ESCC have improved with the development of multidisciplinary treatment modalities [3, 4], postoperative recurrences still occur in 28–53% of patients who undergo curative resection [5, 6]. Locoregional recurrence is the most common type of recurrence. The use of salvage concurrent chemoradiotherapy (CCRT) for such recurrences after curative resection of ESCC has been reported to be more effective than surgery alone as salvage therapy, except for the treatment of cervical lymph node (LN) metastasis [7, 8]. However, since previous reports of salvage CCRT have included heterogeneous modalities, radiation doses, and concurrent chemotherapeutic regimens, the efficacy of a fixed method of salvage CCRT with a high radiation dose in three-dimensional conformal radiotherapy (3D-CRT) combined with 5-fluorouracil (5-FU)/platinum-based chemotherapy—accepted as one of the most effective approaches—is unknown [9,10,11,12,13,14].
In this study, we evaluated the efficacy of salvage CCRT (60 Gy in 30 fractions) with 3D-CRT and 5-FU/platinum-based chemotherapy for locoregional recurrence after curative resection of ESCC.
Patients and methods
Study design and patients
We retrospectively analyzed the outcomes of patients treated with 5-FU/platinum-based CCRT for locoregional recurrence after curative resection of ESCC at the National Cancer Center Hospital East (NCCHE). The correspondence of recurrence after curative resection of ESCC at NCCHE is given in Supplementary Fig. 1. The inclusion criteria were as follows: (1) pathologically proven ESCC; (2) locoregional recurrence defined as recurrence in anastomosis or a regional LN, including the supraclavicular and para-aortic LNs at the upper abdominal level, after curative resection (R0 radical esophagectomy with 2/3-field LN dissection) between April 2002 and December 2014; and (3) treated with salvage CCRT (60 Gy in 30 fractions) with 3D-CRT and 5-FU/platinum-based chemotherapy (cisplatin or nedaplatin). The exclusion criteria were as follows: (1) active cancer in other regions; (2) distant metastases; and (3) receiving radiotherapy and chemotherapy other than 5-FU/platinum-based regimens. Tumors were staged according to the American Joint Committee on Cancer/International Union Against Cancer tumor–node–metastasis (TNM) staging system (seventh edition) [15]. Although tumor response was primarily assessed according to the Response Evaluation Criteria in Solid Tumors (version 1.1) on computed tomography (CT) [16] and the modified criteria of the Japanese Society for Esophagus Diseases on endoscopy [17], for this study the definition of LN metastasis was > 10 mm size of the LN, and the definition of complete response (CR) was the disappearance of all visible lesions except scarred LNs. Adverse events (hematological and non-hematological toxicities of grade 3 or higher) and late adverse events were assessed using the Common Terminology Criteria for Adverse Events (version 4.0) [18].
The study was performed in accordance with the ethical principles based on the Declaration of Helsinki. The study design was approved by the Institutional Review Board of the National Cancer Center, Japan (approval number: 2017–120). Each patient provided written informed consent for diagnosis and treatment before the procedure was performed.
Treatment and follow-up
External radiotherapy was administered using the 6- or 10-MV X-ray of a linear accelerator with a cumulative dose of 60 Gy (30 fractions at 2 Gy each). The gross tumor volume (GTV) was defined as a recurrence within anastomosis and/or one of the regional LNs. The clinical target volume (CTV) was determined with GTV plus 1 cm around GTV avoiding normal organs, and the planning target volume was defined as a 0.5–1.5-cm margin around the CTV to compensate for set-up variations and internal organ motion. 3D-CRT was used in all cases. CCRT consisted of 5-FU (700 mg/m2 on days 1–4 every 4 weeks) and cisplatin (70 mg/m2 on day 1 every 4 weeks) or 5-FU (800 mg/m2 on days 1–4 every 4 weeks) and nedaplatin (80 mg/m2 on days 1–4 every 4 weeks) for two cycles.
Tumor responses were assessed by CT and endoscopy after CCRT and were re-evaluated every 3–6 months for those who achieved a CR. Patients who achieved a partial response or stable disease were treated with an additional two cycles of the same chemotherapy until a CR was achieved or the disease progressed. Patients with disease progression received palliative chemotherapy or the best supportive care.
Statistical analyses
We calculated the survival time from the start of salvage CCRT. We evaluated progression-free survival (PFS), defined as the time from the date of starting salvage CCRT to the date of disease progression or death, whichever came first, and overall survival (OS), defined as the time from the date of starting salvage CCRT to the date of death, using the Kaplan–Meier method, and analyzed PFS and OS according to the achievement of a CR. The log-rank test was used for the univariate analysis of the differences in the median OS. As in previous studies [13, 19, 20], we also analyzed OS according to sex, age (< 60 vs. ≥ 60 years), performance status (PS) of Eastern Cooperative Oncology Group (0 vs. 1/2), squamous cell carcinoma tumor marker (< 1.5 vs. ≥ 1.5 ng/mL), history of neoadjuvant or adjuvant chemotherapy, initial pathological stage (0/I/II vs. III/IV [TNM seventh edition]), recurrence interval (< 6.0 vs. ≥ 6.0 months), region of recurrence (single vs. multiple regions), longest metastatic LN diameter (< 25 vs. ≥ 25 mm), and chemotherapy regimen (5-FU plus cisplatin vs. 5-FU plus nedaplatin). Cox regression models were used for the multivariate analysis of the differences in the median OS; baseline variables with P < 0.10 in the univariate analysis were included in the multivariate analysis. All statistical analyses were conducted using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for version 3.6.3 of R (The R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was defined as a two-tailed P < 0.05.
Results
Study flow and patient and tumor characteristics
The consort diagram is shown in Supplementary Fig. 2. Of the 959 patients with ESCC who underwent curative resection between April 2002 and December 2014, 230 patients (24.0%) had distant metastases, and 140 patients (14.6%) had locoregional recurrence only. Of the 140 patients with locoregional recurrence, 58 (41.4%) were treated with salvage CCRT. Seven patients (5.0%) were excluded from this study because they received chemotherapy other than 5-FU/platinum-based regimens or < 60 Gy of radiotherapy. The recurrence patterns of the 51 eligible patients were lymphnodal recurrences in 49 patients (96.7%), while 2 patients (3.3%) had anastomosis and lymphnodal recurrences. Of the 51 patients, 19 (29.4%) received a reduced dose or frequency of chemotherapy due to an underlying disease or chemotherapy toxicity. Radiotherapy was completed in all patients. Table 1 summarizes the patient and tumor characteristics. The median follow-up time was 17.5 (range, 2.8–116.1) months. The cohort predominantly included men (84.3%) with PS 0 (80.4%), a primary tumor located in the thorax (92.2%), and 5-FU plus cisplatin administered as the chemotherapy regimen for CCRT (80.4%).
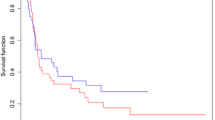
Treatment outcomes
The overall response rate was 74.5% (38/51), with a CR rate of 49.0% (25/51) (Table 2). The median PFS was 8.2 (95% confidence interval [CI] 5.8–10.2) months; the 3-year PFS rate was 22.9% (Fig. 1). The median OS was 23.1 (95% CI 15.9–49.0) months; the 3-year OS rate was 40.7% (Fig. 2). The median PFS was significantly longer in patients with a CR than in those without (15.8 vs. 4.7 [95% CI 9.4–N/A vs. 3.7–6.2] months); the 3-year PFS rate was 49.1% vs. 4.2% (hazard ratio [HR]: 0.242 [95% CI 0.051–0.432]; P < 0.001) (Fig. 3). The median OS was also significantly longer in patients with a CR than in those without (not reached vs. 15.3 [95% CI 23.1–N/A vs. 7.8–17.5] months); the 3-year OS rate was 62.5% vs. 20.3% (HR: 0.206 [95% CI 0.028–0.440]; P < 0.001) (Fig. 4).
Toxicity
Treatment-related adverse events of grade 3 or higher and late adverse events are shown in Table 3. Adverse events of grade 3 or higher were all acute adverse events and no grade 5 toxicities were observed. Grade 4 Leukopenia, neutropenia and thrombocytopenia were observed in two patients (3.9%) each. Grade 3 fatigue was observed in nine patients (17.6%), grade 3 leukopenia in eight patents (15.6%), grade 3 neutropenia in seven patients (13.7%), grade 3 anemia and nausea in four patients (7.8%) each, grade 3 hyponatremia in two patients (3.9%), and grade 3 thrombocytopenia, febrile neutropenia, hyperglycemia, and pneumonia in one patient (2.0%). Among the late adverse events, grade 1 radiation pneumonia was observed in five patients (9.8%), and grade 1 pleural effusion was noted in one patient (2.0%).
Association of tumor response with survival
In univariate analysis, PS 0 and the longest metastatic LN diameter of < 25 mm were associated with significantly better OS (P = 0.009 and 0.001, respectively). Multivariate analysis demonstrated that the independent prognostic factor for OS was the longest metastatic LN diameter of < 25 mm (HR, 3.71; 95% CI, 1.52–9.05) (Table 4).
Discussion
In previous studies, high radiation dose, and 3D-CRT or 5-FU/platinum-based chemotherapy are reported to be effective for salvage CCRT for the treatment of locoregional recurrence after curative resection of ESCC [9,10,11,12,13,14]. This study reported the treatment efficacy of salvage CCRT with a fixed approach at a dose of 60 Gy in 30 fractions of 3D-CRT combined with 5-FU/platinum-based chemotherapy. The median OS was approximately 2 years, and approximately half of the patients achieved a CR, which was associated with longer survival.
The methods involving radiotherapy and chemotherapy for salvage CCRT for locoregional recurrence of ESCC were inconsistent in previous reports (> 20 cases) [9,10,11, 13, 14, 19, 21,22,23], and the overall response rate was > 70% (Supplementary Table 1). However, the 3-year survival rate ranged from 10.5% to 51.8%, with a median OS ranging between 13 and 43 months. More recent reports tended towards better survival outcomes. Our results, with a 3-year OS rate of 40.7% and a median OS of 23.1 months, were consistent with the recent reports.
Compared to two-dimensional radiotherapy, 3D-CRT has improved anatomical imaging, significantly enhancing target delineation and sparing neighboring tissues by optimizing the dose distribution. Thus, 3D-CRT is expected to reduce radiation-induced side effects [24]. Late toxicity events were previously thought to be mainly attributed to radiation-induced side effects. This study reported that 9.8% of the patients showed grade 1 toxicity and reported no toxicities that were grade 3 or more. However, previous reports have shown 2.4%–3.3% of patients with grade 3 toxicity [10, 14] and 4.3%–11.4% with grade 1 or 2 toxicity [11, 14, 21]. Therefore, we assumed that 3D-CRT reduced the radiation-induced side effects despite the high-dose radiation reported in this study. Additionally, late toxicity events in this study using 60 Gy were lower than that of a definitive CCRT study for esophageal cancer using 50.4 Gy regimen [25]. This might be due to differences in target lesion and irradiated area.
Grade 3/4 toxicity of salvage CCRT (60 Gy in 30 fractions of 3D-CRT combined with 5-FU/platinum-based chemotherapy) for locoregional recurrence of ESCC reported in this study was observed in < 15% of patients with hematological toxicities and < 20% of patients with non-hematological toxicities. However, in previous studies, it was observed in 17.4%–36.7% of patients with hematological toxicities and 14.0%–33.3% of patients with non-hematological toxicities [13, 19, 21,22,23]. Therefore, we thought that it did not differ from previously reported studies involving lower-dose radiotherapy or other chemotherapy regimens. Consequently, high-dose salvage CCRT (60 Gy in 30 fractions of 3D-CRT combined with 5-FU/platinum-based chemotherapy) for locoregional recurrence after curative resection of ESCC was well-tolerated.
In previous studies, the prognostic factors for locoregional recurrence after curative resection of ESCC were 3D-CRT, a radiation dose of ≥ 60 Gy, chemotherapy regimens, the time from surgery to recurrence, the size (diameter) of LN metastases, number of LN metastases, and location of LN metastases [12, 13, 19, 22, 23, 26,27,28]. In our study, there was no difference in radiation therapy because all cases received 60 Gy radiation doses in 3D-CRT. Additionally, all cases received radiotherapy with 5-FU and platinum-based chemotherapy. Also, there were no significant differences in the prognostic factors between the patients receiving the regimens that used cisplatin and nedaplatin in this study. Besides chemotherapy and the method of radiation therapy, the examination of prognostic factors revealed that a shorter longest diameter of metastatic LNs was a good prognostic factor.
We reported high 3-year PFS and OS rates in patients owing to the therapeutic effect of CR. Therefore, reaching CR was deemed necessary for long-term survival. The favorable prognosis in the CR cases of salvage CCRT makes it worthwhile considering a treatment regimen that enhances the treatment response. Bao et al. [23] reported a better prognosis and response rate with a docetaxel and cisplatin regimen than with a 5-FU and cisplatin regimen as salvage CCRT. Additionally, Tamaki et al. [29] reported that a combination of docetaxel, cisplatin, and 5-FU had better outcomes than 5-FU and cisplatin in advanced esophageal cancer, with acceptable toxicity profiles. Furthermore, Antonia et al. [30] reported improved therapy when an immune checkpoint inhibitor was added after chemoradiotherapy in patients with lung cancer. Therefore, taxane and platinum-based chemotherapy, 5-FU plus platinum and taxane-based chemotherapy, and the addition of an immune checkpoint inhibitor after chemoradiotherapy in salvage CCRT may be worth considering in the future.
This study has some limitations. First, this is a retrospective, single-center study. Second, this study included patients who received perioperative chemotherapy. However, there was no effect of combination chemotherapy on prognosis in multivariate analysis.
In conclusion, salvage CCRT (60 Gy in 30 fractions of 3D-CRT in combination with 5-FU/platinum-based chemotherapy) for locoregional recurrence after curative resection of ESCC was an effective and safe treatment. Approximately 40% of the cases achieved long-term survival following salvage CCRT, particularly in cases with CR. In addition, cases with a shorter longest diameter of metastatic LNs may be associated with improved long-term survival after salvage CCRT.
References
Smyth EC, Lagergren J, Fitzgerald RC, et al. Oesophageal cancer. Nat Rev Dis Primers. 2017;3:17048.
Malhotra GK, Yanala U, Ravipati A, et al. Global trends in esophageal cancer. J Surg Oncol. 2017;115:564–79.
Mariette C, Piessen G, Triboulet JP. Therapeutic strategies in oesophageal carcinoma: role of surgery and other modalities. Lancet Oncol. 2007;8:545–53.
Allum WH, Stenning SP, Bancewicz J, et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27:5062–7.
Mantziari S, Allemann P, Winiker M, et al. Locoregional tumor extension and preoperative smoking are significant risk factors for early recurrence after esophagectomy for cancer. World J Surg. 2018;42:2209–17.
Hsu PK, Chien LI, Wang LC, et al. Lymphovascular invasion and extracapsular invasion are risk factors for distant recurrence after preoperative chemoradiotherapy and oesophagectomy in patients with oesophageal squamous cell carcinoma. Eur J Cardiothorac Surg. 2017;51:1188–94.
Watanabe M, Mine S, Yamada K, et al. Outcomes of lymphadenectomy for lymph node recurrence after esophagectomy or definitive chemoradiotherapy for squamous cell carcinoma of the esophagus. Gen Thorac Cardiovasc Surg. 2014;62:685–92.
Ma X, Zhao K, Guo W, et al. Salvage lymphadenectomy versus salvage radiotherapy/chemoradiotherapy for recurrence in cervical lymph node after curative resection of esophageal squamous cell carcinoma. Ann Surg Oncol. 2015;22:624–9.
Lu J, Kong C, Tao H. Radiotherapy with or without concurrent chemotherapy for lymph node recurrence after radical surgery of thoracic esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2010;78:710–4.
Kobayashi R, Yamashita H, Okuma K, et al. Salvage radiation therapy and chemoradiation therapy for postoperative locoregional recurrence of esophageal cancer. Dis Esophagus. 2014;27:72–8.
Ma DY, Tan BX, Liu M, et al. Concurrent threedimensional conformal radiotherapy and chemotherapy for postoperative recurrence of mediastinal lymph node metastases in patients with esophageal squamous cell carcinoma: a phase 2 single–institution study. Radiat Oncol. 2014;9:28.
Fakhrian K, Gamisch N, Schuster T, et al. Salvage radiotherapy in patients with recurrent esophageal carcinoma. Strahlenther Onkol. 2012;188:136–42.
Kawamoto T, Nihei K, Sasai K, et al. Clinical outcomes and prognostic factors of chemoradiotherapy for postoperative lymph node recurrence of esophageal cancer. Jpn J Clin Oncol. 2018;48:259–64.
Jingu K, Matsushita H, Takeda K, et al. Long-term results of radiotherapy combined with nedaplatin and 5-fluorouracil for postoperative loco-regional recurrent esophageal cancer: update on a phase II study. BMC Cancer. 2012;12:542.
Sobin LH, Gospodarowicz MK, Wittekind C. International Union Against Cancer. TNM Classification of Malignant Tumours. 7th ed. Chichester, West Sussex, UK; Hoboken, NJ: Wiley-Blackwell; 2010.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Kuwano H, Nishimura Y, Oyama T, et al. Guidelines for diagnosis and treatment of carcinoma of the esophagus April 2012 edited by the Japan Esophageal Society. Esophagus. 2015;12:1–30.
National Cancer Institute (U.S.). Common Terminology Criteria for Adverse Events (CTCAE). Rev. edn. Bethesda, MD.: U.S. Dept. of Health and Human Services, National Institutes of Health, National Cancer Institute, 2009.
Nakamura T, Ota M, Narumiya K, et al. Multimodal treatment for lymph node recurrence of esophageal carcinoma after curative resection. Ann Surg Oncol. 2008;15:2451–7.
Baxi SH, Burmeister B, Harvey JA, et al. Salvage definitive chemo-radiotherapy for locally recurrent oesophageal carcinoma after primary surgery: retrospective review. J Med Imaging Radiat Oncol. 2009;52:583–7.
Maruyama K, Motoyama S, Anbai A, et al. Therapeutic strategy for the treatment of postoperative recurrence of esophageal squamous cell carcinoma: clinical efficacy of radiotherapy. Dis Esophagus. 2011;24:166–71.
Zhang J, Peng F, Li N, et al. Salvage concurrent radio-chemotherapy for post-operative local recurrence of squamous cell esophageal cancer. Radiat Oncol. 2012;7:93.
Bao Y, Liu S, Zhou Q, et al. Three-dimensional conformal radiotherapy with concurrent chemotherapy for postoperative recurrence of esophageal squamous cell carcinoma: clinical efficacy and failure pattern. Radiat Oncol. 2013;8:241.
Deng JY, Wang C, Shi XH, et al. Reduced toxicity with three-dimensional conformal radiotherapy or intensity-modulated radiotherapy compared with conventional two-dimensional radiotherapy for esophageal squamous cell carcinoma: a secondary analysis of data from four prospective clinical trials. Dis Esophagus. 2017;30:1–7.
Kato K, Nakajima TE, Ito Y, et al. Phase II study of concurrent chemoradiotherapy at the dose of 50.4 Gy with elective nodal irradiation for Stage II-III esophageal carcinoma. Jpn J Clin Oncol. 2013;43:608–15.
Shioyama Y, Nakamura K, Ohga S, et al. Radiation therapy for recurrent esophageal cancer after surgery: clinical results and prognostic factors. Jpn J Clin Oncol. 2007;37:918–23.
Kosuga T, Shiozaki A, Fujiwara H, et al. Treatment outcome and prognosis of patients with lymph node recurrence of thoracic esophageal squamous cell carcinoma after curative resection. World J Surg. 2011;35:798–804.
Nemoto K, Ariga H, Kakuto Y, et al. Radiation therapy for locoregionally recurrent esophageal cancer after surgery. Radiother Oncol. 2001;61:165–8.
Tamaki Y, Hieda Y, Nakajima M, et al. Concurrent chemoradiotherapy with docetaxel, cisplatin, and 5-fluorouracil improves survival of patients with advanced esophageal cancer compared with conventional concurrent chemoradiotherapy with cisplatin and 5-fluorouracil. J Cancer. 2018;9:2765–72.
Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–29.
Acknowledgements
We wish to thank all the staff involved in esophageal cancer treatment. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization: RI, YN, and TK; Methodology: RI, YN, and TK, Formal analysis and investigation: RI, YN, and TK; Writing-original draft preparation: RI; Writing-review and editing: YN, TK, HS, HF, HH, NN, TF, TY, HD, TA, and TY; Supervision: TK.
Corresponding author
Ethics declarations
Ethical Statement
The study was performed in accordance with the ethical principles based on the Declaration of Helsinki.
Conflict of interest
Author Renma Ito, Author Hironori Sunakawa, Author Hisashi Fujiwara, Author Hidehiro Hojo, Author Naoki Nakamura, Author Takeo Fujita, Author Hiroyuki Daiko, and Author Tetsuo Akimoto declare that they have no conflict of interest. Author Yoshiaki Nakamura received grants from Genomedia, grants from Guardant Health, grants from Chugai, grants from Taiho, outside the submitted work. Author Tomonori Yano received grants from Fujifilm, grants and personal fees from Olympus, grants from HOYAPENTAX, grants from SHIMAZU, grants from RAKUTEN Medical, personal fees from MeijiSeika Pjarma, outside the submitted work. Author Takayuki Yoshino received grants from Taiho Pharmaceutical, grants from Sumitomo Dainippon Pharma, grants from Ono Pharmaceutical, grants from Chugai Pharmaceutical, grants from Amgen, grants from PAREXEL International, grants from MSD, grants from Daiichi Sankyo, grants from Sanofi, outside the submitted work. Author Takashi Kojima received grants and personal fees from Ono Pharmaceutical, grants and personal fees from MSD, grants from Astellas Amgen BioPharma, grants from Taiho Pharmaceutical, grants from Shionogi, personal fees from Oncolys BioPharma, personal fees from Astellas Pharma, personal fees from BMS, personal fees from Merk, grants from Japan Agency for Medical Research and Development (AMED), outside the submitted work.
Human rights statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
Informed consent
Informed consent or substitute for it was obtained from all patients for being included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ito, R., Nakamura, Y., Sunakawa, H. et al. Tumor response and survival outcomes of salvage concurrent chemoradiotherapy with three-dimensional conformal radiotherapy and 5-fluorouracil/platinum-based chemotherapy for postoperative locoregional recurrence of esophageal squamous cell carcinoma. Esophagus 19, 645–652 (2022). https://doi.org/10.1007/s10388-022-00936-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10388-022-00936-3