Abstract
Purpose
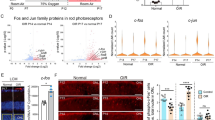
We aimed to analyze the changes in the visual cortex of a ferret model of ocular hypertension (OH) using cytochrome oxidase (CO) staining.
Study design
Experimental.
Methods
OH was induced in 9 ferrets by means of injection of cultured conjunctival cells into the anterior chamber of the right eye. Three ferrets were used as the controls. CO staining was performed to assess the metabolic intensity at the II-III and IVC layers of the visual cortex.
Results
The intensities of CO staining in the right and left II-III layers of the primary visual cortex (V1) in the OH ferrets were 39.8 ± 10.3 and 41.9 ± 9.2 arbitrary units, respectively. In the control ferrets, the intensity was 88.1 ± 8.1 arbitrary units. The intensity of CO staining of the II-III layers obtained from the OH eyes was significantly lower than that from the control eyes (unpaired t test, P < .01). The intensities of CO staining in the right and left IVC layers of V1 in the OH ferrets were 60.3 ± 12.8 and 60.0 ± 13.5 arbitrary units, respectively. In the control ferrets, the intensity was 111.4 ± 9.6 arbitrary units. The CO staining intensity of the IVC layer obtained from the OH eyes was significantly lower than that from the control eyes (unpaired t test, P < .01).
Conclusion
The CO staining intensity was reduced in the visual cortex from OH eyes. This study revealed that OH causes metabolic change in the visual cortex.





Similar content being viewed by others
References
Weinreb RN, Friedman DS, Fechtner RD, Cioffi GA, Coleman AL, Girkin CA, et al. Risk assessment in the management of patients with ocular hypertension. Am J Ophthalmol. 2004;138:458–67.
Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7.
Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998;126:498–505.
The Advanced Glaucoma Intervention Study (AGIS0). The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 2000;130:429–40.
Inoue K, Ishida K, Tomita G, Noma H. A scoping review and network meta-analysis for efficacy and safety of glaucoma medication in Japanese patients. Jpn J Ophthalmol. 2020;64:103–13.
Joh HJ, Jin SW. Comparison of different combinations of maximum medical therapy for lowering intraocular pressure in primary open angle glaucoma: 12-month retrospective consecutive case series. Jpn J Ophthalmol. 2019;63:322–7.
Omoto T, Fujishiro T, Asano-Shimizu K, Sugimoto K, Sakata R, Murata H, et al. Comparison of the short-term effectiveness and safety profile of ab interno combined trabeculotomy using 2 types of trabecular hooks. Jpn J Ophthalmol. 2020;64:407–13.
Aihara M, Kuwayama Y, Miyata K, Ohtani S, Ideta R, Hashimoto Y, et al. Twelve-month efficacy and safety of glaucoma filtration device for surgery in patients with normal-tension glaucoma. Jpn J Ophthalmol. 2019;63:402–9.
Gupta N, Ly T, Zhang Q, Kaufman PL, Weinreb RN, Yucel YH. Chronic ocular hypertension induces dendrite pathology in the lateral geniculate nucleus of the brain. Exp Eye Res. 2007;84:176–84.
Yücel Y, Zhang Q, Weinreb RN, Kaufman A, Gupta N. Effects of retinal ganglion cell loss on magno-, parvo-, koniocellular pathways in the lateral geniculate nucleus and visual cortex in glaucoma. Prog Retin Eye Res. 2003;22:465–81.
Ito Y, Shimazawa M, Chen YN, Tsuruma K, Yamashima T, Araie M, et al. Morphological changes in the visual pathway induced by experimental glaucoma in Japanese monkeys. Exp Eye Res. 2009;89:246–55.
Sasaoka M, Nakamura K, Shimazawa M, Ito Y, Araie M, Hara H. Changes in visual fields and lateral geniculate nucleus in monkey laser-induced high intraocular pressure model. Exp Eye Res. 2008;86:770–82.
Weber AJ, Zelenak D. Experimental glaucoma in the primate induced by latex microspheres. J Neurosci Methods. 2001;111:39–48.
Yücel YH, Zhang Q, Gupta N, Kaufman PL, Weinreb RN. Loss of neurons in magnocellular and parvocellular layers of the lateral geniculate nucleus in glaucoma. Arch Ophthalmol. 2000;118:378–84.
Fujishiro T, Kawasaki H, Aihara M, Saeki T, Ymagishi R, Atarashi T, et al. Establishment of an experimental ferret ocular hypertension model for the analysis of central visual pathway damage. Sci Rep. 2014;4:6501.
Fujishiro T, Honjo M, Kawasaki H, Asaoka R, Yamagishi R, Aihara M. Structural changes and astrocyte response of the lateral geniculate nucleus in a ferret model of ocular hypertension. Int J Mol Sci. 2020;21:1339.
Kawasaki H, Crowley JC, Livesey FJ, Katz LC. Molecular organization of the ferret visual thalamus. J Neurosci. 2004;24:9962–70.
Iwai L, Ohashi Y, van der List D, Usrey WM, Miyashita Y, Kawasaki H. FoxP2 is a parvocellular-specific transcription factor in the visual thalamus of monkeys and ferrets. Cereb Cortex. 2013;23:2204–12.
Dunn DG, Harris RK, Meis JM, Sweet DE. A histomorphologic and immunohistochemical study of chordoma in twenty ferrets (Mustela putorius furo). Vet Pathol. 1991;28:467–73.
Hoffmann KP, Garipis N, Distler C. Optokinetic deficits in albino ferrets (Mustela putorius furo): a behavioral and electrophysiological study. J Neurosci. 2004;24:4061–9.
Christopher D. Neuroanatomy of the ferret brain with focus on the cerebral cortex. In: Fox JG, Marini RP, eds. Biology and diseases of the ferret. 3rd ed. Wiley-Blackwell; 1998:69–80.
Mizuguchi K, Horiike T, Matsumoto N, Ichikawa Y, Shinmyo Y, Kawasaki H. Distribution and morphological features of microglia in the developing cerebral cortex of gyrencephalic mammals. Neurochem Res. 2018;43:1075–85.
Thompson ID, Morgan JE. The development of retinal ganglion cell decussation patterns in postnatal pigmented and albino ferrets. Eur J Neurosci. 1993;5:341–56.
Montiani-Ferreira F, Mattos BC, Russ HH. Reference values for selected ophthalmic diagnostic tests of the ferret (Mustela putorius furo). Vet Ophthalmol. 2006;9:209–13.
Sapienza JS, Porcher D, Collins BR, Gum GG, Brooks DE. Tonometry in clinically normal ferrets (Mustela putorius furo). Prog Vet Comp Ophthalmol. 1991;1:291–4.
Blanco R, Martinez-Navarrete G, Pérez-Rico C, Valiente-Soriano FJ, Aviles-Trigueros M, Vicente J, et al. A chronic ocular-hypertensive rat model induced by injection of the sclerosant agent polidocanol in the aqueous humor outflow pathway. Int J Mol Sci. 2019;20:3209.
Di Girolamo N, Andreani V, Guandalini A, Selleri P. Evaluation of intraocular pressure in conscious ferrets (Mustela putorius furo) by means of rebound tonometry and comparison with applanation tonometry. Vet Rec. 2013;172:396.
Hernández-Guerra AM, Rodilla V, López-Murcia MM. Ocular biometry in the adult anesthetized ferret (Mustela putorius furo). Vet Ophthalmol. 2007;10:50–2.
Cucchiaro JB. Early development of the retinal line of decussation in normal and albino ferrets. J Comp Neurol. 1991;312:193–206.
Lund JS. Anatomical organization of macaque monkey striate visual cortex. Annu Rev Neurosci. 1988;11:253–88.
Crawford ML, Harwerth RS, Smith EL 3rd, Shen F, Carter-Dawson L. Glaucoma in primates: cytochrome oxidase reactivity in parvo- and magnocellular pathways. Invest Ophthalmol Vis Sci. 2000;41:1791–802.
Crawford ML, Harwerth RS, Smith EL 3rd, Mills S, Ewing B. Experimental glaucoma in primates: changes in cytochrome oxidase blobs in V1 cortex. Invest Ophthalmol Vis Sci. 2001;42:358–64.
Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979;171:11–28.
Wong-Riley MTT. Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Trends Neurosci. 1989;12:94–101.
Wong-Riley MT, Hevner RF, Cutlan R, Earnest M, Egan R, Frost J, et al. Cytochrome oxidase in the human visual cortex: distribution in the developing and the adult brain. Vis Neurosci. 1993;10:41–58.
Wong-Riley MT, Tripathi SC, Trusk TC, Hoppe DA. Effect of retinal impulse blockage on cytochrome oxidase-rich zones in the macaque striate cortex: I. Quantitative electron-microscopic (EM) analysis of neurons. Vis Neurosci. 1989;2:483–97.
Wong-Riley MT, Trusk TC, Tripathi SC, Hoppe DA. Effect of retinal impulse blockage on cytochrome oxidase-rich zones in the macaque striate cortex: II. Quantitative electron-microscopic (EM) analysis of neuropil. Vis Neurosci. 1989;2:499–514.
Deyoe EA, Trusk TC, Wong-Riley MT. Activity correlates of cytochrome oxidase-defined compartments in granular and supragranular layers of primary visual cortex of the macaque monkey. Vis Neurosci. 1995;12:629–39.
Nakagawa A, Sakai O, Tokushige H, Fujishiro T, Aihara M. Development and characterization of a new rat ocular hypertension model induced by intracameral injection of conjunctival fibroblasts. Sci Rep. 2019;9:6593.
Zhou W, Muir ER, Nagi KS, Chalfin S, Rodriguez P, Duong TQ. Retinotopic fMRI reveals visual dysfunction and functional reorganization in the visual cortex of mild to moderate glaucoma patients. J Glaucoma. 2017;26:430–7.
Wang J, Li T, Sabel BA, Chen Z, Wen H, Li J, et al. Structural brain alterations in primary open angle glaucoma: a 3T MRI study. Sci Rep. 2016;6:18969.
Chen Z, Wang J, Lin F, Dai H, Mu K, Zhang H. Correlation between lateral geniculate nucleus atrophy and damage to the optic disc in glaucoma. J Neuroradiol. 2013;40:281–7.
Acknowledgments
This research was funded by the Japan Society for the Promotion of Science, kakenhi grant number JP 19K09946.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
T. Fujishiro, None; M. Honjo, None; H. Kawasaki, None; M. Aihara, None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Corresponding Author: Makoto Aihara
About this article
Cite this article
Fujishiro, T., Honjo, M., Kawasaki, H. et al. Visual cortex damage in a ferret model of ocular hypertension. Jpn J Ophthalmol 66, 205–212 (2022). https://doi.org/10.1007/s10384-022-00901-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-022-00901-8