Summary
A gallbladder polyp (GP) is defined as an elevation of the gallbladder mucosa that protrudes into the gallbladder lumen. Gallbladder polyps (GPs) have an estimated prevalence in adults of 0.3–12.3%. However, only 5% of polyps are considered “true” GPs that have malignant potential or are even already cancerous. The most important imaging method for diagnosis and follow-up of GPs is transabdominal ultrasound, but it fails to discriminate between true and pseudo polyps at a clinically relevant level. Although gallbladder cancer (GBC) arising from polyps is a rare event, malignancy is significantly more common among polyps from a size of 10 mm. In light of this, the consensus, which is reflected in current guidelines, is that surgery should be considered for polyps of 10 mm or greater. However, 10 mm is an arbitrary cutoff, and high-quality evidence to support this is lacking. Lowering the threshold for cholecystectomy when patients have additional risk factors for gallbladder malignancy may improve the cancer detection rate in polyps smaller than 10 mm. Nevertheless, the evidence behind this is also weak. This review shows the shortcomings in the available evidence and underlines the decision-making process regarding the surgical indication, surveillance, or both.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gallbladder polyps (GPs) have an estimated prevalence of approximately 0.3–12.3% [1, 2], which varies widely in different regions of the world. They are rarely symptomatic [3, 4] and are mainly incidental findings. Until recently it was generally assumed that polyps in the gallbladder have the potential to grow and become cancerous over many decades [5]. However, a recent population-based study found out that the natural history of polyps is to grow over time, and polyps even more than 10 mm in size are rarely associated with gallbladder cancer (GBC; [6]). The majority of GPs are classified as pseudo or cholesterol polyps. They have no malignant potential and, therefore, do not require follow-up or intervention. Past studies assumed that “true” GPs occur in 5% of cases [1, 2, 7] and are subject to the adenoma–carcinoma sequence [8]. Although rare, malignancy is significantly more common among polyps of 10 mm or greater in size [2, 7]. It is therefore generally accepted that from this size up, GPs have to be removed surgically, preferably by laparoscopic cholecystectomy [2, 9, 10]. Nevertheless, it remains uncertain how many polyps in the gallbladder ultimately progress and become cancerous. The aforementioned recently published study involving more than 600,000 patients reported a very low incidence of GBC in GP carriers, which was interestingly comparable to patients without GPs [6].
This review discusses recent findings on the central role of imaging in diagnosis and discrimination between “true” and pseudo polyps and their treatment. Given the low incidence of true polyps, the decision for laparoscopic cholecystectomy must be balanced against its postoperative morbidity, which is moderate but severe when bile duct injuries occur. The recommendations of recent guidelines are not uniform and rely on a low level of evidence due to the small numbers, short follow-up, and current data not being taken into account in the referenced studies. The white paper of the American College of Radiology, for example, recommends yearly follow-up for polyps sized 7–9 mm and consultation for cholecystectomy in the case of a polyp growth [9]. The American Society for Gastrointestinal Endoscopy also recommends indefinite yearly monitoring for GPs [11], and a joint guideline from various European societies recommended surgery for all polyps larger than 10 mm and those larger than 6 mm with risk factors as well as 5 years of follow-up for all other polyps [10]. In view of recent findings, there appears to be a need for a revision of existing guidelines, in particular concerning periodic ultrasonography of GPs to detect GBC proactively.
Diagnosis
Accurate imaging plays a significant role in the diagnosis and treatment of GPs. In addition to the differential diagnostic delineation of gallstones, sludge, or physiological variants of the gallbladder mucosa [12, 13], imaging should be able to differentiate between true and pseudo polyps. It is reported that ultrasonography is not accurate (less than 70% sensitivity) in differentiating polyps (true or pseudo polyps) from gallstones [12]. Yet the distinction between adenomas and non-adenomas is still made after the operation. The preoperative examination, therefore, is required to show a high degree of accuracy in order to make a reliable treatment recommendation. Also, radiation exposure plays a role in the repeated use of imaging, for example, during follow-up observations. The primary modalities discussed include ultrasound, computed tomography, and magnetic resonance imaging.
Transabdominal ultrasound examination
Conventional ultrasound (US), as well as its high-resolution version (hrUS), are widely used and therefore easily accessible examination methods. Additionally, they are inexpensive, repeatable, allow for a functional assessment under certain conditions, and are—not to be overlooked—a noninvasive technique [14]. In terms of their sensitivity and specificity in the diagnosis of gallbladder pathologies, especially of GPs, they are, however, subject to interobserver variability. With a low-frequency transducer between 2 and 5 MHz, acceptable specificity (71–98%) and sensitivity (50–90%) are, nevertheless, achieved for all types of GPs [15]. However, numerous factors, such as obesity or fixed, incrusted gallbladder stones [2, 8, 16], affect accuracy [4]. As a result, the reported prevalence of transabdominal US is 3–7%, compared with 2–12% in cholecystectomy specimens [17,18,19].
The echogenicity of the lesion, which is essential for differentiating between true polyps and pseudo polyps, distinguishes it from gallstones. There is a typical feature, the so-called comet-tail artifact, which develops behind many, but not all, pseudo polyps [20]. Nevertheless, the differentiation according to malignancy, with a sensitivity of only 47–67% and specificity of only 36–100% for all polyps, does not meet the expectations of clinical decision-making. For polyps of 10 mm or more in size, the sensitivity and specificity increase to 78–100% and 52–87%, respectively, to a clinically more useful range [15]. Besides this, even negative results of conventional US have been reported. Only 50% of the polyps were identified by imaging when compared with corresponding histopathological findings [19]. These findings call into question a follow-up based on transabdominal conventional US.
High-resolution US works with a frequency between 5 and 7 MHz and is particularly suitable for the determination of the T‑level in GBC. It has higher accuracy than the conventional US and can detect more precisely hypoechogenic foci, which are a predictive factor for the presence of neoplastic GPs [7, 21, 22]. Other methods of percutaneous application of US, such as three-dimensional reconstruction and contrast-enhanced ultrasound (ceUS), have not brought about any further improvements in accuracy in the diagnosis and differentiation between true and pseudo polyps, especially in the case of a polyp size of less than 10 mm [7, 23]. Although all of these advanced percutaneous ultrasound-based methods are generally superior to computed tomography (CT; [24]), they are not recommended for routine use.
Endoscopic ultrasound
By increasing the spatial resolution with endoscopic ultrasound (eUS), the important determination of the echogenicity of the lesion can be improved [25] at the price of invasiveness. The potential risk of complications should be taken into account, which, although clinically hardly relevant, are increased compared with the transabdominal procedure [26]. A systematic review demonstrated higher sensitivity (67–86%) and specificity (84–91%) of eUS in detecting malignant polyps when compared with conventional US [15]. However, studies have also shown that differentiation concerning malignancy depends on polyp size [27, 28] with the cutoff repeatedly being around 10 mm. There are studies with a deficient number of cases for contrast-enhanced eUS [29, 30] or other methods (e.g., real-time color Doppler flow eUS; [31]).
Additional imaging modalities
Computed tomography imaging plays a role in the staging of GBC [4]. Still, it is not suitable for differentiating between true and pseudo polyps and for long-term monitoring and is not superior to US [24, 32]. Diffusion-weighted magnetic resonance imaging (MRI) showed significant differentiation in only ten benign and 13 malignant polyps [33]. Otherwise, however, there is a lack of relevant MRI studies supported by a corresponding number of cases that focus on the differentiation between benign and malignant GPs. The role of positron emission tomography (PET) has not been sufficiently investigated. Percutaneous transhepatic cholecystoscopy is a method that cannot be used in practice because of its invasiveness, but it has shown promising results [4]. Intravenous cholecystoscopy is obsolete [29].
Indication for cholecystectomy
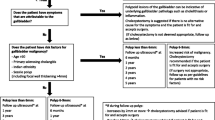
Malignant polyps are generally larger than benign ones (mean diameter 27.97 ± 2.46 mm vs. 8.56 ± 0.36 mm, respectively [14, 15]). Even though the development of malignant GPs seems to be overestimated, polyp size is the major risk factor. From a diameter of 10 mm, the risk of malignancy increases with a probability of 37–55% [34]. Since, as mentioned above, the sensitivity and specificity of the most appropriate examination also increase with the size of the polyp, the diameter of the GPs is the decisive factor in their management. It is accepted practice and is reflected in relevant guidelines, such as the European Society of Gastrointestinal and Abdominal Radiology (ESGAR; [10]), that patients with polyps of 10 mm or more in size should be treated with cholecystectomy [7, 15, 35]. However, it should be emphasized that evidence from controlled randomized studies is still lacking. There are also conflicting findings in reviews. It was shown that a significant number of polyps smaller than 10 mm have malignant potential, or even already show carcinoma [36, 37]. However, none of the cancerous polyps was smaller than 5 mm. Some authors, therefore, call for a cholecystectomy at a size of 6 mm. Due to the postoperative morbidity following cholecystectomy, there is a risk of overtreatment, which is why this procedure has not been generally accepted.
Follow-up imaging may have a limited benefit. In the most extensive population-based study to date, most GBCs were diagnosed in the first year after the detection of polyps and GBC did not appear to depend on the presence of polyps [6]. However, it is frequently believed that true polyps grow faster and therefore can be detected and removed much earlier through careful follow-up so that radiological monitoring is recommended in the case of a polyp size of less, but also more, than 10 mm [15]. Nevertheless, there is no consensus about the size of the polyps nor the frequency or duration of follow-up examinations. Small sample sizes limit studies of the evolution of GPs, and polyp growth seems to be a part of its natural history [6]. A large proportion of polyps remain constant in size for years, even over more extended periods of observation [15, 35]. A sudden increase in the size of true as well as pseudo polyps was only observed in studies with minimal case numbers [4].
Risk factors for malignancy
As already discussed, the size of the polyps is the determining factor for the potential presence of GBC. However, since polyps smaller than 10 mm can also be malignant, the indication for a cholecystectomy is only given in the presence of risk factors for malignancy.
The number of polyps and their morphology could be risk factors. In some studies, malignant polyps are more likely to be solitary even when smaller than 10 mm [14, 35]. Nevertheless, the authors concluded that this difference does not justify a recommendation for cholecystectomy. Further studies that show no correlation between a solitary polyp and malignancy support this assumption [30]. Yet, the current ESGAR guidelines use the strength of evidence to recommend cholecystectomy for all sessile polyps between 6 and 9 mm in size. Especially in a combination of a solitary and sessile polyp smaller than 10 mm, the proportion of malignant polyps was 24.8% [35].
As with any entity, age plays a role. Increased risk of malignancy varies and starts at the age of 50–65 years [30, 35, 38, 39]. The ESGE guidelines recommend that patients with polyps of 6–9 mm should undergo cholecystectomy at the age of 50 [10]. The increased risk of malignancy in patients with a family history appears to be well established [40,41,42] although exposure to similar environmental influences may lead to a bias. Overall, there is limited evidence of a genetic predisposition to GBC, and studies regarding GPs have not been conducted.
In the presence of cholecystolithiasis, the risk assessment in studies varies considerably, too, and is also of relatively low quality. Increased risk (HR: 3.2; 95% CI: 1.42–7.22; [43]) but also no association [30] was described. In patients with symptoms due to gallstones, a cholecystectomy is already recommended, and thus the decision process is simple.
For the tumor markers CEA and CA19‑9, there is insufficient evidence that they may be critical in assessing the risk of malignancy.
It is important to note that primary sclerosing cholangitis (PSC) is a recognized risk factor for the malignancy of GPs [44, 45]. Cholecystectomy is currently recommended for these patients with a GP regardless of polyp size [46], as the largest study to date involving 286 PSC patients found that in 18 patients with a GP, ten already had a malignant polyp as small as 5 mm. Nine patients without a radiologically detectable lesion already showed dysplasia of the gallbladder mucosa [47].
Discussion
It remains unclear whether polyps in the gallbladder have the potential to grow and become cancerous over many decades. Gaps in the available evidence to support the current guidelines for the treatment of GPs are outlined. The relevant radiological examination with the most reliable evidence is conventional percutaneous US, which is widely available, repeatable, and without radiation exposure. Optional additions such as the hrUS or eUS are promising modalities to further discriminate between true and pseudo polyps, and thus to assess the risk of malignancy and to support clinicians in their decision-making process.
Although the risk of a malignant polyp is related to size, it remains uncertain how many polyps ultimately progress and become cancerous. The indication for cholecystectomy, which is strongly recommended for polyps of 10 mm in size or greater, means that a considerable number of unnecessary operations are accepted. The evidence for smaller GPs is of even lower quality. Studies suggest that asymptomatic polyps smaller than 10 mm do have the capacity to become cancerous and surgery is recommended for risk factors such as PSC, solitary and sessile polyps, even from a size of 5 mm. For polyps smaller than 10 mm without risk factors, a follow-up at least every 2 years with percutaneous US seems to be appropriate. The indication for cholecystectomy should be discussed with the patient in the case of dynamic polyp growth. To date, no studies have been conducted to assess the adherence and effects of following these guidelines. Therefore, more extensive retrospective and prospective studies must be done to determine the success of GP treatment according to current guidelines.
References
Lin W‑R, Lin D‑Y, Tai D‑I, Hsieh S‑Y, Lin C‑Y, Sheen I‑S, et al. Prevalence of and risk factors for gallbladder polyps detected by ultrasonography among healthy Chinese: analysis of 34 669 cases. J Gastroenterol Hepatol. 2008;23(6):965–9.
Okamoto M, Okamoto H, Kitahara F, Kobayashi K, Karikome K, Miura K, et al. Ultrasonographic evidence of association of polyps and stones with gallbladder cancer. Am J Gastroenterol. 1999;94(2):446–50.
Aliyazicioglu T, Carilli S, Emre A, Kaya A, Bugra D, Bilge O, et al. Contribution of gallbladder polyp surgery to treatment. Eur Surg. 2017;49(1):23–6. https://doi.org/10.1007/s10353-016-0422-4.
Andrén-Sandberg Å. Diagnosis and management of gallbladder polyps. N Am J Med Sci. 2012;4(5):203.
Kozuka S, Tsubone N, Yasui A, Hachisuka K. Relation of adenoma to carcinoma in the gallbladder. Cancer. 1982;50(10):2226–34.
Szpakowski JL, Tucker LY. Outcomes of gallbladder polyps and their association with gallbladder cancer in a 20-year cohort. JAMA Netw Open. 2020;3(5):e205143.
McCain RS, Diamond A, Jones C, Coleman HG. Current practices and future prospects for the management of gallbladder polyps: a topical review. World J Gastroenterol. 2018;24(26):2844–52.
Aldridge MC, Bismuth H. Gallbladder cancer: the polyp-cancer sequence. Br J Surg. 1990;77(4):363–4.
Sebastian S, Araujo C, Neitlich JD, Berland LL. Managing incidental findings on abdominal and pelvic CT and MRI, part 4: white paper of the ACR incidental findings committee II on gallbladder and biliary findings. J Am Coll Radiol. 2013;10(12):953–6. https://doi.org/10.1016/j.jacr.2013.05.022.
Wiles R, Thoeni RF, Barbu ST, Vashist YK, Rafaelsen SR, Dewhurst C, et al. Management and follow-up of gallbladder polyps: joint guidelines between the European society of gastrointestinal and abdominal radiology (ESGAR), European association for endoscopic surgery and other Interventional techniques (EAES), international society of digestive surgery - European federation (EFISDS) and European society of gastrointestinal endoscopy (ESGE). Eur Radiol. 2017;27(9):3856–66.
Anderson MA, Appalaneni V, Ben-Menachem T, Decker GA, Early DS, Evans JA, et al. The role of endoscopy in the evaluation and treatment of patients with biliary neoplasia. Gastrointest Endosc. 2013;77(2):167–74.
Chattopadhyay D. Outcome of gall bladder polypoidal lesions detected by transabdominal ultrasound scanning: a nine year experience. World J Gastroenterol. 2005;11(14):2171.
Soyer P, Gouhiri M, Boudiaf M, Brocheriou-Spelle I, Kardache M, Fishman EK, et al. Carcinoma of the gallbladder: imaging features with surgical correlation. AJR Am J Roentgenol. 1997;169(3):781–5.
Kwon W, Jang J‑Y, Lee SE, Hwang DW, Kim S‑W. Clinicopathologic features of polypoid lesions of the gallbladder and risk factors of gallbladder cancer. J Korean Med Sci. 2009;24(3):481–7.
Babu BI, Dennison AR, Garcea G. Management and diagnosis of gallbladder polyps: a systematic review. Langenbecks Arch Surg. 2015;400(4):455–62.
Gallahan WC, Conway JD. Diagnosis and management of gallbladder polyps. Gastroenterol Clin North Am. 2010;39(2):359–67.
Chatterjee A, Lopes Vendrami C, Nikolaidis P, Mittal PK, Bandy AJ, Menias CO, et al. Uncommon intraluminal tumors of the gallbladder and biliary tract: spectrum of imaging appearances. Radiographics. 2019;39(2):388–412.
Kitazume Y, Taura S‑I, Nakaminato S, Noguchi O, Masaki Y, Kasahara I, et al. Diffusion-weighted magnetic resonance imaging to differentiate malignant from benign gallbladder disorders. Eur J Radiol. 2016;85(4):864–73.
French DG, Allen PD, Ellsmere JC. The diagnostic accuracy of transabdominal ultrasonography needs to be considered when managing gallbladder polyps. Surg Endosc. 2013;27(11):4021–5.
Shapiro RS, Winsberg F. Comet-tail artifact from cholesterol crystals: observations in the postlithotripsy gallbladder and an in vitro model. Radiology. 1990;177(1):153–6.
Kim JH, Lee JY, Baek JH, Eun HW, Kim YJ, Han JK, et al. High-resolution sonography for distinguishing neoplastic gallbladder polyps and staging gallbladder cancer. AJR Am J Roentgenol. 2015;204(2):W150–9.
Yu MH, Kim YJ, Park HS, Jung SI. Benign gallbladder diseases: Imaging techniques and tips for differentiating with malignant gallbladder diseases. World J Gastroenterol. 2020;26(22):2967–86.
Numata K, Oka H, Morimoto M, Sugimori K, Kunisaki R, Nihonmatsu H, et al. Differential diagnosis of gallbladder diseases with contrast-enhanced harmonic gray scale ultrasonography. J Ultrasound Med. 2007;26(6):763–74.
Furukawa H, Kosuge T, Shimada K, Yamamoto J, Kanai Y, Mukai K, et al. Small polypoid lesions of the gallbladder: differential diagnosis and surgical indications by helical computed tomography. Arch Surg. 1998;133(7):735–9.
Cho JH, Park JY, Kim YJ, Kim HM, Kim HJ, Hong SP, et al. Hypoechoic foci on EUS are simple and strong predictive factors for neoplastic gallbladder polyps. Gastrointest Endosc. 2009;69(7):1244–50.
Wennmacker SZ, Lamberts MP, Di Martino M, Drenth JP, Gurusamy KS, van Laarhoven CJ. Transabdominal ultrasound and endoscopic ultrasound for diagnosis of gallbladder polyps. Cochrane Database Syst Rev. 2018;8:CD12233.
Christensen M, Matzen P, Schulze S, Rosenberg J. Complications of ERCP: a prospective study. Gastrointest Endosc. 2004;60(5):721–31.
Cheon YK, Cho WY, Lee TH, Cho YD, Moon JH, Lee JS, et al. Endoscopic ultrasonography does not differentiate neoplastic from non-neoplastic small gallbladder polyps. World J Gastroenterol. 2009;15(19):2361–6.
de Matos ASB, Baptista HN, Pinheiro C, Martinho F. Gallbladder polyps: how should they be treated and when? Rev Assoc Med Bras. 2010;56(3):318–21.
Park JK, Yoon YB, Kim Y‑T, Ryu JK, Yoon WJ, Lee SH, et al. Management strategies for gallbladder polyps: is it possible to predict malignant gallbladder polyps? Gut Liver. 2008;2(2):88–94.
Kim SY, Cho JH, Kim EJ, Chung DH, Kim KK, Park YH, et al. The efficacy of real-time colour Doppler flow imaging on endoscopic ultrasonography for differential diagnosis between neoplastic and non-neoplastic gallbladder polyps. Eur Radiol. 2018;28(5):1994–2002.
Lou M‑W, Hu W‑D, Fan Y, Chen J‑H, E Z‑S, Yang G‑F. CT biliary cystoscopy of gallbladder polyps. World J Gastroenterol. 2004;10(8):1204–7.
Irie H, Kamochi N, Nojiri J, Egashira Y, Sasaguri K, Kudo S. High b‑value diffusion-weighted MRI in differentiation between benign and malignant polypoid gallbladder lesions. Acta Radiol. 2011;52(3):236–40.
Adsay V, Jang K‑T, Roa JC, Dursun N, Ohike N, Bagci P, et al. Intracholecystic papillary-tubular neoplasms (ICPN) of the gallbladder (neoplastic polyps, adenomas, and papillary neoplasms that are ≥1.0 cm): clinicopathologic and immunohistochemical analysis of 123 cases. Am J Surg Pathol. 2012;36(9):1279–301. https://doi.org/10.1097/PAS.0b013e318262787c.
Bhatt NR, Gillis A, Smoothey CO, Awan FN, Ridgway PF. Evidence based management of polyps of the gall bladder: a systematic review of the risk factors of malignancy. Surgeon. 2016;14(5):278–86.
Pedersen MRV, Dam C, Rafaelsen SR. Ultrasound follow-up for gallbladder polyps less than 6 mm may not be necessary. Dan Med J. 2012;59(10):A4503.
Corwin MT, Siewert B, Sheiman RG, Kane RA. Incidentally detected gallbladder polyps: is follow-up necessary?—long-term clinical and US analysis of 346 patients. Radiology. 2011;258(1):277–82.
Lee KF, Wong J, Li JCM, Lai PBS. Polypoid lesions of the gallbladder. Am J Surg. 2004;188(2):186–90.
Cha BH, Hwang J‑H, Lee SH, Kim JE, Cho JY, Kim H, et al. Pre-operative factors that can predict neoplastic polypoid lesions of the gallbladder. World J Gastroenterol. 2011;17(17):2216–22.
Hsing AW, Bai Y, Andreotti G, Rashid A, Deng J, Chen J, et al. Family history of gallstones and the risk of biliary tract cancer and gallstones: a population-based study in Shanghai, China. Int J Cancer. 2007;121(4):832–8.
Hemminki K, Li X. Familial liver and gall bladder cancer: a nationwide epidemiological study from Sweden. Gut. 2003;52(4):592–6.
Liebe R, Milkiewicz P, Krawczyk M, Bonfrate L, Portincasa P, Krawczyk M. Modifiable factors and genetic predisposition associated with gallbladder cancer. A concise review. J Gastrointestin Liver Dis. 2015;24(3):339–48.
Aldouri AQ, Malik HZ, Wyatt J, Khan S, Ranganathan K, Kummaraganti S, et al. The risk of gallbladder cancer from polyps in a large multiethnic series. Eur J Surg Oncol. 2009;35(1):48–51.
Leung UC, Wong PY, Roberts RH, Koea JB. Gall bladder polyps in sclerosing cholangitis: does the 1‑cm rule apply? ANZ J Surg. 2007;77(5):355–7.
Eaton JE, Thackeray EW, Lindor KD. Likelihood of malignancy in gallbladder polyps and outcomes following cholecystectomy in primary sclerosing cholangitis. Am J Gastroenterol. 2012;107(3):431–9.
European Association for the Study of the Liver. EASL clinical practice guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51(2):237–67.
Said K, Glaumann H, Bergquist A. Gallbladder disease in patients with primary sclerosing cholangitis. J Hepatol. 2008;48(4):598–605.
Funding
Open access funding provided by University of Innsbruck and Medical University of Innsbruck.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
D. Öfner-Velano declares that he has no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Öfner, D. Management of gallbladder polyps. Eur Surg 53, 119–123 (2021). https://doi.org/10.1007/s10353-020-00659-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10353-020-00659-8