Abstract
Purpose
To evaluate the clinical efficacy of 3 % diquafosol sodium ophthalmic solution for dry eye, and to analyze the concentration of tear proteins and mucin-like substances after the treatment.
Methods
Fifty eyes of 25 patients with dry eye syndrome were prospectively enrolled. The patients were treated with diquafosol solution at a dose of 1 drop in each eye 6 times daily for 4 weeks. The parameters of clinical efficacy were tear osmolarity, tear breakup time (BUT), fluorescein staining scores for the cornea and conjunctiva, Schirmer test values, and subjective symptoms evaluated using the ocular surface disease index (OSDI). Tears collected with Schirmer test strips were analyzed by high-performance liquid chromatography, and the concentrations of the total protein and the 4 major tear proteins, namely, secretory IgA, lactoferrin, lipocalin-1, lysozyme, and N-acetyl-neuraminic acid (Neu5Ac), were measured. Neu5Ac is a major sialic acid, a marker of secretory mucins.
Results
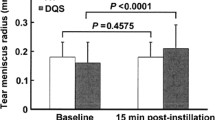
The BUT, keratoconjunctival staining scores, and Schirmer test values were improved with statistical significance after the treatment with diquafosol solution, while changes in the other parameters, including tear osmolarity, corneal staining scores, and OSDI scores were not significant. The Neu5Ac concentration was significantly increased, which was not accompanied by changes in tear proteins.
Conclusions
Topical application of diquafosol significantly improved the clinical parameters of the BUT, keratoconjunctival staining scores, and Schirmer test values and was accompanied by increased sialic acid content in the tears of patients with dry eye.
Similar content being viewed by others
References
Pflugfelder SC, Geerling G, Kinoshita S, Lemo MA, McCulley J, Nelson D, et al. Management and therapy of dry eye disease: report of the Management and Therapy Subcommittee of the International Dry Eye Workshop (2007). Ocul Surf. 2007;5:163–78.
Nichols KK, Yerxa B, Kellerman DJ. Diquafosol tetrasodium: a novel dry eye therapy. Expert Opin Investig Drugs. 2004;13:47–54.
Cowlen MS, Zhang VZ, Warnock L, Moyer CF, Peterson WM, Yerxa BR. Localization of ocular P2Y2 receptor gene expression by in situ hybridization. Exp Eye Res. 2003;77:77–84.
Murakami T, Fujihara T, Horibe Y, Nakamura M. Diquafosol elicits increases in net Cl-transport through P2Y2 receptor stimulation in rabbit conjunctiva. Ophthalmic Res. 2004;36:89–93.
Takaoka-Shichijyou Y, Murakami T, Nakamura M. Stimulatory effect of diquafosol tetrasodium on tear fluid secretion in normal rabbits [in Japanese]. Atarashii Ganka. 2011;28:1029–33.
Jumblatt JE, Jumblatt MM. Regulation of ocular mucin secretion by P2Y2 nucleotide receptors in rabbit and human conjunctiva. Exp Eye Res. 1998;67:341–6.
Fujihara T, Murakami T, Nagano T, Nakamura M, Nakata K. INS365 suppresses loss of corneal epithelial integrity by secretion of mucin-like glycoprotein in a rabbit short-term dry eye model. J Ocul Pharmacol Ther. 2002;18:363–70.
Nakamura M, Imanaka T, Sakamoto A. Diquafosol ophthalmic solution for dry eye treatment. Adv Ther. 2012;29:579–89.
Takamura E, Tsubota K, Watanabe H, Ohashi Y. A randomised, double-masked comparison study of diquafosol versus sodium hyaluronate ophthalmic solutions in dry eye patients. Br J Ophthalmol. 2012;96:1310–5.
Matsumoto Y, Ohashi Y, Watanabe H, Tsubota K. Efficacy and safety of diquafosol ophthalmic solution in patients with dry eye syndrome: a Japanese phase 2 clinical trial. Ophthalmology. 2012;119:1954–60.
Tauber J, Davitt WF, Bokosky JE, Nichols KK, Yerxa BR, Schaberg AE, et al. Double-masked, placebo-controlled safety and efficacy trial of diquafosol tetrasodium (INS365) ophthalmic solution for the treatment of dry eye. Cornea. 2004;23:784–92.
Koh S, Maeda N, Ikeda C, Oie Y, Soma T, Tsujikawa M, et al. Effect of diquafosol ophthalmic solution on the optical quality of the eyes in patients with aqueous-deficient dry eye. Acta Ophthalmol. 2014;92:e671–5.
Arita R, Suehiro J, Haraguchi T, Maeda S, Maeda K, Tokoro H, et al. Topical diquafosol for patients with obstructive meibomian gland dysfunction. Br J Ophthalmol. 2013;97:725–9.
Yokoi N, Kato H, Kinoshita S. Facilitation of tear fluid secretion by 3% diquafosol ophthalmic solution in normal human eyes. Am J Ophthalmol. 2014;157:85–92.
Shigeyasu C, Hirano S, Akune Y, Yamada M. Diquafosol tetrasodium increases the concentration of mucin-like substances in tears of healthy human subjects. Curr Eye Res. 2014;. doi:10.3109/02713683.2014.967871.
Hwang HS, Sung YM, Lee WS, Kim EC. Additive effect of preservative-free sodium hyaluronate 0.1% in treatment of dry eye syndrome with diquafosol 3% eye drops. Cornea. 2014;33:935–41.
Shimazaki J, Tsubota K, Kinoshita S, Ohashi Y, Shimomura Y, Tagawa Y, et al. Definition and diagnosis of dry eye 2006 [in Japanese]. Atarashii Ganka. 2007;24:181–4.
Lemp MA, Baudouin C, Baum J, Dogru M, Foulks GN, Kinoshita S, et al. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Workshop (2007). Ocul Surf. 2007;5:75–92.
Shimmura S, Ono M, Shinozaki K, Toda I, Takamura E, Mashima Y, et al. Sodium hyaluronate eyedrops in the treatment of dry eyes. Br J Ophthalmol. 1995;79:1007–11.
Koh S, Watanabe H, Hosohata J, Hori Y, Hibino S, Nishida K, et al. Diagnosing dry eye using a blue-free barrier filter. Am J Ophthalmol. 2003;136:513–9.
van Bijsterveld OP. Diagnostic tests in the sicca syndrome. Arch Ophthalmol. 1969;82:10–4.
Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118:615–21.
Yamada M, Mochizuki H, Kawai M, Tsubota K, Bryce TJ. Decreased tear lipocalin concentration in patients with meibomian gland dysfunction. Br J Ophthalmol. 2005;89:803–5.
Shigeyasu C, Hirano S, Akune Y, Mochizuki H, Yamada M. Evaluation of the frequency of ophthalmic solution application: washout effects of topical saline application on tear components. Curr Eye Res. 2013;38:722–8.
Yasueda S, Yamakawa K, Nakanishi Y, Kinoshita M, Kakehi K. Decreased mucin concentrations in tear fluids of contact lens wearers. J Pharm Biomed Anal. 2005;39:187–95.
Nakamura Y, Yokoi N, Tokushige H, Kinoshita S. Sialic acid in human tear fluid decreases in dry eye. Jpn J Ophthalmol. 2004;48:519–23.
Kinoshita S, Kiorpes TC, Friend J, Thoft RA. Goblet cell density in ocular surface disease: a better indicator than tear mucin. Arch Ophthalmol. 1983;101:1284–7.
Ralph RA. Conjunctival goblet cell density in normal subjects and in dry eye syndromes. Invest Ophthalmol. 1975;14:299–302.
Corrales RM, de Paiva CS, Li DQ, Farley WJ, Henriksson JT, Bergmanson JP, et al. Entrapment of conjunctival goblet cells by desiccation-induced cornification. Invest Ophthalmol Vis Sci. 2011;52:3492–9.
Argueso P, Balaram M, Spurr-Michaud S, Keutmann HT, Dana MR, Gipson IK. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjogren syndrome. Invest Ophthalmol Vis Sci. 2002;43:1004–11.
Danjo Y, Watanabe H, Tisdale AS, George M, Tsumura T, Abelson MB, et al. Alteration of mucin in human conjunctival epithelia in dry eye. Invest Ophthalmol Vis Sci. 1998;39:2602–9.
Gipson IK, Hori Y, Argueso P. Character of ocular surface mucins and their alteration in dry eye disease. Ocul Surf. 2004;2:131–48.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
C. Shigeyasu, None; M. Yamada, None; Y. Akune, None; K. Tsubota, Lecture fees (Santen Pharmaceutical Co., Ltd.).
About this article
Cite this article
Shigeyasu, C., Yamada, M., Akune, Y. et al. Diquafosol sodium ophthalmic solution for the treatment of dry eye: clinical evaluation and biochemical analysis of tear composition . Jpn J Ophthalmol 59, 415–420 (2015). https://doi.org/10.1007/s10384-015-0408-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10384-015-0408-y