Summary
Background
Large skin defects caused by trauma (e.g., burns) or due to other reasons (e.g., tumor-related skin resections) require sufficient skin replacement. The constant improvement of innovative methods of skin replacement and skin expansion mean that even burn victims with more than 80% body surface burned have a realistic chance of survival. Due to these new developments, not only has survival rate increased, but also quality of life has increased tremendously over the past decades.
Methods
The aim of this review is to present an overview of current standards and future trends concerning the treatment of skin defects. The main focus is placed on the most important technologies and future trends.
Results
Autologous skin grafting was developed more than 3500 years ago. Several approaches and techniques have been discovered and established in burn care and plastic surgery since then. Great achievements were made during the 19th and 20th centuries. Many of these old and new techniques are still part of modern burn and plastic surgery. Today, autologous skin grafting is still considered to be the gold standard for many wounds, but new technologies have been developed, ranging from biological to synthetic skin replacement materials.
Conclusion
Today, old and new technologies are available which allow us new treatment concepts. All this has led to the reconstructive clockwork for reconstructive surgery of the 21st century.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Large skin defects caused by trauma (e.g., burns) or due to other reasons (e.g., tumor-related skin resections) require a sufficient skin replacement. The constant improvement of innovative methods of skin replacement and skin expansion technologies mean that even burn victims with more than 80% body surface burned have a realistic chance of survival. However, due to these new developments, not only has the survival rate increased, but also the quality of life has increased tremendously over the past decades.
Both in the case of extensive third-degree burns and other extensive skin losses, it has been shown that pleasing functional and cosmetic results cannot be achieved through conservative measures, but only through surgical procedures and modern skin replacement strategies.
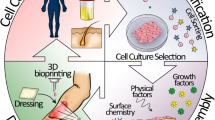
In the past, deep burn wounds (grade 2b, grade 3) were routinely covered with autologous skin grafts directly after necrosectomy. Today, new technologies are available which allow us new treatment concepts. All this has led to the reconstructive clockwork for reconstructive surgery of the 21st century (Fig. 1).
The idea of the reconstructive clockwork is to mirror the integral parts of various reconstructive echelons serving the one goal of addressing the defect, the function, the deformity, or all of them in combination [1].
Materials and methods
The aim of this review is to present an overview of current standards and future trends concerning the treatment of skin defects. The main focus is placed on the most important technologies and future trends.
Results
Skin transplantation
Skin grafts are classified as either split thickness or full thickness based on the strength of their associated dermal portion. If the skin is removed with the dermis completely included, it is called a full-thickness skin graft. A “typical” split-thickness skin graft is thinner and includes only parts of the dermis. The skin appendages located in the deeper dermal layers remain at the site of removal and provide the resources necessary for the defect to heal ([2]; Fig. 2).
Autologous full-thickness skin grafts
The full-thickness skin graft has proven to be the best choice, both functionally and cosmetically, for covering burned areas on the face, hands, and over large joints in particular, since the strong dermal component prevents excessive scarring with subsequent shrinkage.
The limiting factor for use of full-thickness skin grafts, however, is the fact that the removal sites for full-thickness skin grafts always have to be primarily closed; thus, mostly only smaller grafts are available [2,3,4].
Combined reconstruction using a split-thickness skin graft combined with dermal replacement material (matrix, scaffold)
In the case of full-layer skin defects in functionally important regions (e.g., hands), combined skin reconstruction using a split-thickness skin graft (often non-meshed) in combination with a dermal matrix is often used. There are currently several matrices available (e.g., Matriderm® [MedSkin Solutions Dr. Suwelack AG, Germany] and Integra® [Integra Life Sciences, Germany], PolyNovo® [Polynovo Limited, Australia]) [5, 6].
Autologous meshed split-thickness skin grafts (meshed graft)
With the lattice split-skin graft, a defined mesh-like perforation is produced on a roller in combination with a corresponding template using a special arrangement of parallel knives on a roller, which leads to a relative increase in the area of the transplant. Split-skin grafts are particularly useful where large burned areas can only be covered with a remnant of healthy skin. An expansion ratio of 1:1.5 to 1:3 is preferably selected. With larger expansion ratios, the Meek graft is superior to the mesh graft in terms of healing and expansion [7, 8].
Meek technique
In 1958, Meek described a dermatome with which the split skin obtained can be cut into small square islands of equal size. In the 1990s, this method was modified in connection with an easy-to-use transplantation method, which made it possible, in one step, to not only cut the split skin layer, but also to expand it in ratios of up to 1:9 after applying it to a cork and silk support and to transplant. This method, which is somewhat easier to use, has now become established in many burn centers because of the mathematically favorable use of the enlargement factor and is preferred to the mesh graft for very large burns and other skin defects. This grafting technique has also become established for the coverage of chronic wounds [2, 3, 7,8,9,10].
Alternate methods
The use of standard surgical methods depends on the availability of a sufficiently large area of undamaged skin as a donor area for transplantation. In order to circumvent this limitation, efforts have focused on finding alternative methods so that patients with more than 70% of the body surface burned have a realistic chance of survival [9].
Allogeneic transplants (allografts)
If there are not enough donor areas available, allogeneic transplants can be used temporarily as a temporary skin replacement. Allogeneic transplants became more widespread when the so-called sandwich technique was used, in which widely meshed autologous transplants are covered with less widely meshed allografts [2, 10,11,12].
Xenogeneic transplants (xenografts)
Since the mid-1950s, especially in China, pigskin has often been used to temporarily cover large wound areas. After transplantation, the xenograft initially finds a nutritive connection to the basal wound bed. The dermis is initially revascularized, but then usually quickly dissolved and replaced by collagen structures. Especially in countries where allogeneic transplants are not used for ethical reasons, temporary wound covering with xenografts is still an important procedure today. These grafts are not only used in the case of severe burns, particularly in situations where donor sites are scare, but have been used for the treatment of other acute and chronic wounds [2, 3, 13].
Acellular fish skin
Acellular fish skin grafts (Fig. 3) have several advantages in comparison to other xenografts of porcine and bovine origin. Acellular fish skin grafts can be stored at room temperature and have a shelf life of 3 years. Due to the particularly gentle process of decellularization and preservation, the protein and matrix structure of marine omega‑3 wound matrices are extremely similar to the structure of human skin (Fig. 4). Its structure remains intact and enables the ingrowth of cells and capillaries (Fig. 5). Beside this, acellular fish skin grafts are extremely rich in omega‑3 fatty acids. These grafts have anti-inflammatory and anti-infective properties, too. Therefore, omega‑3 wound matrices seem to be suited for the treatment of complicated acute and chronic wounds [14,15,16,17,18,19,20,21].
Image of fibroblast cells and acellular fish skin (Kerecis®; Island) under confocal fluorescence microscopy. Kerecis® fluoresces green. NucBlue bound to the fibroblast nucleus is blue and Alexa-Fluor 546 Phalloidin bound to F‑actin in the cytoplasm is pink. As well as interacting with the two-dimensional structure as shown in the image, the cells populate the three-dimensional spaces in the fish skin medical device throughout its whole depth
Cell culture and tissue engineering
The surgical standard methods have their limits in terms of effectiveness in people who have been burned extensively, since the remaining unburned residual skin resources as donor areas are reduced to a minimum depending on the extent. The development and improvement of new cultivation methods and the introduction of transplantable and resorbable biomaterials using so-called tissue engineering offers a potential way out of the dilemma [22,23,24].
The aim is in vitro generation of tissues that are able to permanently replace specific tissue losses with comparable biomechanical and biochemical quality.
Specifically, the epidermis was the first organ or biological structure that could be successfully cultivated under in vitro conditions and transplanted in vivo [25,26,27,28].
These successes have made it possible, especially during the past 30 years, to treat patients with burns covering more than 60% of the body surface successfully [9]. Today, cultivated allogeneic cell transplants and autologous cell transplantation kits are commercially available.
Cultured autologous epidermis
Transplantation of a cultured epidermal membrane of autologous keratinocytes (cultured epidermal autografts, CEA) was the first successful clinical use of a cultured organ component. Applied cultured epidermis transplants usually consist of three to five cell layers. However, the transplants are very fragile and hard to handle. Another problem is the lack of a dermal component in case of third-degree burns. In order to counteract this problem, the development of dermal analogs of different compositions has been promoted and used clinically with success [2, 23, 24].
Cell suspensions
In 1895, the first successful transplantation of scraped keratinocytes suspended in autologous wound serum was performed. However, this technique was not initially able to establish itself, because of a lack of suitable carrier substances. The use of allogeneic keratinocyte suspensions aims primarily to utilize the paracrine-secreting activity of the cells. In areas with a burn degree of 2a to 2b, the re-epithelialization of the remaining skin appendages can be stimulated and the time until healing can be shortened. The same technology can be used to treat split-skin donor areas, where this possibility of using allogeneic cells is intended to ensure that the donor areas are available again more quickly [2, 3].
Cultured cells
The combination of cultured autologous keratinocytes on alloplastic or mixed synthetic/biological materials as dermal regeneration matrices has been investigated by different groups. In the 1980s, Yannas and Burke produced a skin equivalent by centrifugation of primarily trypsinized keratinocytes and fibroblasts in a collagen–glycosaminoglycan matrix (C-GAG), which healed completely after transplantation in guinea pigs [29,30,31]. Today, this and other matrices have been increasingly used for this purpose, also in humans [23, 24].
In spite of the tremendous advances in skin tissue engineering, a “complete” tissue-engineered skin substitutes is not yet available. Current substitutes are mainly composed of keratinocytes and fibroblasts, but still lack some of the functional components such as nerves, adnexal structures, and pigment cells. [22].
Synthetic materials
In addition to biological materials, more and more purely synthetic materials are on the market. In addition, various polymers/polymer composites (including polycaprolactone, PCL; polyurethane, PU; silicones; polylactic acid compounds PLA/PGLA) and “natural” materials such as silk proteins and bacterial cellulose are used for research and clinical purposes [32,33,34].
Synthetic skin replacement materials should replicate the functions of the natural extracellular matrix as far as possible. These include influencing cell proliferation, cell migration, and cell differentiation. The following factors should be considered in the development and production of synthetic biomaterials: composition and suitability (biocompatibility), biodegradation in vitro and in vivo, production and shaping as well as availability, batch-to-batch variability, production under physiological conditions (e.g., temperature, pH), and easy processing and application in the clinic. The materials should also have physiological properties that are as similar as possible to those of the skin, such as elasticity or biomechanical stability, and provide a 3D structure for tissue regeneration.
Common manufacturing methods
Common methods for the production of biomaterials are freeze drying, salt leaching, gas foaming, and electrospinning. Freeze drying (lyophilization) is a gentle technique for drying sensitive valuable materials (such as proteins) and can be used effectively for the production of collagen mats, for example. A porous 3D structure is created that can either be populated with endogenous cells or allow endogenous cells to grow in from the surrounding tissue and ECM. Salt leaching and gas foaming are techniques in which salt crystals or gas (e.g., CO2) are deliberately introduced into the material mixture and later released. This is how porous 3D membranes are created. With electrospinning, natural (e.g., collagen) or synthetic polymer solutions (e.g., PCL) can be spun into very thin fibers (nanometers to microns) in an electric field. These fibers (e.g., polymer, collagen) can also be processed as bundles as well as mats.
Three-dimensional printing
In spite of the tremendous advances in skin tissue engineering, a “complete” tissue-engineered skin substitute is not yet available. Therefore, skin substitutes that replace the entire function of the skin are urgently required. From this perspective, 3D printing technology, bioink, and artificial skin bioprinting technologies that imitate the skin structure and microenvironment have gained immense attention. [35,36,37,38].
Beside the therapeutic impact, 3D bioprinting has the potential to serve as a platform for studying tissue development and homeostasis and for modeling diseases in pharmaceutical testing [35]. Bioprinting seems to be a technology that could overcome the gap between grafts and skin substitutes.
As very briefly described, 3D bioprinting seems to be very promising, but the next step is already being taken: 4D bioprinting, where the fourth dimension is transformation. It is the 3D printing of smart, stimuli-responsive biomaterials to create constructs that emulate the dynamic processes of biological tissues and organs.
Imagine, for instance, that instead of having to 3D print a skin graft for a burn victim, with all the entailed complexity, you could 4D print a basic skin graft that would, once implanted on the patient, vascularize itself, develop all nerve endings, take on the patient’s complexion, and even grow hair if on the head. In a way, 4D bioprinting is to medicine what artificial intelligence is to computer science (Fig. 6; [35,36,37,38]).
Discussion
Autologous skin grafting was developed more than 3500 years ago. Several approaches and techniques have been discovered and established in burn care and plastic surgery since then. Great achievements were made during the 19th and 20th centuries. Many of these old and new techniques are still part of modern burn and plastic surgery. Today, autologous skin grafting is still considered to be the gold standard for many wounds, but new technologies have been developed, ranging from biological to synthetic skin replacement materials [2,3,4, 9, 39,40,41]. In spite of the tremendous advances in skin tissue engineering, a “complete” tissue-engineered skin substitute is not yet available and there is a need for new innovations and developments [42, 43]. One of these promising new technologies is 3D and 4D bioprinting [35,36,37].
Today, old and new technologies are available which allow us new treatment concepts for patients suffering from large skin defects. All this has led to the reconstructive clockwork for reconstructive surgery of the 21st century [1], and the clock will made more complex by new technologies.
References
Knobloch K, Vogt PM. The reconstructive clockwork of the twenty-first century: an extension of the concept of the reconstructive ladder and reconstructive elevator. Plast Reconstr Surg. 2010;126(4):220e–2e.
Horch RE, Kopp J. Haut und Hautersatz, Tissue Engineering. In: Kamolz LP, Herndon DN, Jeschke MG, editors. Verbrennungen – Diagnose, Therapie und Rehabilitation des thermischen Traumas. 1st ed. Vienna: Springer; 2009. p. 123–143
Kohlhauser M, Luze H, Nischwitz SP, Kamolz LP. Historical evolution of skin grafting—a journey through time. Medicina (Kaunas). 2021;57(4):348.
Beier JP, Boos AM, Kamolz L, Vogt PM, Koller R, Horch RE. Skin tissue engineering—from split skin to engineered skin grafts? Handchir Mikrochir Plast Chir. 2010;42(6):342–53.
Haslik W, Kamolz LP, Nathschläger G, Andel H, Meissl G, Frey M. First experiences with the collagen-elastin matrix matriderm as a dermal substitute in severe burn injuries of the hand. Burns. 2007;33(3):364–8.
Wiedner M, Tinhofer IE, Kamolz LP, et al. Simultaneous dermal matrix and autologous split-thickness skin graft transplantation in a porcine wound model: a three-dimensional histological analysis of revascularization. Wound Repair Regen. 2014;22(6):749–54.
Kamolz LP, Schintler M, Parvizi D, Selig H, Lumenta DB. The real expansion rate of meshers and micrografts: things we should keep in mind. Ann Burns Fire Disasters. 2013;26(1):26–9.
Lumenta DB, Kamolz LP, Keck M, Frey M. Comparison of meshed versus MEEK micrografted skin expansion rate: claimed, achieved, and polled results. Plast Reconstr Surg. 2011;128(1):40e–1e.
Lumenta DB, Kamolz LP, Frey M. Adult burn patients with more than 60 % TBSA involved-Meek and other techniques to overcome restricted skin harvest availability—the Viennese concept. J Burn Care Res. 2009;30(2):231–42.
Astarita C, Arora CL, Trovato L. Tissue regeneration: an overview from stem cells to micrografts. J Int Med Res. 2020;48(6):300060520914794.
Horch RE, Corbei O, Formanek-Corbei B, Brand-Saberi B, Vanscheidt W, Stark GB. Reconstitution of basement membrane after “sandwich-technique” skin grafting for severe burns demonstrated by immunohistochemistry. J Burn Care Rehabil. 1998;19(3):189–202.
Horch R, Stark GB, Kopp J, Spilker G. Cologne burn centre experiences with glycerol-preserved allogeneic skin: part I: clinical experiences and histological findings (overgraft and sandwich technique). Burns. 1994;20(1):S23–6.
Haller HL, Blome-Eberwein SE, Branski LK, et al. Porcine xenograft and epidermal fully synthetic skin substitutes in the treatment of partial-thickness burns: a literature review. Medicina (Kaunas). 2021;57(5):432.
McDaniel JC, Belury M, Ahijevych K, Blakely W. Omega‑3 fatty acids effect on wound healing. Wound Repair Regen. 2008;16(3):337–45.
Dorweiler B, Trinh T, Dürnschede F, et al. The marine Omega3 wound matrix for treatment of complicated wounds. A multicenter experience report. Gefäßchirurgie. 2018;23(2):S46–S55.
Lullove EJ, Liden B, Winters C, et al. A multicenter, blinded, randomized controlled clinical trial evaluating the effect of omega-3-rich fish skin in the treatment of chronic, nonresponsive diabetic foot ulcers. Wounds. 2021;33(7):169–77.
Stone R 2nd, Saathoff EC, Larson DA, et al. Accelerated wound closure of deep partial thickness burns with acellular fish skin graft. Int J Mol Sci. 2021;22(4):1590.
Alam K, Jeffery SLA. Acellular fish skin grafts for management of split thickness donor sites and partial thickness burns: a case series. Mil Med. 2019;184(1):16–20.
Michael S, Winters C, Khan M. Acellular fish skin graft use for diabetic lower extremity wound healing: a retrospective study of 58 ulcerations and a literature review. Wounds. 2019;31(10):262–8.
Kirsner RS, Margolis DJ, Baldursson BT, et al. Fish skin grafts compared to human amnion/chorion membrane allografts: a double-blind, prospective, randomized clinical trial of acute wound healing. Wound Repair Regen. 2020;28(1):75–80.
Woodrow T, Chant T, Chant H. Treatment of diabetic foot wounds with acellular fish skin graft rich in omega-3: a prospective evaluation. J Wound Care. 2019;28(2):76–80.
Sierra-Sánchez Á, Kim KH, Blasco-Morente G, Arias-Santiago S. Cellular human tissue-engineered skin substitutes investigated for deep and difficult to heal injuries. NPJ Regen Med. 2021;6(1):35.
Keck M, Haluza D, Lumenta DB, et al. Construction of a multi-layer skin substitute: simultaneous cultivation of keratinocytes and preadipocytes on a dermal template. Burns. 2011;37(4):626–30.
Kamolz LP, Luegmair M, Wick N, et al. The Viennese culture method: cultured human epithelium obtained on a dermal matrix based on fibroblast containing fibrin glue gels. Burns. 2005;31(1):25–9.
Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6(3):331–43.
Sun TT, Green H. Differentiation of the epidermal keratinocyte in cell culture: formation of the cornified envelope. Cell. 1976;9(4 Pt 1):511–21.
Green H, Rheinwald JG, Sun TT. Properties of an epithelial cell type in culture: the epidermal keratinocyte and its dependence on products of the fibroblast. Prog Clin Biol Res. 1977;17:493–500.
Rheinwald JG, Green H. Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature. 1977;265(5593):421–4.
Yannas IV, Burke JF. Design of an artificial skin. I. Basic design principles. J Biomed Mater Res. 1980;14(1):65–81.
Yannas IV, Burke JF, Gordon PL, Huang C, Rubenstein RH. Design of an artificial skin. II. Control of chemical composition. J Biomed Mater Res. 1980;14(2):107–32.
Dagalakis N, Flink J, Stasikelis P, Burke JF, Yannas IV. Design of an artificial skin. Part III. Control of pore structure. J Biomed Mater Res. 1980;14(4):511–28.
Uhlig C, Rapp M, Hartmann B, Hierlemann H, Planck H, Dittel KK. Suprathel—an innovative, resorbable skin substitute for the treatment of burn victims. Burns. 2007;33(2):221–9.
Nischwitz SP, Popp D, Shubitidze D, et al. The successful use of polylactide wound dressings for chronic lower leg wounds: a retrospective analysis. Int Wound J. 2021; https://doi.org/10.1111/iwj.13713.
Luca-Pozner V, Nischwitz SP, Conti E, et al. The use of a novel burn dressing out of bacterial nanocellulose compared to the French standard of care in paediatric 2nd degree burns—a retrospective analysis. Burns. 2021; https://doi.org/10.1016/j.burns.2021.11.019.
Di Piazza E, Pandolfi E, Cacciotti I, et al. Bioprinting technology in skin, heart, pancreas and cartilage tissues: progress and challenges in clinical practice. Int J Environ Res Public Health. 2021;18(20):1–30.
Antezana PE, Municoy S, Álvarez-Echazú MI, Santo-Orihuela PL, Catalano PN, Al-Tel TH, et al. The 3D bioprinted scaffolds for wound healing. Pharmaceutics. 2022;14(2):464.
Jang KS, Park SJ, Choi JJ, et al. Therapeutic efficacy of artificial skin produced by 3D bioprinting. Materials (Basel). 2021;14(18):5177.
Tarassoli SP, Jessop ZM, Al-Sabah A, Gao N, Whitaker S, Doak S, et al. Skin tissue engineering using 3D bioprinting: an evolving research field. J Plast Reconstr Aesthet Surg. 2018;71(5):615–23.
Bay C, Chizmar Z, Reece EM, et al. Comparison of skin substitutes for acute and chronic wound management. Semin Plast Surg. 2021;35(3):171–80.
Dai C, Shih S, Khachemoune A. Skin substitutes for acute and chronic wound healing: an updated review. J Dermatolog Treat. 2020;31(6):639–48.
Haller HL, Rapp M, Popp D, Nischwitz SP, Kamolz LP. Made in Germany: a quality indicator not only in the automobile industry but also when it comes to skin replacement: how an automobile textile research institute developed a new skin substitute. Medicina (Kaunas). 2021;57(2):143.
Wurzer P, Keil H, Branski LK, et al. The use of skin substitutes and burn care—a survey. J Surg Res. 2016;201(2):293–8.
Bhardwaj N, Chouhan D, Mandal BB. Tissue engineered skin and wound healing: current strategies and future directions. Curr Pharm Des. 2017;23(24):3455–82.
Funding
Open access funding provided by Medical University of Graz.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
L.-P. Kamolz, P. Kotzbeck, M. Schintler, and S. Spendel declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kamolz, LP., Kotzbeck, P., Schintler, M. et al. Skin regeneration, repair, and reconstruction: present and future. Eur Surg 54, 163–169 (2022). https://doi.org/10.1007/s10353-022-00757-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10353-022-00757-9