Abstract
Objectives
We have found that an altered lower esophageal sphincter (LES) accommodation response is an underlying cause of esophagogastric junction outflow obstruction (EGJOO). The objective of this study was to examine the treatment effect of acotiamide, a prokinetic agent which improves impaired gastric accommodation in functional dyspepsia, in patients with EGJOO.
Methods
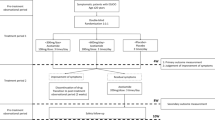
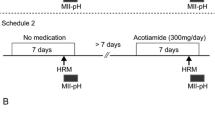
A prospective observational longitudinal study was conducted between October 2014 and March 2020. Acotiamide (100 mg, 3 times a day) was administered to 25 patients with EGJOO for 4 weeks. High-resolution manometry (HRM) was performed just before and after 4 weeks of treatment.
Results
As the primary outcome, the extent of integrated relaxation pressure (IRP) after treatment (14.6, 12.1–22.0 mmHg) was significantly lower than that before treatment (19.4, 17.1–27.4 mmHg). The extent of LES accommodation index after treatment (32.7, 21.0–40.0 mmHg) was also significantly lower than that before treatment (39.3, 31.2–50.2 mmHg). Acotiamide normalized the IRP (< 15 mmHg) in 13 of 25 patients with EGJOO (52%), and the IRP was decreased in 20 of 25 patients with EGJOO (80%). As the secondary outcome, the total FSSG score in 25 patients with EGJOO before and after acotiamide treatment showed no significant difference. In a sub-analysis of 13 patients in whom EGJOO was normalized by acotiamide, however, dysphagia was reported to be significantly improved by acotiamide.
Conclusions
Acotiamide has a treatment effect on patients with EGJOO via a reduction in the IRP level through the lowering of both the basal LES pressure and LES accommodation response. Dysphagia is a key symptom to be evaluated and treated in patients with EGJOO.





Similar content being viewed by others
References
Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27:160–74.
Yadlapati R, Kahrilas PJ, Fox MR, et al. Esophageal motility disorders on high-resolution manometry: Chicago classification version 4.0((c)). Neurogastroenterol Motil. 2021;33:e14058.
Ihara E, Muta K, Fukaura K, et al. Diagnosis and treatment strategy of achalasia subtypes and esophagogastric junction outflow obstruction based on high-resolution manometry. Digestion. 2017;95:29–35.
Trifan A, Shaker R, Ren J, et al. Inhibition of resting lower esophageal sphincter pressure by pharyngeal water stimulation in humans. Gastroenterology. 1995;108:441–6.
Muta K, Ihara E, Hamada S, et al. Physiological and pathological roles of the accommodation response in lower esophageal sphincter relaxation during wet swallows. Sci Rep. 2021;11:7898.
Tack J, Janssen P. Acotiamide (Z-338, YM443), a new drug for the treatment of functional dyspepsia. Expert Opin Investig Drugs. 2011;20:701–12.
Muta K, Ihara E, Fukaura K, et al. Effects of acotiamide on the esophageal motility function in patients with esophageal motility disorders: a pilot study. Digestion. 2016;94:9–16.
Kusunoki H, Haruma K, Manabe N, et al. Therapeutic efficacy of acotiamide in patients with functional dyspepsia based on enhanced postprandial gastric accommodation and emptying: randomized controlled study evaluation by real-time ultrasonography. Neurogastroenterol Motil. 2012;24(540–545):e250-541.
Fukaura K, Ihara E, Ogino H, et al. Mucosally expressed cytokines are associated with the esophageal motility function. Digestion. 2018;98:95–103.
Hamada S, Ihara E, Ikeda H, et al. Clinical characterization of vonoprazan-refractory gastroesophageal reflux disease. Digestion. 2021;102:197–204.
Kusano M, Shimoyama Y, Sugimoto S, et al. Development and evaluation of FSSG: frequency scale for the symptoms of GERD. J Gastroenterol. 2004;39:888–91.
Porter AJ, Wattchow DA, Brookes SJ, et al. The neurochemical coding and projections of circular muscle motor neurons in the human colon. Gastroenterology. 1997;113:1916–23.
Chiocchetti R, Giancola F, Mazzoni M, et al. Excitatory and inhibitory enteric innervation of horse lower esophageal sphincter. Histochem Cell Biol. 2015;143:625–35.
Matsueda K, Hongo M, Tack J, et al. Clinical trial: dose-dependent therapeutic efficacy of acotiamide hydrochloride (Z-338) in patients with functional dyspepsia—100 mg t.i.d. is an optimal dosage. Neurogastroenterol Motil. 2010;22:618-e173.
Yadlapati R, Pandolfino JE, Fox MR, et al. What is new in Chicago classification version 40? Neurogastroenterol Motil. 2021;33:e14053.
Sloan JA, Triggs JR, Pandolfino JE, et al. Treatment experience with a novel 30-mm hydrostatic balloon in esophageal dysmotility: a multicenter retrospective analysis. Gastrointest Endosc. 2020;92:1251–7.
Samo S, Qayed E. Esophagogastric junction outflow obstruction: Where are we now in diagnosis and management? World J Gastroenterol. 2019;25:411–7.
Ichkhanian Y, Sanaei O, Canakis A, et al. Esophageal peroral endoscopic myotomy (POEM) for treatment of esophagogastric junction outflow obstruction: results from the first prospective trial. Endosc Int Open. 2020;8:E1137–43.
Inoue H, Shiwaku H, Iwakiri K, et al. Clinical practice guidelines for peroral endoscopic myotomy. Dig Endosc. 2018;30:563–79.
Kahrilas PJ, Katzka D, Richter JE. Clinical practice update: the use of per-oral endoscopic myotomy in achalasia: expert review and best practice advice from the AGA institute. Gastroenterology. 2017;153:1205–11.
Khashab MA, Familiari P, Draganov PV, et al. Peroral endoscopic myotomy is effective and safe in non-achalasia esophageal motility disorders: an international multicenter study. Endosc Int Open. 2018;6:E1031–6.
Funding
Grant Support: This study was supported in part by the Japan Society for the Promotion of Science KAKENHI (20K08334) and the Project Promoting Clinical Trials for Development of New Drugs and Medical Devices (Japan Medical Association) from the Japan Agency for Medical Research and Development (AMED) (19lk0201088h0001 and 21lk0201144h0001).
Author information
Authors and Affiliations
Contributions
EI, HO, and KM designed this study. KM, SH, MW, and HI performed the HRM study. MW, YH, HI, XB, YM, ME and YT performed EGD. KM, SH, MW, YH, YM, and ME collected the data and performed statistical analysis. EI, HO, and ME wrote the manuscript. TC and YO supervised this study and reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
Conflict of interest
E.I. belongs to an endowed course supported by the companies including Ono pharmaceutical Co., Ltd., Miyarisan Pharmaceutical Co. Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Otsuka Pharmaceutical Factory, Inc., Fujifilm Medical Co., Ltd., Terumo corporation, FANCL corporation, Ohga pharmacy, and Abbott Japan, LLC. E.I. receives lecture honorarium from Takeda Pharmaceutical Company. Y.O. conducts collaborative research with Fujifilm Medical Co., Ltd. and FANCL corporation. H.O., K.M., S.H., M.W., Y.H., H.I., X.B.,Y.M., M.E., Y.T., and T.C. declare no conflict of interest in association with this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ihara, E., Ogino, H., Muta, K. et al. The treatment effects of acotiamide in esophagogastric outflow obstruction: a prospective longitudinal observational study. Esophagus 19, 332–342 (2022). https://doi.org/10.1007/s10388-021-00887-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10388-021-00887-1