Abstract
Purpose
To assess the 12-month efficacy and safety of fixed-combination brimonidine tartrate 0.2%/timolol maleate 0.5% (FCBT) with or without bimatoprost 0.01% (BIM) in primary open-angle glaucoma (POAG), including normal-tension glaucoma (NTG).
Study design
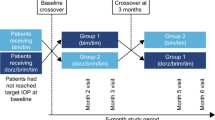
Prospective, multicenter, open-label study.
Methods
FCBT was self-administered twice daily after applicable washout (study eye). Intraocular pressure (IOP) was measured at baseline and months 1, 3, 6, 9, and 12. BIM could be added for IOP ≥ 21 mmHg, IOP reduction from baseline < 20%, or the investigator deemed it necessary. Primary endpoint: mean (11-a.m.) month-12 IOP change from baseline. Secondary endpoints included mean IOP changes from baseline at other visits, median time to achieving and patients (%) achieving target IOP reduction with FCBT, and visual field (VF) progression rate over 12 months. Safety was assessed at each visit.
Results
Of 118 eyes with POAG (NTG, n = 93), 87 used FCBT; 31 required FCBT + BIM. Mean IOP changes from baseline (16.8 and 15.3 mmHg) to month 12 were − 4.1 mmHg (FCBT, n = 62) and − 3.5 mmHg (FCBT + BIM, n = 15), respectively (both P < 0.0001). Patients who achieved target IOP reduction with FCBT did so in 1 month (median). VF progression rates were 0.17%/year (FCBT, P = 0.8367) and − 0.08%/year (FCBT + BIM, P = 0.9410). Ocular treatment-emergent adverse events occurred in 42.5% (FCBT) and 71.0% (FCBT + BIM) of patients; most were mild and included ocular hyperemia (9.2% and 41.9%, respectively).
Conclusions
Despite low mean baseline IOP, ≥ 20% IOP reduction from baseline persisted over 12 months with FCBT and FCBT + BIM, without clinically significant VF progression. Tolerability was consistent with reported drug safety profiles.






Similar content being viewed by others
References
Enoch J, McDonald L, Jones L, Jones PR, Crabb DP. Evaluating whether sight is the most valued sense. JAMA Ophthalmol. 2019;137:1317–20.
European Glaucoma Society. Terminology and guidelines for glaucoma (4th edition). https://bjo.bmj.com/content/bjophthalmol/101/4/1.full.pdf. Accessed 22 Dec 2020.
Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998;126:498–505.
Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126:487–97.
Burgoyne CF, Downs JC, Bellezza AJ, Suh JK, Hart RT. The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog Retin Eye Res. 2005;24:39–73.
Kass MA, Heuer DK, Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–13 (Discussion 829-30).
Craven ER, Walters TR, Williams R, Chou C, Cheetham JK, Schiffman R. Brimonidine and timolol fixed-combination therapy versus monotherapy: a 3-month randomized trial in patients with glaucoma or ocular hypertension. J Ocul Pharmacol Ther. 2005;21:337–48.
Sherwood MB, Craven ER, Chou C, DuBiner HB, Batoosingh AL, Schiffman RM, et al. Twice-daily 0.2% brimonidine-0.5% timolol fixed-combination therapy vs monotherapy with timolol or brimonidine in patients with glaucoma or ocular hypertension: a 12-month randomized trial. Arch Ophthalmol. 2006;124:1230–8.
Lewis RA, Gross RL, Sall KN, Schiffman RM, Liu CC, Batoosingh AL. The safety and efficacy of bimatoprost/timolol fixed combination: a 1-year double-masked, randomized parallel comparison to its individual components in patients with glaucoma or ocular hypertension. J Glaucoma. 2010;19:424–6.
Realini T, Nguyen QH, Katz G, DuBiner H. Fixed-combination brinzolamide 1%/brimonidine 0.2% vs monotherapy with brinzolamide or brimonidine in patients with open-angle glaucoma or ocular hypertension: results of a pooled analysis of two phase 3 studies. Eye (Lond). 2013;27:841–7.
Brandt JD, Cantor LB, Katz LJ, Batoosingh AL, Chou C, Bossowska I. Bimatoprost/timolol fixed combination: a 3-month double-masked, randomized parallel comparison to its individual components in patients with glaucoma or ocular hypertension. J Glaucoma. 2008;17:211–6.
Higginbotham EJ, Olander KW, Kim EE, Grunden JW, Kwok KK, Tressler CS. Fixed combination of latanoprost and timolol vs individual components for primary open-angle glaucoma or ocular hypertension: a randomized, double-masked study. Arch Ophthalmol. 2010;128:165–72.
Whitson JT, Realini T, Nguyen QH, McMenemy MG, Goode SM. Six-month results from a Phase III randomized trial of fixed-combination brinzolamide 1% + brimonidine 0.2% versus brinzolamide or brimonidine monotherapy in glaucoma or ocular hypertension. Clin Ophthalmol. 2013;7:1053–60.
Aung T, Laganovska G, Hernandez Paredes TJ, Branch JD, Tsorbatzoglou A, Goldberg I. Twice-daily brinzolamide/brimonidine fixed combination versus brinzolamide or brimonidine in open-angle glaucoma or ocular hypertension. Ophthalmology. 2014;121:2348–55.
Yamamoto T, Ikegami T, Ishikawa Y, Kikuchi S. Randomized, controlled, phase 3 trials of carteolol/latanoprost fixed combination in primary open-angle glaucoma or ocular hypertension. Am J Ophthalmol. 2016;171:35–46.
Goñi FJ, Brimonidine/Timolol Fixed Combination Study Group. 12-week study comparing the fixed combination of brimonidine and timolol with concomitant use of the individual components in patients with glaucoma and ocular hypertension. Eur J Ophthalmol. 2005;15:581–90.
Prum B, American Academy of Ophthalmology. Preferred Practice Patterns − Primary open-angle glaucoma. Ophthalmology. 2016;123:P41–111.
Spaeth GL, Bernstein P, Caprioli J, Schiffman RM. Control of intraocular pressure and fluctuation with fixed-combination brimonidine-timolol versus brimonidine or timolol monotherapy. Am J Ophthalmol. 2011;151:93-9.e4.
Kim JM, Kim TW, Kim CY, Kim HK, Park KH. Comparison of the intraocular pressure-lowering effect and safety of brimonidine/timolol fixed combination and 0.5% timolol in normal-tension glaucoma patients. Jpn J Ophthalmol. 2016;60:20–6.
Cho SW, Kim JM, Park KH, Choi CY. Effects of brimonidine 0.2%-timolol 0.5% fixed-combination therapy for glaucoma. Jpn J Ophthalmol. 2010;54:407–13.
Rudnicka AR, Mt-Isa S, Owen CG, Cook DG, Ashby D. Variations in primary open-angle glaucoma prevalence by age, gender, and race: a Bayesian meta-analysis. Invest Ophthalmol Vis Sci. 2006;47:4254–61.
Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7.
Cho HK, Kee C. Population-based glaucoma prevalence studies in Asians. Surv Ophthalmol. 2014;59:434–47.
Kim JH, Kang SY, Kim NR, Lee ES, Hong S, Seong GJ, et al. Prevalence and characteristics of glaucoma among Korean adults. Korean J Ophthalmol. 2011;25:110–5.
Allergan Korea Ltd. Combigan® (Brimonidine tartrate/timolol maleate 0.2%/0.68 % ophthalmic solution) Product Information. 2014. https://nedrug.mfds.go.kr/pbp/CCBBB01/getItemDetail?itemSeq=200700952. Accessed 25 Mar 2020.
Mathew A, Pandey M, Murthy NS. Survival analysis: caveats and pitfalls. Eur J Surg Oncol. 1999;25:321–9.
SAS Institute. SAS/STAT® 9.3 User’s Guide: the LIFETEST procedure (Chapter). NC, USA: Cary; 2011.
Cho JW, Sung KR, Yun SC, Na JH, Lee Y, Kook MS, et al. Progression detection in different stages of glaucoma: mean deviation versus visual field index - A visual field index for calculation of glaucoma rate of progression. Jpn J Ophthalmol. 2012;56:128–33.
Bengtsson B, Heijl A. A visual field index for calculation of glaucoma rate of progression. Am J Ophthalmol. 2008;145:343–53.
Allergan plc. Highlights of prescribing information - Lumigan® 0.01% (bimatoprost ophthalmic solution). 2017. https://media.allergan.com/actavis/actavis/media/allergan-pdf-documents/product-prescribing/20170729-LUMIGAN-0-01%25-USPI-71807US15.pdf. Accessed 22 Dec 2020.
Allergan plc Combigan® (brimonidine tartrate/timolol maleate ophthalmic solution) 0.2%/0.5% - highlights of prescribing information. 2015. https://media.allergan.com/actavis/actavis/media/allergan-pdf-documents/product-prescribing/20151015-Combigan-USPI-72060US15.pdf. (Accessed 6 Jan 2020)
Higginbotham EJ. Considerations in glaucoma therapy: fixed combinations versus their component medications. Clin Ophthalmol. 2010;4:1–9.
Joh HJ, Jin SW. Comparison of different combinations of maximum medical therapy for lowering intraocular pressure in primary open angle glaucoma: 12-month retrospective consecutive case series. Jpn J Ophthalmol. 2019;63:322–7.
Heijl A, Leske MC, Bengtsson B, Hyman L, Bengtsson B, Hussein M, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–79.
Doughty MJ, Zaman ML. Human corneal thickness and its impact on intraocular pressure measures: a review and meta-analysis approach. Surv Ophthalmol. 2000;44:367–408.
Dueker DK, Singh K, Lin SC, Fechtner RD, Minckler DS, Samples JR, et al. Corneal thickness measurement in the management of primary open-angle glaucoma: a report by the American Academy of Ophthalmology. Ophthalmology. 2007;114:1779–87.
Gaspar R, Pinto LA, Sousa DC. Corneal properties and glaucoma: a review of the literature and meta-analysis. Arq Bras Oftalmol. 2017;80:202–6.
Guo ZZ, Chang K, Wei X. Intraocular pressure fluctuation and the risk of glaucomatous damage deterioration: a meta-analysis. Int J Ophthalmol. 2019;12:123–8.
Acknowledgements
This study was sponsored by Allergan (prior to its acquisition by AbbVie). The funding body was involved in the study design, data analysis and interpretation, manuscript revision for intellectual content, and decision to submit the manuscript for publication. Writing and editorial assistance were provided to the authors by Michele Jacob, PhD, CMPP, of Evidence Scientific Solutions, Philadelphia, PA, and funded by Allergan, an AbbVie company. Neither honoraria nor payments were made for authorship. Assistance with study monitoring and statistical/data management was provided by LSK Global Pharma Services Co., Ltd. The authors also thank Youkyung Lee, MD (employee of Allergan at the time the study was conducted) for her contribution to study protocol and clinical study report. Study group investigators: Ki Ho Park (Seoul National University Hospital), Tae-Woo Kim (Seoul National University Bundang Hospital), Joon Mo Kim (Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine), Yung Hoon Hwang (Kim’s Eye Hospital), Chan Kee Park (The Catholic University of Korea Seoul St. Mary’s Hospital), Kyoung Nam Kim (Chungnam National University Hospital), Soon Cheol Cha (Yeungnam University Medical Center), Sang Woo Park (Chonnam National University Hospital), Ji Woong Lee (Pusan National University Hospital).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The conflicts of interest for S. W. Park, None; J. M. Kim, None; J. W. Lee, None; J. Maglambayan, Employee (AbbVie); S. Simonyi, Employee (AbbVie); K. H. Park, None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This work was partially presented at the 122nd Annual Meeting of the Korean Ophthalmology Society (KOS) 2019 meeting (November 1–3, 2019, Seoul, Korea), Association for Research in Vision and Ophthalmology (ARVO) 2019 Annual Meeting (April 28–May 2, 2019, Vancouver, Canada), and Controversies in Ophthalmology 5th Asia-Australia (COPHy–AA) Congress (February 22 –23, 2019, Shanghai, China).
Corresponding Author: Ki Ho Park.
About this article
Cite this article
Park, S.W., Kim, J.M., Lee, J.W. et al. Efficacy and safety of fixed-combination brimonidine tartrate/timolol maleate in primary open-angle glaucoma, including normal-tension glaucoma. Jpn J Ophthalmol 65, 295–305 (2021). https://doi.org/10.1007/s10384-020-00796-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-020-00796-3