Summary
Background
The effects of cytotoxic chemotherapy on the expression of programmed death ligand 2 (PD-L2) are unknown and little is known about how the tumor microenvironment changes following neoadjuvant chemotherapy in locally advanced gastroesophageal adenocarcinomas (AEG). Recently, a number of studies reported that cytotoxic chemotherapy affects the expression levels of programmed cell death protein 1 (PD-1) and its ligand 1 (PD-L1). Regarding PD-L2, the second known ligand of PD‑1, no data on potential changes in expression patterns in patients with preoperatively treated AEG are available. The aim of this study was to investigate the impact of cytotoxic chemotherapy on PD-L2 expression in patients with resectable AEG.
Methods
Consecutive patients with locally advanced AEG treated with preoperative cytotoxic chemotherapy were included. PD-L2 expression by cancer cells (CCs) and tumor-infiltrating lymphocytes (TILs) was investigated in samples of paired diagnostic biopsies and resected tumor specimens by immunohistochemistry using two different anti-PD-L2 antibodies.
Results
Included were 40 patients with AEG and available paired tumor tissue samples. PD-L2 expression was observed in one diagnostic biopsy sample by CCs and in one diagnostic biopsy sample by TILs. There was no difference concerning the expression levels measured by the two antibodies.
Conclusion
In contrast to previously published studies reporting PD-L2 expression rates of up to 50% in AEGs, in our cohort, PD-L2 expression seems to play no significant role in AEG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastroesophageal adenocarcinoma (AEG) is one of the ten most common causes of cancer deaths worldwide [1, 2]. Even though the use of multimodal therapies combining surgery, cytotoxic chemotherapy, radiotherapy, and targeted therapy has improved patientsʼ survival rates, 5‑year survival rates remain poor, at 10–15% [3,4,5,6,7]. The majority of patients present with locally advanced AEG and are therefore eligible for preoperative treatment [8, 9]. Perioperative chemo(radio)therapy along with surgery is now the standard of care treatment for patients with locally advanced AEG [10, 11]. However, due to the development of resistance to cytotoxic chemotherapy, these conventional therapies are limited in efficacy. Recently, targeted therapy strategies have become a promising approach to overcome resistance to cytotoxic chemotherapies. Human epidermal growth factor receptor 2 (HER-2) has been confirmed to play an important role in the treatment of AEG. Cytotoxic chemotherapy in combination with trastuzumab, a monoclonal antibody against HER‑2, significantly improves the prognosis in patients with HER-2-positive AEG [12]. Despite these promising data, recent studies also point out emerging resistance mechanisms to anti-HER2 therapy [13]. Therefore, new approaches for molecular-targeted therapeutics are needed to further improve patients’ survival. Cancer immunotherapy has revolutionized the field of oncology and immune checkpoint therapy provides promising treatment results in various cancer entities, including esophageal cancer [14, 15]. The therapeutic modulation of the PD‑1 pathway has emerged as a promising target in the treatment of melanoma, renal cancer, non-small cell lung cancer, and urothelial cancer [16]. Programmed cell death 1 (PD-1) is one of the most potent immune-checkpoint molecules, expressed mainly on the surface of T‑cells during activation. Binding of this co-receptor results in limited activation and function of the T‑cell and is therefore of great importance in avoiding hyperactivation [17]. Cancer cells can take advantage of this mechanism and misuse it to hide from immunosurveillance [18]. Programmed death ligand 1 (PD-L1) and programmed death ligand 2 (PD-L2) are cell surface glycoproteins belonging to the B7 family and are the two known ligands for PD‑1 [19, 20].
In advanced esophageal squamous cell cancer (ESCC), pembrolizumab, a monoclonal antibody targeting PD-L1, has been approved by the United States Food and Drug Administration (FDA) and significantly improves patient survival rates [21]. Recently, several studies could show that cytotoxic chemotherapy can affect the expression of PD-L1. So far, our group has investigated the prognostic value of PD‑1, PD-L1, and PD-L2 regarding survival rates of patients with AEG [22, 23]. Since neoadjuvant chemotherapy has become indispensable in the treatment of patients with locally advanced AEG, it is necessary to understand its impact on immune characteristics. To the best of our knowledge, there are no data on the neoadjuvant chemotherapeutic effect on the expression of PD-L2 in AEG until today. Therefore, the aim of this study was to investigate the potential change in the expression level of PD-L2 in neoadjuvantly treated AEG patients.
Materials and methods
Patients
Patients with histologically confirmed and locally advanced AEG who underwent neoadjuvant chemotherapy (NCT) followed by curative resection between 2003 and 2016 at the Department of Surgery of the Medical University of Vienna were enrolled in this study. Patients’ demographic and histopathological characteristics were collected from a prospectively maintained database. The pathological tumor–node–metastasis (TNM) classification of the Union for International Cancer Control (UICC), 8th edition, was used to determine the clinical tumor stage pre-NCT and post-surgery [24]. Patients with complete response to the neoadjuvant chemotherapy, distant metastasis at the time of surgery, positive resection margins, or malignancies other than AEG were excluded. All patients were discussed in multidisciplinary tumor board meetings and either received an oxaliplatin/capecitabine-based or a cisplatin/5-fluoruacil-based neoadjuvant chemotherapy according to the standards of the Comprehensive Cancer Center of the Medical University of Vienna. Depending on the primary tumor localization, patients either underwent abdominothoracic en-bloc esophagectomy or trans-hiatal extended gastrectomy.
Paired diagnostic biopsies and surgical specimens were acquired from each patient included in the study. Biopsy specimen were obtained during esophagogastroduodenoscopy (EGD) according to the standards of the European Society of Gastrointestinal Endoscopy (ESGE) [25, 26]. PD-L2 expression was analyzed by two different immunohistochemical staining methods. This study was approved by the Ethics Committee of the Medical University of Vienna, Austria, and was performed in accordance with the Declaration of Helsinki (EK 1056/2016).
Assessment of response to NCT
The response to neoadjuvant chemotherapy was analyzed through histopathological investigation of the resection specimens according to the Mandard grading system [27]. In brief, this classification divides the histopathological effects into five tumor regression grades (TRGs) based on the ratio of vital tumor tissue and fibrosis: TRG 1—complete regression (=fibrosis without detectable tumor tissue); TRG 2—fibrosis with scattered tumor cells; TRG 3—fibrosis and tumor cells with predominance of fibrosis; TRG 4—fibrosis and tumor cells with predominance of tumor cells; TRG 5—tumor tissue without changes of regression [27].
Immunohistochemistry
Paraffin-embedded specimens fixed in 4% buffered formalin were cut into 4‑µm-thick slides and subjected to immunohistochemical analyses. The following antibodies were utilized for PD-L2 detection: rabbit anti-human PD-L2 antibody (Cell Signaling Technology, Cambridge, United Kingdom, #82723, clone D7U8C, dilution 1:50) and the anti-PD-L2 antibody (Proteintech, Manchester, United Kingdom, #18251-1-AP, dilution: 1:200). The sections were deparaffinized and rehydrated in graded series: X‑TRA-Solv 8 (Medite, Burgdorf, Germany, # 41-5212-00) for 15 min at 68 °C; xylol—5 min RT, 100% EtOH—5 min RT; 96% EtOH—5 min RT; 80% EtOH—5 min RT; distilled water 2 min RT. The antigens were retrieved via cooking at 100 ºC for 45 min with Leica, Vienna, Austria, buffer nr. 2. Goat anti-rabbit IgG (H+L), HRP-conjugated antibody (Abcam, Cambridge, United Kingdom, #ab97051) was used as a secondary antibody. The stainings with the primary and secondary antibodies were performed according to the manufacturer’s protocols. In order to depict the cell nuclei, sections were counterstained with Mayer’s hematoxylin.
For each patient, histologic expression of PD-L2 was analyzed separately for tumor cells and TILs. The percentage of tumor cells and lymphocytes showing immunoreactivity to PD-L2 was rated (positive staining, 0–100%) and classified as follows: 0: no positive cells; +: 5–25% of cells; ++: 26–50% of cells; +++: 51–75% of cells; and ++++: 76–100% of cells. Histological analyses were performed by two pathologists who were blinded to the clinical characteristics of each patient.
Statistical analysis
Statistical analysis was performed using the R 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria, www.r-project.org). Correlations between clinicopathological factors and expression of PD-L2 were analyzed with the Kendall’s correlation coefficient (tau-b).
Results
A total of 40 paired sets of diagnostic biopsies and surgical specimens from patients with neoadjuvantly treated AEG were included in this study. The median age of the patients in the study population was 64 years (range: 35.0–80.5 years) and the ratio of female to male was 1:2.3. The most frequent tumor localization (28 patients, 70.0%) was AEG type I according to the Siewert classification [28]. Before neoadjuvant chemotherapy, the majority of patients were diagnosed with UICC stage III (30 patients, 75.0%). After surgical resection, UICC stage III (25 patients, 62.5%) remained the most frequent staging. 23 (57.5%) patients presented with a moderately differentiated tumor (G2). Regarding the response to administered chemotherapy, 18 patients (45.0%) showed partial response (TRG 2–3), 15 patients (37.5%) showed minor regression (TRG 4), and 7 patients (17.5%) had no signs of regression at all (TRG 5). 34 (85.0%) patients presented with an ECOG (Eastern Cooperative Oncology Group) score of 0 before neoadjuvant chemotherapy and 15 patients (37.5%) were classified as ASA (American Society of Anesthesiologists) 1 before surgery. Clinico-pathological data are presented in Tab. 1.
PD-L2—cancer cells (CC) and tumor-infiltrating lymphocytes (TILs)
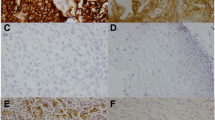
A total of 33 cases were evaluated regarding the change of PD-L2 expression by CC and TILs in diagnostic biopsies. On 7 specimens of diagnostic biopsies, IHC could not be performed due to technical problems. All of the 40 surgical specimens were successfully stained. Among the specimens of diagnostic biopsies, one sample showed 1+ (1–25%) PD-L2 staining by cancer cells and another sample 2+ (26–50%) PD-L2 staining by TILs (same results for both PD-L2 antibodies; Fig. 1a, b). Both cases showed Mandard TRG 2. None of the surgical specimens showed positive staining for PD-L2 with either of the two used antibodies (Fig. 1c, d). Based on these results, no further meaningful investigations of the changes of PD-L2 expression have been conducted (Tab. 2).
Representative images of diagnostic biopsies (a, b) and surgical specimen (c, d) of adenocarcinomas of the gastroesophageal junction stained for programmed death ligand 2 (PD-L2). a Positive signals of PD-L2 expression in cancer cells and b in tumor-infiltrating lymphocytes. c No signal or low expression of PD-L2 in cancer cells and d in tumor-infiltrating lymphocytes. Scale bar = 20 µm. Original magnification ×400 all
Discussion
The incidence of adenocarcinoma of the esophagogastric junction (AEG) has increased markedly over the past years in the western world. Its aggressive nature leads to early local invasion and systemic spreading [1, 2]. In recent years, cancer immunotherapy has become another pillar in cancer therapy. The clinical use of monoclonal antibodies targeting the PD‑1 pathway has achieved major improvements in patient survival in multiple malignancies. The immune suppressive proteins PD-L1 and PD-L2 limit T‑cell activation and cytokine production, which contributes to immunosurveillance [14, 29,30,31,32]. Compared to PD-L1, the relevance of PD-L2 tumor-expression has not been explored intensively. Regarding AEG, only little information is available on its immune characteristics. Derks et al. were able to detect PD-L2 expression by cancer cells in 52% of patients with esophageal adenocarcinoma [33]. Recently, our group investigated the role of PD1, PD-L1, and PD-L2 expression as a prognostic factor in patients with resectable AEG. In that study, PD-L2 expression was only occasionally found on TILs and cancer cells (1.8 and 3.5%, respectively) [22, 23].
The standard approach for the treatment of patients with locally advanced AEG is cytotoxic chemotherapy or chemoradiotherapy followed by surgery. The use of cytotoxic chemotherapy is inevitable for treating advanced cancers. These drugs target nucleic acid metabolism or interfere with the microtubule network of proliferating tumor cells. In recent years, various studies reported that PD-L1 expression can be either decreased or increased by neoadjuvant chemotherapy according to the cancer type. These results suggested that the immune response, altered by the chemotherapeutical drugs, could be inhibited by upregulated PD-L1, which may play a role in chemoresistance [30, 31, 34,35,36,37,38,39,40]. The potential change in PD-L2 expression has hardly been researched; regarding AEG, no data were available until now. With chemoresistance being a growing problem, it is important to further investigate the effects of chemotherapy on PD-L2 expression to gain more understanding of this issue in anti-cancer therapy.
In this study, we aimed to investigate the potential effect of oxaliplatin/capecitabine- and cisplatin/5-fluorouracil-based neoadjuvant therapy on the expression of PD-L2 in cancer cells and TILs in neoadjuvantly treated AEG using immunohistochemistry.
In our study, PD-L2 expression by AEG cancer cells and TILs was detected in only one out of 33 specimens of diagnostic biopsies. All the 40 surgical (post neoadjuvant therapy) specimens were rated as negative regarding PD-L2 expression. Therefore, we were not able to further investigate/illustrate a potential correlation between neoadjuvant treatment and the expression pattern of PD-L2. The positive expression by 6% of the specimens of diagnostic biopsies is a result that needs to be validated in a larger study population.
We have to consider certain limitations of our study. There is a potential selection bias, caused by only partial availability of tumor tissue, especially of diagnostic biopsies. The small amount of tissue obtained from a biopsy might not be sufficient to represent the PD-L2 expression rate of the tumor. Further, the retrospective nature of this single-center study represents another limitation. In general, the immunohistochemical methods for PD-L2 are not yet well established compared to those for PD-L1. Dhupar et al. have proposed that different criteria for positive staining of PD-L2 are a major reason for the generally inconsistent results [41]. Another aspect would be that patient cohorts from different studies vary regarding their demographic and pathologic factors, which can also affect the results of PD‑L expression analysis. Regarding the evaluation of the immunohistochemical expression grade, there is always a chance of interobserver variability, especially in borderline cases. Last but not least, an important limitation to mention, is the small study population.
Surprisingly, we found no PD-L2 expression in postsurgical specimens in this study. These findings are in contrast with our previously published data on the expression of PD-L2 as a potentially prognostic factor in AEG, describing 3.5% positive PD-L2 cases in patients with primarily resected and neoadjuvantly treated AEG [22]. The different expression rate may result from the inconsistent study populations, but it could also be interpreted as a small indication for a negative effect of the chemotherapy on PD-L2 expression. On the other hand, the non-existent PD-L2 expression in the present study is possibly related to the used antibodies. The issue of controversies attributed to different antibody specificity that leads to both underestimating and overestimating the results has been reported in various studies investigating PD-L1 and PD-L2 expression [41,42,43].
To the best of our knowledge, this is the first study investigating the expression of PD-L2 in neoadjuvantly treated AEG patients.
In conclusion, we were not able to demonstrate a significant change in the expression of PD-L2 in AEG patients given neoadjuvant treatment. Our results can be interpreted as a tendency and definitely need further investigation. It is of great importance to gain better understanding into how cytotoxic chemotherapy affects PD-L2 expression and function to potentially contribute to develop new and highly effective anti-cancer therapies for patients suffering from AEG.
References
Berlth F, Hoelscher AH. History of esophagogastric junction cancer treatment and current surgical management in western countries. J Gastric Cancer. 2019;19(2):139–47.
Arnold M, Ferlay J, van Berge Henegouwen MI, Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. 2020;69(9):1564–71.
Zhang S, Orita H, Fukunaga T. Current surgical treatment of esophagogastric junction adenocarcinoma. World J Gastrointest Oncol. 2019;11(8):567–78.
Holscher AH, Vallbohmer D, Schroder W, Bollschweiler E. Limited surgery for ‘early’ cancer of the esophagus. Eur Surg. 2007;39(5):273–80.
Lindenmann J, Maier A, Matzi V, Neuboeck N, Anegg U, Porubsky C, et al. Photodynamic therapy for esophageal carcinoma(*). Eur Surg. 2011;43(6):355–65.
Falk GL, Archer L, Gooley SC. Is fundoplication advisable in repair of para-oesophageal hernia? “Little operation” or “big operation”? Eur Surg. 2020;52(6):277–81.
Kersebaum JN, Moller T, Becker T, Egberts JH. Robotic resection for esophageal cancer. Eur Surg. 2020. https://doi.org/10.1007/s10353-020-00675-8
Chandrasoma P. New evidence defining the pathology and pathogenesis of lower esophageal sphincter damage. Eur Surg. 2019;51(6):282–90.
Mitchell DP, Yeluri S, Van der Wall H, Falk GL. The real risk of nodal disease in T1 oesophageal adenocarcinoma A systematic review. Eur Surg. 2020;52(3):110–7.
Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393(10184):1948–57.
Kleinberg L, Brock M, Gibson M. Management of locally advanced adenocarcinoma of the esophagus and gastroesophageal junction: finally a consensus. Curr Treat Options Oncol. 2015;16(7):35.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97.
Gerson JN, Skariah S, Denlinger CS, Astsaturov I. Perspectives of HER2-targeting in gastric and esophageal cancer. Expert Opin Investig Drugs. 2017;26(5):531–40.
Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17(8):807–21.
Friedl J, Riss S, Stift A. Is immunotherapy a reasonable approach for the treatment of esophageal cancer? Eur Surg. 2007;39(3):158–66.
Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD‑1 expression. Clin Cancer Res. 2004;10(15):5094–100.
Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20(11):651–68.
Pedoeem A, Azoulay-Alfaguter I, Strazza M, Silverman GJ, Mor A. Programmed death‑1 pathway in cancer and autoimmunity. Clin Immunol. 2014;153(1):145–52.
Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. PD-L2 is a second ligand for PD‑1 and inhibits T cell activation. Nat Immunol. 2001;2(3):261–8.
Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101(49):17174–9.
Kato K, Shah MA, Enzinger P, Bennouna J, Shen L, Adenis A, et al. KEYNOTE-590: Phase III study of first-line chemotherapy with or without pembrolizumab for advanced esophageal cancer. Future Oncol. 2019;15(10):1057–66.
Kollmann D, Ignatova D, Jedamzik J, Chang YT, Jomrich G, Baierl A, et al. PD-L1 expression is an independent predictor of favorable outcome in patients with localized esophageal adenocarcinoma. OncoImmunology. 2018;7(6):e1435226.
Kollmann D, Ignatova D, Jedamzik J, Chang YT, Jomrich G, Paireder M, et al. Expression of programmed cell death protein 1 by tumor-infiltrating lymphocytes and tumor cells is associated with advanced tumor stage in patients with esophageal Adenocarcinoma. Ann Surg Oncol. 2017;24(9):2698–706.
Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg. 2017;6(2):119–30.
Pimentel-Nunes P, Libanio D, Marcos-Pinto R, Areia M, Leja M, Esposito G, et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy. 2019;51(4):365–88.
Weusten B, Bisschops R, Coron E, Dinis-Ribeiro M, Dumonceau JM, Esteban JM, et al. Endoscopic management of Barrett’s esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2017;49(2):191–8.
Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73(11):2680–6.
Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg. 1998;85(11):1457–9.
Festino L, Botti G, Lorigan P, Masucci GV, Hipp JD, Horak CE, et al. Cancer treatment with anti-PD-1/PD-L1 agents: is PD-L1 expression a biomarker for patient selection? Drugs. 2016;76(9):925–45.
Forde PM, Chaft JE, Pardoll DM. Neoadjuvant PD‑1 blockade in resectable lung cancer. N Engl J Med. 2018;379(9):e14.
Garcia-Aranda M, Perez-Ruiz E, Redondo M. Bcl‑2 inhibition to overcome resistance to chemo- and immunotherapy. Int J Mol Sci. 2018. https://doi.org/10.3390/ijms19123950
Gatalica Z, Snyder C, Maney T, Ghazalpour A, Holterman DA, Xiao N, et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev. 2014;23(12):2965–70.
Derks S, Nason KS, Liao X, Stachler MD, Liu KX, Liu JB, et al. Epithelial PD-L2 expression marks Barrett’s esophagus and esophageal Adenocarcinoma. Cancer Immunol Res. 2015;3(10):1123–9.
Gilad Y, Eliaz Y, Yu Y, Han SJ, O’Malley BW, Lonard DM. Drug-induced PD-L1 expression and cell stress response in breast cancer cells can be balanced by drug combination. Sci Rep. 2019;9(1):15099.
Guo L, Song P, Xue X, Guo C, Han L, Fang Q, et al. Variation of programmed death Ligand 1 expression after platinum-based neoadjuvant chemotherapy in lung cancer. J Immunother. 2019;42(6):215–20.
Huang AC, Orlowski RJ, Xu X, Mick R, George SM, Yan PK, et al. A single dose of neoadjuvant PD‑1 blockade predicts clinical outcomes in resectable melanoma. Nat Med. 2019;25(3):454–61.
Jomrich G, Silberhumer GR, Marian B, Beer A, Mullauer L. Programmed death-ligand 1 expression in rectal cancer. Eur Surg. 2016;48(6):352–6.
Lim SH, Hong M, Ahn S, Choi YL, Kim KM, Oh D, et al. Changes in tumour expression of programmed death-ligand 1 after neoadjuvant concurrent chemoradiotherapy in patients with squamous oesophageal cancer. Eur J Cancer. 2016;52:1–9.
Mesnage SJL, Auguste A, Genestie C, Dunant A, Pain E, Drusch F, et al. Neoadjuvant chemotherapy (NACT) increases immune infiltration and programmed death-ligand 1 (PD-L1) expression in epithelial ovarian cancer (EOC). Ann Oncol. 2017;28(3):651–7.
Yu Y, Ma X, Zhang Y, Zhang Y, Ying J, Zhang W, et al. Changes in expression of multiple checkpoint molecules and infiltration of tumor immune cells after neoadjuvant chemotherapy in gastric cancer. J Cancer. 2019;10(12):2754–63.
Dhupar R, Van Der Kraak L, Pennathur A, Schuchert MJ, Nason KS, Luketich JD, et al. Targeting immune checkpoints in esophageal cancer: a high mutational load tumor. Ann Thorac Surg. 2017;103(4):1340–9.
Parra ER, Villalobos P, Mino B, Rodriguez-Canales J. Comparison of different antibody clones for Immunohistochemistry detection of programmed cell death Ligand 1 (PD-L1) on non-small cell lung carcinoma. Appl Immunohistochem Mol Morphol. 2018;26(2):83–93.
Takada K, Toyokawa G, Shoji F, Okamoto T, Maehara Y. The significance of the PD-L1 expression in non-small-cell lung cancer: trenchant double swords as predictive and prognostic markers. Clin Lung Cancer. 2018;19(2):120–9.
Funding
Open access funding provided by Medical University of Vienna.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
This study was partially supported by the Promedica Stiftung (1406/M and 1412/M), the Swiss Cancer Research Foundation (KFS-4243-08-2017), the Swiss National Science Foundation (PMPDP3_151326), the Clinical Research Priority Program (CRPP) of the University of Zurich, and the European Academy of Dermatology and Venereology (PPRC-2019-20). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. A. Ilhan-Mutlu participated in advisory boards from MSD, BMS, and Servier; received lecture honoraria from Eli Lilly, MSD, and Servier; is the local PI for clinical trials sponsored by BMS and Roche; and received travel support from BMS, Roche, and Servier. M. Preusser has received honoraria for lectures, consultation, or advisory board participation from the following for-profit companies: Bayer, Bristol-Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, Astra Zeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dome, Tocagen, Adastra. G. Jomrich, D. Kollmann, L. Wilfing, S. Radosavljevic, D. Ramazanova, R. Ristl, R.P. Grose, C. Fassnacht, Y.-C. Tsai, E. Guenova, and S.F. Schoppmann declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jomrich, G., Kollmann, D., Wilfing, L. et al. Programmed death ligand 2 expression plays a limited role in adenocarcinomas of the gastroesophageal junction after preoperative chemotherapy. Eur Surg 53, 287–293 (2021). https://doi.org/10.1007/s10353-021-00700-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10353-021-00700-4