Summary
Patients from the general practice who had severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) infection between January 2020 and March 2021 participated in an online survey to report their symptoms. This observational study describes the broad range of mild to moderate symptoms experienced by 160 symptom-based coronavirus disease 2019 (COVID-19) nonhospitalized patients, including 89 laboratory-confirmed cases. Compared to confirmed cases, a higher number of presumed and suspected patients had sore throat, shortness of breath, tightness in the chest, fatigue, or severe neck tension. Unexpected COVID-19-associated clinical features, such as alteration of blood consistency, hormonal imbalance, increased muscle tone and/or aches of skeletal muscles, joint pain, or dermatological disorders were observed by the participants. In the early period of the pandemic, COVID-19 diagnosis of patients was based on medical symptoms rather than polymerase chain reaction (PCR) or serological testing. These real-world data are essential to understand the pathophysiology of this virus infection and to develop innovative therapeutic approaches.
Zusammenfassung
Patienten der Arztpraxis, die zwischen Januar 2020 und März 2021 eine SARS-CoV-2(„severe acute respiratory syndrome coronavirus type 2“)-Infektion durchgemacht haben, berichteten in einem Online-Fragebogen über ihre Symptome. Die vorliegende Beobachtungsstudie zeigt das breite Spektrum der COVID-19 („coronavirus disease 2019“)-Symptome bei 160 nichthospitalisierten COVID-19-Patienten – davon 89 laborbestätigte Fälle – mit milder bis mittelschwerer Erkrankung auf. Im Vergleich zu den bestätigten Fällen zeigte ein höherer Anteil bei den Verdachtsfällen die Symptome Halsweh, Atemnot, Engegefühl in der Brust, Müdigkeit und schwere Nackenverspannung. Unter den Probanden sind unerwartete COVID-19-assoziierte Krankheitsbilder, wie Viskositätsänderungen des Bluts, hormonelle Dysbalancen, ein erhöhter, schmerzhafter Muskeltonus der Skelettmuskulatur, Gelenkschmerzen sowie dermatologische Beschwerden beobachtet worden. In der Frühphase der Pandemie erfolgte die Diagnose einer COVID-19-Infektion anhand der spezifischen klinischen Symptome anstatt durch eine Polymerasekettenreaktion (PCR) oder Antikörpertestung. Diese Daten aus der Praxis sind zum Verständnis der Pathophysiologie dieser Virusinfektion unerlässlich sowie zur Entwicklung innovativer Konzepte für präventive und therapeutische Ansätze.
Similar content being viewed by others
Introduction
Up until 2019, six pathogenic human coronaviruses (HCoV) were known to be responsible for infections of the respiratory system. Whereas some of these HCoVs mainly caused mild respiratory tract infections, two additional epidemic coronaviruses were responsible for new disease patterns named severe acute respiratory syndrome (SARS) and middle east respiratory syndrome (MERS) [1]. The latter two HCoVs caused the pandemic in 2002–2003 (SARS-CoV-1) and the outbreak in 2012 (MERS CoV) [2]. SARS is categorized through the following main symptoms: fever (in 100% of affected patients), cough (65%), myalgia (60%), cold (50%), tachycardia (46%), dyspnea (45%), tachypnea (39%), and headache (38%) [3]. In addition to these symptoms, the most common initial radiographic abnormalities are residual ground-glass opacities which have been noted on follow-up chest radiographs (x-ray) and computed tomography (CT) scans in 80–95% of patients. Furthermore, changes in laboratory findings (lymphocytopenia, thrombocytopenia, increased D‑dimers level, alteration of coagulation parameters) have also been described [3].
Since 2019, a novel coronavirus called severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) has been responsible for the clinical picture of a severe acute respiratory syndrome and described as the source for the coronavirus disease 2019 (COVID-19), which was declared as a pandemic by the World Health Organisation (WHO) in March 2020. Whereas approximately 14% of COVID-19 infected patients present a severe spectrum of the disease (hospitalization for respiratory distress and oxygen requirement) and 5% are critically ill (hospitalized for more than 30 days with ventilator use and tracheal intubation), the vast majority of infected individuals (81%) experience a mild to moderate severity of symptoms not requiring hospitalization [4].
Among the affected patients, COVID-19 was first characterized by acute respiratory distress, similar to the ARDS observed with SARS-CoV‑1 [3]. According to the Robert Koch Institute (RKI) in Germany, the leading symptoms of a COVID-19 infection are cough (in 40% of patients), cold (28%), fever (27%), smell and taste disorders (21%), and pneumonia (1%) [5]. Of note, olfactory and gustatory dysfunctions in mild COVID-19 forms were recognized as leading symptoms relatively late after the beginning of the pandemic [6, 7]. Moreover, additional nonspecific symptoms related to this viral disease have been also described [5].
Concerning the diagnostic methods, real-time polymerase chain reaction (RT-PCR) has been universally used to detect SARS-CoV‑2 RNA, whereas the purpose of serological testing is the detection of antibodies against COVID-19 [8].
As first reported with this new coronavirus infection, so-called “long COVID” cases have been described as follows: patients who were suspected of having COVID-19, regardless of whether they had been tested or not, and who had still not recovered several weeks or months following the start of symptoms [9]. Those patients show very different patterns of clinical presentations, and so far, no consistent definition for the prolonged symptoms or delayed effects exists [10]. For instance, long COVID patients might be characterized as presenting not only respiratory symptoms, but also neurological, psychological or gastrointestinal symptoms, as well as dermatological disorders [9].
The aims of this observational study are (i) to describe the entire range of symptoms associated with a COVID-19 infection, (ii) to compare the reported symptoms in patients with a confirmed, a presumed or suspected COVID-19 infection, (iii) to report on unexpected symptoms and signs observed in our COVID-19 patients, and (iv) to formulate a hypothesis based on our observations on the pathophysiology of the COVID-19 disease, which shows a new and independent disease pattern compared to other previously described coronavirus-associated infections.
Materials and methods
Study design
An observational study was conducted among adults and children through an online survey designed by a general medical practice in Vienna (Austria) in German and English. All patients had become ill with COVID-19 between January 2020 and March 2021 and self-reported through an online questionnaire their disease-related symptoms. All our confirmed, presumed, or suspected COVID-19 cases were invited to participate in this observational study; the response rate reached 90%. The general aim of this study was to describe the broad spectrum of typical and atypical symptoms and signs experienced by nonintensive care patients with mild or moderate disease severity.
Study population
No study selection criteria were applicable. It should be noted that none of our patients required hospitalization, as they presented a broad range of mild to moderate symptoms. The patients were initially considered to have a COVID-19 infection based on their clinical symptoms (symptom-based medical diagnosis); depending upon whether a diagnostic test for SARS-CoV‑2 was conducted and on its outcome, the patients were then classified into three groups: (i) confirmed cases were tested patients who had at least one positive diagnostic test (polymerase-chain reaction [PCR] or antibody tests); (ii) presumed cases were patients tested negative in PCR or antibody tests; (iii) suspected cases were patients who were never tested.
Data collection
Participation in the online survey allowed patients to self-report their clinical observations during the acute phase of their COVID-19 disease as well as in the weeks thereafter. The survey was open online between June 1, 2020, and March 31, 2021. Patients who experienced COVID-19 infection between January 1, 2020, and March 31, 2021 were invited to complete the online questionnaire in the weeks or months following their illness. The data collected included self-reported demographics and clinical symptom-based characteristics of a COVID-19 infection.
Ethical considerations
This observational study was conducted in accordance with the principles of Good Clinical Practice and following the Declaration of Helsinki. As the participation in the questionnaire was voluntary, patients’ consent was implicit; for pediatric patients, consent was collected from at least one parent prior to completing the survey. Most outcomes are presented in an aggregated form; for patients’ pictures, written consent for publication has been obtained. Participants’ privacy and confidentiality was guaranteed following European laws and regulations (General Data Protection Regulation [GDPR]).
Statistics
Demographics and clinical characteristics were summarized using descriptive statistics. For the quantitative description of the data, the following indicators were used: mean, range, number, and percentage.
Results
Study population
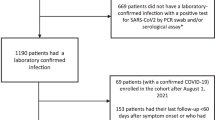
In total, 160 patients presenting with a COVID-19 symptom-based medical diagnosis between January 2020 and March 2021, who participated in the online survey, were documented in this observational study. All patients affected by COVID-19 presented mild to moderate disease severity; only 3 patients (2%) presented unusual findings after x‑ray or CT scans in the form of monolateral or bilateral atypical pneumonia and 3 patients (2%) had elevated CRP (C-reactive protein) levels. Fig. 1 presents the period of their first COVID-19 symptoms appearance. Among the patients visiting our general practice in Vienna, a first wave of 69 patients (43%) with a COVID-19 infection between January and April 2020 (peak in March 2020, 51 patients, 32%) was observed, followed by a second wave of 70 affected patients (44%) between September and December 2020 (peak in November 2020, 26 patients, 16%). Patients who reported having felt sick in January 2020 in Austria mentioned having travelled to COVID-19-suspected endemic regions—e.g., Maldives, New York (USA) or northern Italy—shortly before the appearance of the first symptoms.
At the time of COVID-19 diagnosis, the mean age of the overall analyzed participants was 46 years (range 3–83 years), with 69% aged between 31 and 60 years old (Table 1). Participants were predominantly female (n = 123, 77%); the elevated number of women might be, however, explained by the gynecologic specialty of our practice. Only 5 children (below 18 of age) were among our patient pool. Among the COVID-19 affected respondents, the more frequent blood groups were O (n = 59, 42%) and A (n = 54, 38%), while a positive rhesus was present in 78% (n = 80) of them.
Respondents were analyzed in three subgroups: 89 confirmed cases (56%) who had at least one positive diagnostic test (PCR or antibody test); 38 presumed cases (24%) with symptom-based medical diagnosis (negative PCR or antibody tests); and 33 suspected cases (approximately 20%) with symptom-based medical diagnosis but no diagnostic test performed. When considering the three patient subgroups, no relevant demographic difference was observed compared to the overall patient pool, except that more women (92%) were observed among the presumed cases than in the overall respondent pool.
Symptoms experienced by all respondents
Most of the COVID-19-affected patients (n = 86, 55%) had symptoms lasting for less than 30 days, and up to 60 days in 22% of them (n = 35; Fig. 2). Long-term consequences of COVID-19 symptoms were experienced in 23% (n = 36) of affected patients: 15 patients up to 90 days and 21 for more than 90 days. Among the 21 patients experiencing symptoms for more than 90 days, the mean age was 45 years (range 19–70); those long-COVID patients had BMI (body mass index) in a normal range and did not have relevant pre-existing comorbidities (data not shown).
All clinical symptoms experienced by the symptom-based COVID-19 patients are categorized in Table 2; in addition, the main symptoms presented by more than 35% of the patients are summarized in Fig. 3. Overall, a high proportion of affected patients (n = 108, 68%) reported a phased appearance of symptoms with patients feeling better for a couple of days and then falling ill again (relapse), as well as symptoms coming in intervals, attacks “out of nowhere” or unexpectedly (n = 92, 58%); most of them (n = 102, 64%) described never having experienced such symptoms before.
Regarding the upper respiratory tract, patients reported a sore throat (n = 102, 64%) with a dry irritable cough (n = 80, 50%) and a feeling of dryness in the mouth and throat (n = 83, 52%), while having paradoxically nasal congestion (n = 74, 46%) or mucus in the throat and paranasal sinus (n = 68, 42%). One of the key symptoms associated with a mild progression of the disease was a change (dysosmia) or loss (anosmia) of smell (n = 75, 47%), which, in some cases, was the only symptom reported.
For the lower respiratory tract/lung symptoms, the common symptom was cough (n = 105, 66%). It typically appeared as either a nonproductive cough, or a cough with a foamy white mucus difficult to expectorate. Key pulmonal symptoms were shortness of breath (dyspnea) and labored breathing (n = 95, 59%). Further, patients described difficult and/or blocked deep inhalation (n = 76, 47%), talking causing exhaustion (n = 67, 42%), a feeling of lack of oxygen (n = 62, 39%) or feeling of pressure behind the breastbone (retrosternal; n = 61, 38%), a tightness in the chest as though a heavy object lays on the chest (n = 60, 37%) as well as panic, anxiety, and an increased heart rate (n = 46, 29%).
Regarding the cardiovascular symptoms, the most frequently reported were the tendency to circulatory collapse, dizziness, vertigo (n = 73, 46%), as well as changes in pulse rate (n = 52, 32%) or in blood pressure, i.e., newly increased blood pressure (n = 28, 17%) which was difficult to adjust for weeks.
Digestive manifestations experienced were nausea (n = 76, 47%), a strong feeling of thirst (n = 67, 42%), noticeable weight loss (n = 68, 43%) in a short time (mostly fat and muscle mass reduction, despite appetite), and abdominal pain (n = 67, 42%). The key symptom in this category was a change (dysgeusia) or loss (ageusia) of taste (n = 81, 51%) in all its variations, ranging from metallic taste (n = 47, 29%), bland sticky taste (n = 62, 39%) to a feeling of disgust (n = 37, 23%).
A large majority of COVID-19 patients reported having constitution problems. The leading symptom was fatigue, lethargy, extreme exhaustion, and sleep attacks (n = 104, 65%). Especially repeated tiredness after getting up in the morning and/or shortly after breakfast (n = 85, 53%), fatigue attacks after lunch (n = 83, 52%) or increased fatigue in the late afternoon (n = 82, 51%) or a necessity to lie down/falling asleep while sitting (n = 74, 46%) were mentioned. The body temperature altered from feeling cold with the desire for extreme heat supply (such as a hot shower or hot water bottle; n = 73, 46%), to fever (n = 66, 41%) and extreme temperature fluctuations (n = 54, 34%). Night sweating was also stated by 70 patients (44%).
Another key indicator of the neurologic system was headache (n = 118, 74%), from mild to extremely strong, often combined with severe neck tension, in most cases worse on the right side (n = 70, 44%). COVID-19 patients also suffered from mental symptoms, the most severe reported being restlessness (n = 91, 57%), concentration difficulties/forgetfulness (“brain fog”; n = 85, 53%), sleeping disorders/insomnia (n = 70, 44%) or sadness/emotional distress/depression (n = 63, 39%).
Besides the organic systems, the eyes (worsening of eyesight in 59 patients, 37%), as well as the urogenital tract (interruption in menstrual cycle in 31 patients [25%] and worsening of menstrual disorders in 22 patients [18%]) were affected.
The most common alterations concerning the skin appeared both in the acute phase (< 30 days) as well as among patients presenting long-term symptoms, as already described previously [11]; they included mostly general itching (n = 40, 25%), but also blisters, burning sensations of the skin, changes in the vascular lymphatic system (hematoma, edema) and skin rashes.
Looking at the extremities, the definition of “COVID joints” has been developed [12]; it describes a painful swelling, especially in the small joints (toes and fingers) but also in other joints like elbow, shoulder, and knee. Usually, the exposure to heat (hot shower, hot water bottle) brought relief to these symptoms. A great number of patients reported having muscle aches (n = 65, 41%) or muscle weakness in the extremities (n = 45, 28%).
Independent of the manifestations asked in the online survey, we also observed in our practice an increased number of urinary tract infections or hair loss among our COVID-19 patients (about 1/3 of patients), especially in those experiencing long-term symptoms.
Clinical characteristics and diagnostic testing
We analyzed our symptom-based COVID-19 patients according to the results of their SARS-CoV‑2 tests (confirmed or presumed cases) or the absence of testing (suspected cases; Table 2). To assess whether the validity of the COVID-19 tests can be used as the only diagnostic criterium, we compared the symptoms self-reported by all patients to those of the three categories previously defined (see also “Materials and methods” section). Some differences and similarities were observed in the proportion of patients presenting specified symptoms among confirmed, presumed or suspected cases; nevertheless, the percentage of the main COVID-19 symptoms previously reported by respondents were nearly equally distributed in each of the three groups, showing only minor differences for the following symptoms: those which come in intervals and attacks “out of the nowhere”, shortness of breath, vertigo, strong feeling of thirst, headache, mental symptoms, fatigue and muscle aches, as highlighted in Table 2. The results showed that nontested patients (33 suspected cases) presented a remarkably similar disease pattern compared to the confirmed cases.
More surprisingly, one of the key symptoms—change (dysosmia) or loss of smell (anosmia)—was much more frequently observed in confirmed cases (n = 53, 59%) compared to presumed cases (n = 14, 37%) or suspected cases (n = 8, 24%). Similarly, change (dysgeusia) or loss of taste (ageusia) was reported in 60% (n = 53) of confirmed cases, 47% (n = 14) of presumed cases, and 30% (n = 10) of suspected cases. Demonstratively, all symptoms related to smelly excretion—like urine, feces and sweat—were much more represented in presumed and suspected cases compared to confirmed ones. Diarrhea with a strange unknown smell was reported by only 6% of confirmed cases, but by 42% of presumed and 36% of suspected cases; however, this outcome might be easily explained through the higher rate in change or loss of smell reported in the confirmed cases.
Unexpected symptoms and clinical features among COVID-19 patients
All patients treated in our practice for pulmonary key symptoms—acute shortness of breath, pressure behind the breastbone (retrosternal chest pain) and a feeling of lack of oxygen mostly coupled with a panic feeling—showed neither increased inflammation markers nor any abnormalities in their x‑ray or computed tomography (CT) scans. Therefore, no signs of a potential pneumonia or other signs of vascular damage of the lung tissue, i.e., pulmonary embolism, could be identified. Contrary to severely affected cases, only 2% of our patients with mild to moderate symptomatic showed the typical lung symptomatic for this disease. During the treatment of our COVID-19 patients who presented pulmonal symptoms, painful, uncontrolled increased muscle tone of the upper back muscles, intercostal and auxiliary respiratory muscles, especially on the right side, was noticed.
Another key symptom—strong headache affecting 74% of patients—was often coupled with severe tension in cervical muscles, particularly the trapezius and sternocleidomastoid muscles. After treating several patients with manual techniques to reduce muscle tension, we reached a significant improvement of the symptoms in terms of recovering a normal, deep breathing and a substantial reduction of headache symptoms.
One of the highly effective methods in our practice to release muscle tension or joint pain is the use of the blood cupping technique [13]. After performing a light and superficial scarification of the epidermis with a thin needle, a cupping glass is applied on the area to be treated; because of the negative pressure through suction, a small amount of blood appears in the cupping glass and simultaneously, the painful tension is released. In non-COVID-19 patients, the cupped blood is dark-red colored and fluid, and the cupping glass stays clear (Fig. 4a,b). In all COVID-19 patients we treated with the cupping therapy, the blood showed an altered viscosity in the form of a foamy or jelly-like, firm consistency, and condensation appeared on the cupping glass (Fig. 4c,d). Of note, these abnormal blood features were observed in our COVID-19 patients independently of the SARS-CoV‑2 testing outcome. We also found these abnormal blood alterations during the treatment of COVID joints like shoulders, elbows, or knees (Fig. 4e,f). Surprisingly, the blood consistency was jelly-like among young patients and foamy in elderly cases. Interestingly, as soon as the patients were symptom-free, the blood viscosity returned to normal (Fig. 4h compared to Fig. 4g, as well as Fig. 4k compared to Fig. 4i,j). During our 12 years of practicing the cupping therapy on many patients, we had never experienced such a phenomenon, which seems highly likely to be associated with the COVID-19 infection.
Cupping technique. a,b 37-year-old woman, non-coronavirus disease 2019 (COVID-19) patient, suffering from muscle tension in her cervical spine and showing dark-red colored and fluid blood. c,d 70-year-old woman (confirmed case) presenting with first disease manifestations on November 5, 2020, and still experiencing long-term COVID-19 symptoms (lower back pain) in March 2021; after applying the cupping glass on her back, condensation appeared on the cupping glass and the blood had a foamy, firm consistency. e 49-year-old woman (suspected case) suffering from elbow pain one month post mild COVID-19 infection in March 2020 and showing foamy blood consistency upon cupping technique for symptom relief. f 14-year-old boy (presumed case), who experienced sudden elbow pain 4 weeks after a COVID-19 infection in March 2020 (moderate symptoms, including severe sore throat and labored breathing); after cupping glass application, his blood showed a thick blood texture. g Firm blood pattern observed after cupping glass application on the upper back of a 19-year-old (confirmed case) long COVID patient, who experienced in an acute phase in October 2020 shortness of breath (dyspnea), labored breathing, blocked deep inhalation, a feeling of lack of oxygen, panic, and anxiety; h a second application of cupping technique at the same location on March 11, 2021—after symptom resolution—did not show blood abnormalities anymore. i,j 60-year-old man (confirmed case) presenting with first COVID-19 symptoms at beginning of December 2020 and experiencing severe headache as leading post-COVID infection manifestation. After cupping treatment on December 16, 2020, to release neck tension, his blood showed abnormal features and some condensation was observed on the cupping glass; k a further cupping glass application on February 11, 2021, on the same patient after symptom resolution did not show any abnormal blood pattern
Another unexpected feature was a significant change in hormone levels. Post COVID-19 infection, many female patients showed abnormal hormones analysis in form of highly increased estrogen levels, including in cases already in menopause, who already had a decreased estrogen level prior to the infection. In addition, abnormal low testosterone levels in young male patients were observed. Furthermore, in both genders a high decrease in thyroid stimulating hormone (TSH), leading to a newly presentation of hyperthyroidism, was noticed. Moreover, in patients with previously known hypercholesterolemia and hypertriglyceridemia, an increase of the cholesterol and triglyceride values was detected following their COVID-19 infection.
Interestingly, after a COVID-19 vaccination (regardless of the vaccine used), several of our patients experienced the same typical symptoms and features—such as alteration of blood viscosity and hormonal imbalances—as those from our practice who undergone a COVID-19 infection. However, the number of post-vaccination-reported symptomatic cases might be too small to draw any conclusions about the side effects of COVID-19 vaccines.
Discussion
This observational study reports symptoms experienced by 160 patients from our general practice in Vienna (Austria) who had a confirmed, presumed, or suspected COVID-19 infection. When considering the number of COVID-19 symptom-based patients treated in our practice, our data are very similar to the official statistics from the Austrian COVID-19 dashboard on confirmed COVID-19 infections; this verifies the representativity of the collected study data [14].
Göker et al. described an increased susceptibility of patients with blood group A towards COVID-19 infection, whereas the blood group O might be protective to some extent [15]. The distribution of the blood groups and rhesus factors among our COVID-19 patients was quite similar to the repartition in the Austrian population [16]; therefore, our data do not infer a higher representation of any blood group in COVID-19 infected patients.
The sensitivity of RT-PCR testing as routine COVID-19 diagnostic method has been reported to be between 38 and 83% [17, 18]; nevertheless, the sensitivity of the PCR test might be strongly dependent of many factors such as the actual state of infection and the virus load, the quality of test kits and samples (sampling, transportation and handling) [19] as well as stage of the pandemic, geographic data, and sociologic parameters. Several publications strongly suggest that a negative diagnostic test should not be considered as a reliable exclusion criterion for an acute or undergone COVID-19 infection, as false-negative results occur [20, 21]; with suspicion of SARS-CoV‑2 infection and initial negative PCR outcome, resampling is therefore recommended [22]. These results might explain the negative diagnostic tests (molecular or serological) in 24% (n = 38) of our symptom-based COVID-19 patients, most likely resulting from inadequate sampling and/or timing of the specimen collection (too late or too early regarding illness onset).
According to our results, almost double of our confirmed cases showed as key symptom a change or loss of smell and/or taste compared to negative tested or suspected cases. Hence, it can be presumed that patients with above-mentioned symptom had a higher probability of being tested positive, either by a PCR or an antibody test.
Our study confirms that COVID-19 patients, regardless of whether they have been tested or not, experienced a broad range of symptoms far beyond the ones defined by the RKI [5]. In the early period of the pandemic covered by this study and considering the shortage of available PCR testing at that time, clinical diagnosis was based on some characteristic symptoms (anosmia/dysgeusia, fever, chest pain, muscle ache) that were used as a surrogate. Our patients showed quite different patterns of clinical presentations and thus far, no consistent explanation for some of these newly described symptoms or unexpected manifestations exists.
In an attempt to gain a better understanding of the pathophysiology of the COVID-19 clinical patterns, various explanatory models have been proposed, which describe the broad range of symptoms and their different intensities, including the alterations in blood viscosity. One of these models suggests an overactive immune response induced by cytokine storms, mostly seen in hospitalized or critically ill patients. This can cause blood coagulation abnormalities, leading to the formation of microthrombi, which damages organ tissues (i.e., multiorgan failure) [23]. However, latest research has shown that the cytokine storm model is still evolving and is unlikely to be regarded as the exclusive explanatory model of the pathophysiology of the SARS-CoV‑2 [24].
In relation to the SARS-CoV‑1, the impact of the angiotensin-converting enzyme 2 (ACE2) has already been described extensively; a high level of ACE2 expression was found in symptomatic patients presenting with ARDS, which caused cardiopulmonal, gastrointestinal, neurologic, and renal disorders [25, 26]. However, all symptoms observed in our COVID-19 patients cannot be ascribed solely to the ACE2 model.
To explain the broad range and the different intensity of symptoms experienced by patients during the acute and/or long-term phase of the COVID-19 disease, we might hypothesize with a high probability that the coronavirus directly impacts the hypothalamic–pituitary–adrenal (HPA) axis, which might explain the affected olfactory and gustatory system, as well as the hormonal disbalance. Indeed, the hypothalamic–pituitary system has a major influence on the metabolism, blood sugar level, weight, body temperature, brain, psychological condition, sleep, immune system, cardiovascular system, muscle and skeletal system, intestinal motility and sexual organs, and be involved as well in permeability alterations of vascular walls and in circulatory disturbances [27,28,29]. This hypothesis might not only explain most observed symptoms, but also their occurrence in waves, as hormones are released in phases through the HPA axis. The high variability of the experienced symptoms, as well as the different duration and intensity, might be explained due to individual responses to hormonal alterations (i.e., hormone substitution, premenstrual syndrome, pregnancy, menopause).
A SARS-CoV‑2 viral invasion and replication in both the olfactory bulb and the hypothalamus was described postmortem in COVID-19 patients [30]. Moreover, Alzahrani et al. already described the impact of the COVID-19 viral infection on the HPA [31]. An association between SARS-CoV‑2 and a low level of reproductive hormones in male patients has been also identified [32, 33] and COVID-19 possibly has an impact on sexual steroids, fertility, and other functions as well [34]. For instance, in the Italian THYRCOV study, COVID-19 patients experienced newly diagnosed hyperthyroidism (for 20% of them) and hypothyreosis (5%) [35]. The above findings underline the influence of this coronavirus on hormones, which was confirmed by the results of the hormonal laboratory analysis performed in our COVID-19-infected patients.
According to our observations, COVID-19 patients not recovering within several months following disease onset were still showing hormonal disbalances and abnormalities in blood viscosity; on the other hand, both latter disorders returned to normal after COVID-19 symptoms resolved. These abnormal blood features seem, therefore, clearly related to a COVID-19 infection; however, patients with modified blood viscosity showed normal blood analysis, including a normal coagulation factor. An altered glucose or fat metabolism due to a hormonal imbalance might also have caused the blood viscosity changes observed [36]. Alternatively, this hormonal disbalance might have induced a change in the concentration of the plasma proteins, leading to abnormal blood features; similar observations were mentioned for the ovarian hyperstimulation syndrome [37]. Further research should be done to understand fully the association between the measured hormonal disbalances, and the symptoms reported by the affected patients.
In total, 10–20% of our COVID-19 infected patients reported lasting symptoms for more than 4 weeks, irrespective of the extent of their initial COVID-19 infection. These long-term manifestations have been reported world-wide, also in young and healthy individuals, as well as asymptomatic or mildly affected COVID-19 patients. Whereas the German RKI described the main risks for developing long-term consequences after a COVID-19 infection as being a higher age and a higher body mass index (BMI) [5], we did not observe any association between patients showing prolonged post-COVID effects and those parameters in our patient pool.
This observational study presents some limitations. The long-term consequences of the COVID-19 infection were underestimated as some patients (n = 10, 6%) who felt ill in February or March 2021 and did not have the possibility to report long-lasting manifestations before the termination of the online survey end of March 2021. No comparison with a control group of non-COVID-19 infected patients has been performed; asymptomatic COVID-19 patients or false-negative tested patients might have compromised any attempt to define a reliable control group. Regarding self-reported symptoms such as intense neck tension and back pain, insomnia, or mental symptoms, we cannot completely exclude that the post-COVID associated symptoms experienced were not also related to higher stress levels because of the trauma of their disease and social isolation.
In conclusion, our observational study among 160 symptom-based coronavirus disease 2019 (COVID-19) patients (including 89 confirmed cases) suggests that a COVID-19 disease is much more than a typical respiratory distress syndrome; it affects different parts of the body simultaneously and/or subsequently, like a multisystem disease. In addition, COVID-19 is mainly associated with a broad range of symptoms, which appear not only in the acute phase of the infection, but quite often recurring weeks or months after the illness onset. The diagnosis of the severe acute respiratory syndrome coronavirus type 2 (SARS-CoV‑2) should not be based only on the testing outcome, but rather focused on the leading and further symptoms of the disease as well as their frequency. Needless to say, COVID-19 needs to be considered as an independent clinical syndrome rather than an equivalent to SARS-CoV-1- and MERS-associated pathologies. Additional research in understanding the pathophysiology of COVID-19 is therefore crucial; such real-life data from a general medical practice might help to assess the COVID-19 symptomatic experienced in a broad population outside any selected clinical study conditions.
References
Maine Center for disease control and prevention. Division of disease surveillance. https://www.maine.gov/dhhs/mecdc/infectious-disease/epi/airborne/mers-and-sars.shtml. Accessed 08 Feb 2022.
Chen B, Tian EK, He B, Tian L, Han R, Wang S, et al. Overview of lethal human coronaviruses. Signal Transduct Target Ther. 2020;5(1):89.
Peiris JS, Guan Y, Yuen KY. Severe acute respiratory syndrome. Nat Med. 2004;10(12 Suppl):S88–S97.
Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–42.
Koch Institute R. Coronavirus SARS-CoV‑2. https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Steckbrief.html. Accessed 08 Feb 2022.
Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251–61.
Breyer MK, Breyer-Kohansal R, Hartl S, Kundi M, Weseslindtner L, Stiasny K, et al. Low SARS-CoV‑2 seroprevalence in the Austrian capital after an early governmental lockdown. Sci Rep. 2021;11(1):10158.
Li C, Ren L. Recent progress on the diagnosis of 2019 novel Coronavirus. Transbound Emerg Dis. 2020;67(4):1485–91.
Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, Cuadrado ML, Florencio LL. Defining post-COVID symptoms (post-acute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health. 2021;18(5):2621. https://doi.org/10.3390/ijerph18052621.
Research NIfH. Living with Covid19 2020. https://evidence.nihr.ac.uk/themedreview/living-with-covid19/. Accessed 08 Feb 2022.
British Association of Dermatologists. COVID-19 skin patterns. https://covidskinsigns.com/. Accessed 08 Feb 2022.
Burke K, McGinnis K, Petronic-Rosic V. COVID Toes. Clin Dermatol. 2021;39(3):380–3.
Furhad S, Bokhari AA. Cupping therapy. Treasure Island: StatPearls Publishing; 2021.
AGES dashboard COVID-19. https://covid19-dashboard.ages.at/dashboard.html. Accessed 08 Feb 2022.
Göker H, Aladağ Karakulak E, Demiroğlu H, Ceylan AÇM, Büyükaşik Y, Inkaya A, et al. The effects of blood group types on the risk of COVID-19 infection and its clinical outcome. Turk J Med Sci. 2020;50(4):679–83.
Blood groups in Austria. https://www.gesundheit.gv.at/labor/laborwerte/blutgruppenserologie-transfusion/blutgruppenuntersuchung1-kh. Accessed 08 Feb 2022.
Liu R, Han H, Liu F, Lv Z, Wu K, Liu Y, et al. Positive rate of RT-PCR detection of SARS-CoV‑2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin Chim Acta. 2020;505:172–5.
Long C, Xu H, Shen Q, Zhang X, Fan B, Wang C, et al. Diagnosis of the Coronavirus disease (COVID-19): rRT-PCR or CT? Eur J Radiol. 2020;126:108961.
Zhang Y, Wang C, Han M, Ye J, Gao Y, Liu Z, et al. Discrimination of false negative results in RT-PCR detection of SARS-coV‑2 RNas in clinical specimens by using an internal reference. Virol Sin. 2020;35(6):758–67.
La Marca A, Capuzzo M, Paglia T, Roli L, Trenti T, Nelson SM. Testing for SARS-CoV‑2 (COVID-19): a systematic review and clinical guide to molecular and serological in-vitro diagnostic assays. Reprod Biomed Online. 2020;41(3):483–99.
Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–23.
Austrian Society for Laboratory Medicine and Clinical Chemistry. Laboratory diagnostics for coronavirus SARS-CoV‑2. 2020. https://www.oeglmkc.at/down/en_OeGLMKC%20recommendations%20COVID19_20201107.pdf. Accessed 08 Feb 2022.
Hojyo S, Uchida M, Tanaka K, Hasebe R, Tanaka Y, Murakami M, et al. How COVID-19 induces cytokine storm with high mortality. Inflamm Regen. 2020;40:37.
Leisman DE, Ronner L, Pinotti R, Taylor MD, Sinha P, Calfee CS, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8(12):1233–44.
Kaseb AO, Mohamed YI, Malek AE, Raad II, Altameemi L, Li D, et al. The impact of angiotensin-converting enzyme 2 (ACE2) expression on the incidence and severity of COVID-19 infection. Pathogens. 2021;10(3):379. https://doi.org/10.3390/pathogens10030379.
Li Y, Zhou W, Yang L, You R. Physiological and pathological regulation of ACE2, the SARS-CoV‑2 receptor. Pharmacol Res. 2020;157:104833.
Abdulla J, Torpy B, Feingold KR, Anawalt B, Boyce A, et al. Chronic fatigue syndrome. South Dartmouth: MDText.com; 2017.
Lim CT, Khoo B, Feingold KR, Anawalt B, Boyce A, et al. Normal physiology of ACTH and GH release in the Hypothalamus and anterior pituitary in man. South Dartmouth: MDText.com; 2000.
Armstrong M, Asuka E, Fingeret A. Physiology, thyroid function. Treasure Island: StatPearls Publishing; 2021.
Nampoothiri S, Sauve F, Ternier G, Fernandois D, Coelho C, Imbernon M, et al. The hypothalamus as a hub for SARS-CoV‑2 brain infection and pathogenesis. bioRxiv. 2020:2020.06.08.139329. 2020.
Alzahrani AS, Mukhtar N, Aljomaiah A, Aljamei H, Bakhsh A, Alsudani N, et al. The impact of COVID-19 viral infection on the hypothalamic-pituitary-adrenal axis. Endocr Pract. 2021;27(2):83–9.
Dutta S, Sengupta P. SARS-CoV‑2 infection, oxidative stress and male reproductive hormones: can testicular-adrenal crosstalk be ruled-out? J Basic Clin Physiol Pharmacol. 2020;31(6). https://doi.org/10.1515/jbcpp-2020-0205.
Haghpanah A, Masjedi F, Alborzi S, Hosseinpour A, Dehghani A, Malekmakan L, et al. Potential mechanisms of SARS-coV‑2 action on male gonadal function and fertility: current status and future prospects. Andrologia. 2021;53(1):e13883.
Selek A, Güçlü M, Bolu ŞE. COVID-19 pandemic: what about the gonads? Hormones. 2021;20(2):259–68.
Lania A, Sandri MT, Cellini M, Mirani M, Lavezzi E, Mazziotti G. Thyrotoxicosis in patients with COVID-19: the THYRCOV study. Eur J Endocrinol. 2020;183(4):381–7.
Olarescu N, Gunawardane K, Hansen T, Feingold KR, Anawalt B, Boyce A, et al. Normal physiology of growth hormone in adults. South Dartmouth: MDText.com; 2000.
Bielfeld A, Krüssel J, Baston-Büst D‑M. Ovarielles Überstimulationssyndrom. https://www.springermedizin.de/emedpedia/reproduktionsmedizin/ovarielles-ueberstimulationssyndrom?epediaDoi=10.1007%2F978-3-662-55601-6_32. Accessed 08 Feb 2022.
Acknowledgements
The authors would like to thank all patients for their participation in the online survey.
Funding
The authors do not have any disclosures to report.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Conceptualization of the study was performed by CB; data collection was performed by CB and VH; formal data analysis was performed by CB and FB. The first draft of the manuscript was written by CB and FB. All authors reviewed and edited previous versions of the manuscript; all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
C. Bouwensch, V. Hahn and F. Boulmé declare that they have no competing interests.
Ethical standards
The study was conducted according to the guidelines of the Declaration of Helsinki. Ethical review and approval were waived for this observational study based on an online survey aiming to determine patients’ insights from their experience.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bouwensch, C., Hahn, V. & Boulmé, F. Analysis of 160 nonhospitalized COVID-19 patients with mild to moderate symptoms from an Austrian general medical practice: from typical disease pattern to unexpected clinical features. Wien Med Wochenschr 172, 198–210 (2022). https://doi.org/10.1007/s10354-022-00919-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10354-022-00919-0