Abstract
Purpose
This study aimed to investigate differences in microvasculature dropout (MvD) between the superior and inferior hemispheres in glaucoma patients.
Study design
Retrospective and cross-sectional.
Methods
Fifty-eight eyes of 58 open-angle glaucoma patients (age 61.12 ± 10.19 years, mean deviation − 7.32 ± 6.36 dB) were included. MvD was detected with en face images from swept-source optical coherence tomography angiography. Blood flow at the optic nerve head was measured with laser speckle flowgraphy, represented as the mean blur rate in tissue (MBRT). Logistic and linear regression models adjusted for age, intraocular pressure, axial length, and circumpapillary retinal nerve fiber layer thickness were used to investigate the relationship between various factors and MvD angle in each hemisphere.
Results
The presence of inferior MvD was related to peripapillary atrophy-β area (odds ratio = 14.10 [2.49–234.00], P = 0.019). Superior MvD angle was significantly related to MBRT in the superior quadrant (β = −0.31 [− 0.60 – −0.02], P = 0.037). Inferior MvD angle was significantly related to peripapillary atrophy-β area (β = 0.49 [0.21–0.77], P = 0.001).
Conclusions
Only superior MvD demonstrated a significant relationship with reduced ocular blood flow. In contrast, inferior MvD was associated with mechanical stress. These findings may suggest a potential difference in pathophysiology between superior and inferior MvD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glaucoma is the second most common cause of blindness worldwide [1, 2]. In general, it is considered that high intraocular pressure (IOP) leads to damage to the lamina cribrosa where the optic nerve runs through it. Therefore, therapies aimed at reducing IOP, whether through medication or surgical intervention, are considered effective treatments to prevent glaucoma progression [3, 4]. However, despite successful IOP reduction, many patients, particularly those with normal-tension glaucoma, still demonstrate visual field progression [5]. Additionally, risk factors other than elevated IOP have been identified [6]. One important non-IOP risk factor is low ocular blood flow [7]. We reported that low ocular blood flow precedes the reduction of circumpapillary retinal nerve fiber layer thickness (cpRNFLT) in some glaucoma cases [8]. Therefore, it is also important to evaluate ocular blood flow in glaucoma patients.
The advent of swept-source optical coherence tomography (SS-OCT) enabled more precise investigation in deeper layers of the retina and choroid. SS-OCT employs a relatively long wavelength (1050 nm), enabling deep penetration, and produces high-resolution images of these deeper layers, including the choroidal layer [9, 10]. SS-OCT also has faster scanning speed and allows patients to maintain good fixation due to its low glare, providing highly detailed images. Thus, SS-OCT has the advantage of producing clear capillary images of these deeper layers.
Recently, choroidal microvasculature dropout (MvD), which can be observed in the peripapillary atrophy-β (PPAβ) zone, has been reported as a characteristic marker associated with glaucoma progression [11]. MvD represents localized vascular dropout associated with retinal nerve fiber layer defects (RNFLDs) [11], disc hemorrhage [12], and central visual field dysfunction [13]. Studies using indocyanine green angiography show that the short posterior ciliary artery supplies the lamina cribrosa through capillaries in the PPAβ [13]. Thus, MvD indicates localized vascular dysfunction, which reduces the blood supply in the lamina cribrosa [14, 15] and might be a key to revealing the relationship between ocular blood flow and glaucoma progression.
Differences in initial glaucomatous damage between the superior and inferior hemispheres have been documented. It is reported that IOP fluctuations are associated with greater damage to the inferior optic disc [16]. In contrast, our previous findings indicate that in older patients, a higher pulse rate is associated with an increased risk of reduced ocular blood flow, which precedes cpRNFL thinning in the superior and temporal quadrants [8]. We also reported that reduced optic nerve head blood flow in the superior quadrant at baseline was significantly associated with subsequent inferior visual field progression [17]. Based on these findings, we hypothesized that the mechanism of glaucoma progression may vary depending on the specific quadrant. In the present study, we aimed to investigate whether superior and inferior MvD were associated with ocular/systemic parameters. Our results highlight the disparities between superior and inferior MvD and the implications of these disparities.
Materials and methods
Subjects
This study included 58 eyes of 58 patients with primary open-angle glaucoma (POAG) or normal-tension glaucoma (NTG). The initial diagnosis was made by a glaucoma specialist (T.N.) between January 2016 and September 2020 at Tohoku University Hospital. Glaucoma was defined in this study by the presence of an abnormal glaucomatous optic disc (with diffuse or focal thinning of the neuroretinal rim) and a corresponding glaucomatous visual field defect, defined according to the Anderson-Patella criteria [18] by the presence of one or more of the following: (1) a cluster of three points with reduced sensitivity at a probability of < 5% on the pattern deviation map in at least one hemifield (including ≥ 1 point at a probability of < 1% or a cluster of two points at a probability of < 1%), (2) glaucomatous hemifield test results outside the normal limits, and (3) a pattern standard deviation beyond 95% of normal limits, as confirmed in at least two reliable examinations. A peak IOP of 21 mmHg or less without any glaucoma medication was diagnosed as NTG, and a peak IOP of more than 21 mmHg was diagnosed as POAG. The number of hypertension and diabetes drugs was self-reported for this study. Eyes with cataract (a lens nucleus grade 3 or more by Emery-Little classification), vitreoretinal or optic nerve diseases other than glaucoma were excluded from the current study. All patients had PPAβ, atrophy of the retinal pigment epithelium and choriocapillaris [19], and RNFLDs with clear boundaries in en face images of 12 × 9 mm OCT wide scans. When both eyes of a patient were eligible, the eye with the wider RNFLD angle was included. This study adhered to the tenets of the Declaration of Helsinki, and the protocols were approved by the Clinical Research Ethics Committee of the Tohoku University Graduate School of Medicine (study 2021-1-430).
Measurement of variables
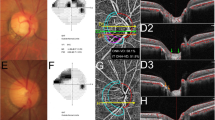
Best-corrected visual acuity (BCVA) was measured with a standard Japanese decimal visual acuity chart and converted to the logarithm of the minimum angle of resolution (logMAR). IOP was measured with Goldmann applanation tonometry. Axial length was measured with ocular biometry (OA-2000, Tomey Corporation). Central corneal thickness (CCT) was measured with anterior-segment OCT (CASIA2, Tomey Corporation). Optic nerve head (ONH) blood flow was assessed with laser speckle flowgraphy (LSFG; LSFG-NAVI, Softcare Co, Ltd.), which measures mean blur rate (MBR) in arbitrary units (AU). In the current study, we used MBR in the tissue area (MBRT) to assess capillary blood flow; we measured overall MBRT as well as MBRT in the superior, temporal, and inferior quadrants (Fig. 1a). Mean deviation (MD) was measured with the Humphrey field analyzer (HFA; Carl Zeiss Meditec) with the Swedish interactive threshold algorithm (SITA)–standard strategy of the 24 − 2 program. Only reliably measured MD values were used (< 20% fixation errors, < 15% false-positive results, and < 33% false-negative results). All the examination data were obtained within a two-month period, without interrupting the use of medication for glaucoma.
Representative laser speckle flowgraphy measurements and an en face image obtained with optical coherence tomography angiography. (a) We measured the blood flow within the optic nerve head (ONH) in the tissue area (white ellipse), as well as in the separate quadrants. (b) The yellow line illustrates the margin of the peripapillary atrophy-β (PPAβ) zone. Microvasculature dropout (MvD) was detected when it was more than two times greater than the width of visible capillaries in the PPAβ zone (red line). The MvD area was measured within the red line. The MvD angle was determined by identifying the two points where its extreme borders met the ONH border as angular circumferential margins and drawing two lines connecting the ONH center (white line). To determine the positional coordinates of MvD, we set the temporal side as 0 degrees
Clinical parameters for each patient were recorded, including age, sex, and body mass index (BMI). BMI was calculated by the following formula: weight (kg) / height2 (m2). Blood pressure (BP) and pulse rate (PR) were conventionally measured in the brachial artery at the height of the heart (HBP-1300, Omron Colin Co.). Mean arterial pressure (MAP) and ocular perfusion pressure (OPP) were calculated with the following formulas, respectively: MAP = diastolic BP + 1/3 (systolic BP − diastolic BP); OPP = 2/3MAP − IOP.
OCT examination
CpRNFL thickness (cpRNFLT) was measured in a 12 × 9 mm wide scan with swept-source OCT (SS-OCT; DRI OCT Triton, Topcon Corp.). Retinal nerve fiber layer defect (RNFLD) angle was measured in en face images according to a method described in previous reports. Briefly, the RNFLD angle was measured as the intersection between the RNFLD and a circle centered on the disc with a radius half the distance between the disc and the fovea, using software we have developed [20, 21].
Measurement of MvD and PPAβ area
In this study, 4.5 × 4.5 mm OCT angiography (OCTA) images centered on the ONH were acquired with SS-OCTA (DRI OCT Triton, Topcon Corp.). Only good-quality images with image quality > 30 were included [22]. The PPAβ border was marked in projection images using Label Studio, an open-source data labeling tool (Heartex Inc.). We overlaid the PPAβ margin on the en face image, which represented a layer starting at 130 μm below the internal limiting membrane (ILM) and reaching 390 μm below Bruch’s membrane; this layer includes the full thickness of the choroid and the scleral flange [12, 23]. MvD was evaluated on the overlaid en face image using Label Studio and was defined as local, sectorial capillary dropout without any visible microvascular network; this capillary dropout was more than two times greater than the width of the visible capillaries [11, 24]. Based on the labeled data, we calculated the area of both the PPAβ zone and MvD. MvD angle was determined by identifying the two points where its extreme borders met the ONH border as angular circumferential margins and drawing two lines connecting the ONH center to these margins (Fig. 1b). To determine the positional coordinates of MvD, we set the temporal side at 0 degrees, with the superior side at 90 degrees (moving clockwise), the nasal side at 180 degrees, and the inferior side at 270 degrees.
Both the PPAβ zone and MvD margins were evaluated by two glaucoma specialists (N.T. and K.O.), who were masked to all clinical data, including cpRNFL and visual field sensitivity. Any uncertainty was resolved by another glaucoma specialist (T.N.). If an eye had more than one MvD, all the MvDs were calculated separately then added for the superior and inferior hemispheres. If an MvD extended across both the superior and inferior hemispheres, the MvD angle was measured in each hemisphere separately.
Statistical analysis
Correlations were calculated with the Pearson correlation coefficient. Logistic regression analysis was performed to examine the presence of MvD in the superior and inferior hemispheres. Linear regression models were used to evaluate the magnitude of MvD. All models were adjusted for potential confounding factors, including age, IOP, axial length, and cpRNFLT [22, 25]. Analyses were performed using JMP software (version 17.0.0; SAS Institute Japan Inc.) or R (version 4.1.2; R Foundation for Statistical Computing). P values < 0.05 were considered statistically significant. No adjustments were made for multiple comparisons, and 95% confidence intervals (CIs) are provided for all results to enable comparison of parameters.
Results
Table 1 shows the characteristics of the participants in this study. The average age was 61.12 ± 10.19 years, axial length was 25.06 ± 1.34 mm, MD was − 7.32 ± 6.36 dB, PPAβ area was 1.24 ± 0.98 mm2, MvD angle was 32.95 ± 20.12, and MvD area was 0.21 ± 0.23 mm2. Table 2 shows the correlation between the MvD angle/area and the various ocular/systemic parameters. MvD angle showed significant correlation with glaucoma severity (cpRNFLT: β = − 0.48 [− 0.71 – −0.24], P < 0.001; MD: β = − 0.55 [− 0.77 – −0.32], P < 0.001; RNFLD angle: β = 0.68 [0.49–0.88], P < 0.001). Both axial length and PPAβ area were significantly correlated with MvD area (β = 0.35 [0.10–0.60], P = 0.008, β = 0.75 [0.58–0.93], P < 0.001, respectively); however, glaucoma severity was not. Consequently, we used MvD angle in subsequent analyses. Table 3 shows the results of multivariable regression models adjusted for confounding factors (i.e., age, IOP, axial length, and cpRNFLT). The models show that MvD angle was significantly related to systolic BP, MAP, OPP, and PPAβ area (β = −0.29 [− 0.55 – −0.04], P = 0.024; β = −0.26 [− 0.50 – −0.01], P = 0.040; β = −0.25 [− 0.50 – −0.01], P = 0.040; β = 0.41 [0.15–0.67], P = 0.003, respectively). Figure 2 presents a rose diagram illustrating the locations of MvD. As shown in the figure, MvD was most frequently located inferotemporally and next most frequently located superotemporally. We did not observe any MvDs that extended across the superior and inferior regions in this study.
Next, we performed a logistic regression analysis to investigate which factors were related to superior or inferior MvD (Table 3). The analysis showed that superior MvD was not significantly, but was likely related to, low systolic BP, low MAP, and low OPP (OR = 0.96 [0.92–1.00], P = 0.062; OR = 0.96 [0.91–1.00], P = 0.092; OR = 0.94 [0.87–1.00], P = 0.092, respectively), after adjusting for age, IOP, AL, and cpRNFLT. On the other hand, inferior MvD was significantly related to high PPAβ area (OR = 14.10 [2.49–234.00], P = 0.019) in the multivariable model.
Our investigation of the relationship between MvD angle and other factors in both the superior and inferior hemispheres had the following results. For superior MvD, a significant relationship was found with MBRT (β = −0.32 [− 0.58 – −0.07], P = 0.015), particularly superior MBRT (β = −0.42 [− 0.66 – −0.17], P = 0.001) and temporal MBRT (β = −0.30 [− 0.56 – −0.04], P = 0.023); for inferior MvD, there was a significant relationship with PPAβ area (β = 0.32 [0.07–0.58], P = 0.014). These relationships remained significant even after adjusting for age, IOP, AL, and cpRNFLT: superior MvD with superior MBRT (β = −0.31 [− 0.60 – −0.02], P = 0.037) and inferior MvD with PPAβ area (β = 0.49 [0.21–0.77], P = 0.001).
Discussion
In this study, we categorized MvD based on its location in the superior or inferior hemispheres to explore potential differences in pathology between MvD in these two locations. Our findings indicate a significant association between lower MvD and PPAβ after adjusting for potential confounding factors (as shown in Table 4). In terms of MvD angle, a notable relationship was identified between superior MvD and superior MBRT. Conversely, while inferior MvD angle was significantly related with PPAβ area, no significant relationships were observed between inferior MvD and any of the other circulation parameters, as detailed in Table 5. Overall, these results imply that superior MvD is uniquely associated with reduced ocular circulation.
First, we measured both the MvD angle and area. As evident from the results in Table 2, the MvD angle, when compared to the MvD area, had a stronger correlation with more parameters of glaucoma severity. Consequently, we chose the MvD angle for our subsequent investigations. On the other hand, the MvD area demonstrated a strong correlation with axial length and PPAβ area, suggesting that the MvD area measurements were predominantly influenced by these anatomical parameters.
Reduced ocular circulation, indicative of low BP and OPP, was significantly associated with a wider MvD angle (Table 3). This is consistent with Lee et al.’s identification of low MAP and OPP as risk factors for MvD [23]. While our multivariable logistic models did not demonstrate significant relationships between the superior/inferior hemispheres and ocular circulation parameters (Table 4), the borderline p-values suggest that further investigation is warranted. Although low BP can be a risk factor of MvD, hypertension is concurrently a significant risk factor for glaucoma [26]. Elevated blood pressure not only affects IOP but, due to microvascular damage from systemic hypertension, is a risk factor for POAG [27]. Therefore, both high and low blood pressure are reported to be harmful in glaucoma patients [28], highlighting the need for optimal blood pressure regulation.
In the linear regression models examining the association between MvD angle in the superior/inferior hemispheres and other parameters, a significant association was observed only between the superior hemisphere and reduced superior MBRT. A previous report demonstrated that MvD in the peripapillary region is frequently adjacent to MvD in the optic disc [29], which aligns with the findings of this study. However, the specific reasons for the difference in ocular circulation between the superior and inferior hemispheres remain unclear. Previous reports show that inferior MBRT also has a significant association with the corresponding (i.e., superior) visual field [17]. Therefore, it is unlikely that measuring inferior MBRT would be challenging. On the other hand, inferior MvD has shown a strong association with PPAβ, suggesting that the expansion of MvD due to disc rotation may be a physical cause, which could explain the lack of association with inferior MBRT. Our findings of an association in the superior hemisphere are supported by several past studies. Suzuki et al. found that normal-tension glaucoma patients who had signs of ischemic changes in brain MRI predominantly had inferior pericentral visual field disturbances [30]. Tomita et al. observed that blood flow in the superior retina decreased significantly compared to the inferior retina following postural changes in healthy subjects [31]. Similarly, Kiyota et al. indicate that older open-angle glaucoma patients with disturbances in the superior and temporal cpRNFL had an increased risk of ocular blood flow reduction preceding cpRNFL loss [8]. The specific mechanism rendering the superior hemisphere more vulnerable to reduced blood flow remains unclear, but these studies reinforce our observations.
The presence and magnitude of inferior MvD were significantly associated with the PPAβ area (Tables 4 and 5). PPAβ enlargement has been shown to be associated with axial elongation caused by myopia [32]. This elongation may increase the distance between the Zinn-Haller arterial circle and the optic disc, potentially leading to blood vessel ruptures [33] and the consequent development of inferior MvD. While our findings did not show a relationship between inferior MvD and IOP, other studies suggest such a relationship. For example, Midgett et al. found pronounced distortion in the inferior quadrant when increased pressure was applied to the lamina cribrosa in human donor eyes [34]. Thus, a possible association between inferior MvD and mechanical stresses, such as axial elongation or elevated IOP, could exist, but this question warrants further investigation.
There were several limitations in this study. (1) The study had a limited number of participants who were all of a single ethnicity. Being a single-center and cross-sectional study further limits its generalizability. (2) By its very design, MvD can only be detected within the PPA zone, making it a challenge to assess deep vessels outside this area. This limitation is particularly pronounced in glaucoma patients with smaller PPA zones. (3) The cross-sectional nature of the study means that we cannot determine if the development of superior MvD is a result of reduced ocular blood flow or a precursor to it. A longitudinal study would provide clarity on the causality of this relationship. (4) Poor OCTA image quality could hinder the detection of MvD. To ensure accuracy, we excluded images with an image quality score below 30. (5) Our method for generating en face images from OCTA was consistent, drawing from established practices in prior studies [12, 23]. However, using a slab reference 130 μm downward from the ILM might occasionally overlook MvD or misjudge its size. This calls for a potential re-evaluation of the slab creation methodology. (6) The study’s retrospective nature and the limited number of cases without inferior MvD might mean potential underlying factors associated with the formation of inferior MvD went unnoticed. In light of these considerations, future studies might benefit from larger, more diverse samples, more refined imaging techniques, and a longitudinal design. (7) In this study, we did not perform adjustment for multiple comparisons. This is particularly relevant in the analysis of quartiles of MBRT, where there may have been a need for multiple comparisons. As Rothman has noted [35], while corrections for multiple comparisons reduce the risk of type I errors, they simultaneously increase the risk of type II errors. Our primary objective was to report new findings that could be clinically useful. Therefore, to facilitate comparison of parameters, we have provided the 95% confidence intervals for all β coefficients [36].
In conclusion, our findings suggest distinct mechanisms for superior and inferior MvD development. While superior MvD appears to be influenced by reduced blood flow, the size of the PPAβ area may be pivotal for inferior MvD. Such observations underscore the importance of stratifying glaucoma based on its underlying pathophysiology. This study builds on our previous work, in which we found that various risk factors, such as decreased blood flow and aging, were associated with visual acuity decline in glaucoma patients [37], and suggests that understanding the disease’s pathophysiology in a more segmented manner offers promise. This could very well lay the groundwork for a tailored approach to treating glaucoma, moving toward a more personalized paradigm in medicine.
References
Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S, Glaucoma. Lancet. 2017;390:2183–93.
Resnikoff S, Pascolini D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–51.
Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the early manifest Glaucoma trial. Arch Ophthalmol. 2002;120:1268–79.
The advanced glaucoma intervention study (AGIS). 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–40.
Yokoyama Y, Maruyama K, Konno H, Hashimoto S, Takahashi M, Kayaba H, et al. Characteristics of patients with primary open angle glaucoma and normal tension glaucoma at a university hospital: a cross-sectional retrospective study. BMC Res Notes. 2015;8:360.
Ernest PJ, Schouten JS, Beckers HJ, Hendrikse F, Prins MH, Webers CA. An evidence-based review of prognostic factors for glaucomatous visual field progression. Ophthalmology. 2013;120:512–9.
Bowe A, Grunig M, Schubert J, Demir M, Hoffmann V, Kutting F, et al. Circadian variation in arterial blood pressure and Glaucomatous Optic Neuropathy–A systematic review and Meta-analysis. Am J Hypertens. 2015;28:1077–82.
Kiyota N, Shiga Y, Omodaka K, Pak K, Nakazawa T. Time-Course changes in Optic nerve Head Blood Flow and retinal nerve Fiber layer thickness in eyes with open-angle Glaucoma. Ophthalmology. 2021;128:663–71.
Adhi M, Liu JJ, Qavi AH, Grulkowski I, Fujimoto JG, Duker JS. Enhanced visualization of the choroido-scleral interface using swept-source OCT. Ophthalmic Surg Lasers Imaging Retina. 2013;44:S40–2.
Mansouri K, Nuyen B, Weinreb N. Improved visualization of deep ocular structures in glaucoma using high penetration optical coherence tomography. Expert Rev Med Dev. 2013;10:621–8.
Kim JA, Lee EJ, Kim TW. Evaluation of Parapapillary Choroidal Microvasculature Dropout and Progressive Retinal nerve Fiber layer thinning in patients with Glaucoma. JAMA Ophthalmol. 2019;137:810–6.
Kim CY, Lee EJ, Kim JA, Kim H, Kim TW. Progressive retinal nerve fibre layer thinning and choroidal microvasculature dropout at the location of disc haemorrhage in glaucoma. Br J Ophthalmol. 2020;674 – 80.
Lee KM, Kim JM, Lee EJ, Kim TW. Anterior Optic nerve Head Perfusion is dependent on adjacent Parapapillary Choroidal perfusion. Sci Rep. 2019;9:10999.
Kiyota N, Kunikata H, Shiga Y, Omodaka K, Nakazawa T. Relationship between laser speckle flowgraphy and optical coherence tomography angiography measurements of ocular microcirculation. Graefes Arch Clin Exp Ophthalmol. 2017;255:1633–42.
Kiyota N, Kunikata H, Takahashi S, Shiga Y, Omodaka K, Nakazawa T. Factors associated with deep circulation in the peripapillary chorioretinal atrophy zone in normal-tension glaucoma with myopic disc. Acta Ophthalmol. 2018;96:e290–7.
Asaoka R, Sakata R, Yoshitomi T, Iwase A, Matsumoto C, Higashide T, et al. Differences in factors Associated with Glaucoma progression with lower normal intraocular pressure in Superior and Inferior halves of the Optic nerve head. Transl Vis Sci Technol. 2023;12:19.
Kiyota N, Shiga Y, Yasuda M, Aizawa N, Omodaka K, Tsuda S, et al. Sectoral differences in the Association of Optic Nerve Head Blood Flow and Glaucomatous Visual Field defect severity and progression. Invest Ophthalmol Vis Sci. 2019;60:2650–8.
Anderson DR, Patella VM. Automated Static Perimetry. 2nd ed. Mosby; 1999.
Jonas JB, Nguyen XN, Gusek GC, Naumann GO. Parapapillary chorioretinal atrophy in normal and glaucoma eyes. I. Morphometric data. Invest Ophthalmol Vis Sci. 1989;30:908–18.
Miura N, Omodaka K, Kimura K, Matsumoto A, Kikawa T, Takahashi S, et al. Evaluation of retinal nerve fiber layer defect using wide-field en-face swept-source OCT images by applying the inner limiting membrane flattening. PLoS ONE. 2017;12:e0185573.
Takahashi N, Omodaka K, Nakazawa A, Kikawa T, Ninomiya T, Kiyota N, et al. Correlation between enlargement of retinal nerve Fiber defect Angle in En Face Imaging and Visual Field Progression. Transl Vis Sci Technol. 2022;11:8.
Park HL, Kim JW, Park CK. Choroidal Microvasculature Dropout is Associated with Progressive Retinal nerve Fiber layer thinning in Glaucoma with disc hemorrhage. Ophthalmology. 2018;125:1003–13.
Lee EJ, Kim TW, Kim JA, Kim JA. Central visual field damage and Parapapillary Choroidal Microvasculature Dropout in Primary Open-Angle Glaucoma. Ophthalmology. 2018;125:588–96.
Son KY, Han JC, Kee C. Parapapillary deep-layer microvasculature dropout is only found near the retinal nerve fibre layer defect location in open-angle glaucoma. Acta Ophthalmol. 2022;100:e174–80.
Micheletti E, Moghimi S, Nishida T, El-Nimri N, Mahmoudinedzah G, Kamalipour A, et al. Factors associated with choroidal microvascular dropout change. Br J Ophthalmol. 2023;107:1444–51.
Orzalesi N, Rossetti L, Omboni S, Group OS, Conproso. Vascular risk factors in glaucoma: the results of a national survey. Graefes Arch Clin Exp Ophthalmol. 2007;245:795–802.
Costa VP, Arcieri ES, Harris A. Blood pressure and glaucoma. Br J Ophthalmol. 2009;93:1276–82.
Zhao D, Cho J, Kim MH, Guallar E. The association of blood pressure and primary open-angle glaucoma: a meta-analysis. Am J Ophthalmol. 2014;158:615–27. e9.
Akagi T, Zangwill LM, Shoji T, Suh MH, Saunders LJ, Yarmohammadi A, et al. Optic disc microvasculature dropout in primary open-angle glaucoma measured with optical coherence tomography angiography. PLoS ONE. 2018;13:e0201729.
Suzuki J, Tomidokoro A, Araie M, Tomita G, Yamagami J, Okubo T, et al. Visual field damage in normal-tension glaucoma patients with or without ischemic changes in cerebral magnetic resonance imaging. Jpn J Ophthalmol. 2004;48:340–4.
Tomita R, Iwase T, Ueno Y, Goto K, Yamamoto K, Ra E, et al. Differences in blood Flow between Superior and Inferior Retinal hemispheres. Invest Ophthalmol Vis Sci. 2020;61:27.
Toda R, Mochizuki H, Sone T, Kiuchi Y. Changes in optic disc shape and size in two patients with suspected glaucoma during a two- and three-year follow-up period. Jpn J Ophthalmol. 2010;54:94–6.
Jonas JB, Holbach L, Panda-Jonas S. Peripapillary arterial circle of Zinn-Haller: location and spatial relationships with myopia. PLoS ONE. 2013;8:e78867.
Midgett DE, Pease ME, Jefferys JL, Patel M, Franck C, Quigley HA, et al. The pressure-induced deformation response of the human lamina cribrosa: analysis of regional variations. Acta Biomater. 2017;53:123–39.
Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–6.
Saville DJ. Multiple comparison procedures: the practical solution. Am Stat. 1990;44:174–80.
Takahashi N, Omodaka K, Kikawa T, Ninomiya T, Kiyota N, Tsuda S, et al. Factors Associated with Visual Acuity decline in Glaucoma patients with loss of Ganglion Cell Complex Thickness. Transl Vis Sci Technol. 2023;12:2.
Acknowledgements
The authors thank Mr. Tim Hilts for editing this manuscript and Mr. Takehiro Miya of the Tohoku University Graduate School of Medicine for technical support. We would also like to express our sincere gratitude to Akiko Hanyuda, MD, PhD, MPH, for her support with statistical methods. Dr. Hanyuda is affiliated with both Keio University and Tohoku University.
Funding
This study was supported in part by a JST grant from JSPS Kakenhi Grants-in-Aid for Scientific Research (B) (T.N. 20H03838), by the JST Center for Revitalization Promotion and KAKENHI Grants-in-Aid for Scientific Research (C) (K.O. 21K09690), by COI-NEXT (JPMJPF2201), and by the Kitazawa Yoshiaki Glaucoma Research Award.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
N. Takahashi, None; K. Omodaka, None; T. Kikawa, Employee (Topcon); T. Ninomiya, None; N. Kiyota, None; S. Tsuda, None; T. Nakazawa, None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Author: Naoki Takahashi
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Takahashi, N., Omodaka, K., Kikawa, T. et al. Comparative features of superior versus inferior hemisphere microvasculature dropout in open-angle glaucoma. Jpn J Ophthalmol (2024). https://doi.org/10.1007/s10384-024-01071-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10384-024-01071-5