Abstract
The proliferation of gull populations in urban areas has led to an increase in human-gull conflicts, especially in highly populated cities where these opportunistic predators are often considered a nuisance. There is a lack of data regarding the selection of nesting sites by gulls, so management measures at nesting level cannot be implemented. Here, we investigated the main environmental factors that explain the nesting preferences of the yellow-legged gull (Larus michahellis) in urban areas, using the population in the city of Barcelona (NE Spain) as study model. We conducted an integrated analysis that combines micro-scale habitat selection assessments of 148 urban nesting sites with a macro-scale assessment of gull movements derived from GPS tracking of breeding yellow-legged gulls. We also analysed the type and abundance of litter in nests and main factors related to this. Nests were mainly found on flat roofs or above a structure on the main roof located at corner placements and situated on gravel substrate. Nest selection showed a negative relation to port distance and a building height beyond 12 m. The presence of litter was detected in more than 80% nests and was related to population density. Understanding the ecology of yellow-legged gulls in urban areas has implications for urban population management to prevent nest establishment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The growth of the human population has led to an increase in anthropogenic impacts on natural ecosystems (McKinney and Lockwood 1999; He and Silliman 2019). Among these human impacts, the process of urbanization has notably affected biodiversity, displacing species from their natural habitats or reducing the size of their populations (McKinney 2006; McCauley et al. 2015; Goddard et al. 2010). However, although a majority of wildlife suffer adverse consequences due to urbanization (Chace and Walsh 2006), some successful species have benefited from these human-altered environments (McKinney and Lockwood 1999). Gulls are examples of wildlife capable of thriving in anthropogenic environments, including urban ecosystems, where they have established breeding populations (Rock et al. 2016; Spelt et al. 2019; Méndez et al. 2020; Coccon et al. 2022). These successful species often show an opportunistic and generalist behaviour and are able to exploit accessible and abundant trophic resources in cities and surroundings (Méndez et al. 2020; Carmona et al. 2021; Lopes et al. 2020; Coccon et al. 2022).
The notable proliferation of gull populations in urban areas has led to an increase in human-gull conflicts, especially in highly populated cities (Vidal et al. 1998; Spelt et al. 2019; Benussi and Fraissinet 2020; Carmona et al. 2021). This is the case of the yellow-legged gull (Larus michahellis), whose populations have exponentially growth in southern regions of Europe (Vidal et al. 1998; Duhem et al. 2008; Benussi and Fraissinet 2020; Ramírez et al. 2020; Coccon et al. 2022). Due to its high abundance, ecological adaptability, and aggressive behaviour, it is often considered a nuisance in urban settings (Vidal et al. 1998; Navarro et al. 2019). Consequently, the colonization and population expansion of urban gulls have raised significant concern in terms of conservation and management (Vidal et al. 1998; Navarro et al. 2017). These concerns encompass issues such as attacks to defend the nesting site, noise pollution, infrastructure damage, as well the dispersal of pathogens and invasive plant species (Belant 1997; Senar et al. 2016; Vergara et al. 2017; Navarro et al. 2019; Martín-Vélez et al. 2022, 2024).
In order to mitigate human-wildlife conflicts in urban ecosystems, it is of high importance to identify the main factors driving population growth and to develop effective prevention and control measures balanced with the role of urban populations to population dynamics of the species. Different management actions have been proposed to reduce the abundance of gulls in urban areas, such as limiting access to food resources, removing breeding individuals, eggs or/and chicks, or implementing direct actions to prevent nesting (Belant 1997). However, while a lot of information exists regarding the trophic ecology of urban yellow-legged gulls (e.g., Méndez et al. 2020; Carmona et al. 2021; Martín-Vélez et al. 2022; Vez-Garzón et al. 2023; Lopes et al. 2021; Pais de Faria et al. 2023), there is lack of data regarding the identification of the main factors that modulate the nesting selection by these urban predators. We only found a recent study with an urban population of herring gull (Larus argentatus) in Ireland showing that this species selects particular rooftop types, avoiding the ground floor, based on their slope or the presence of protective structures, such as chimneys, pipes, or ventilation grilles and corners to build their nests (Dalla Pria et al. 2022). In the case of the yellow-legged gull, the information about urban nesting is limited to studies describing how this species include human litter (mainly plastics) in the construction of urban nests, suggesting that in non-natural environments, litter could be a substitute of natural nesting material (Galimany et al. 2023; Lopes et al. 2020).
In this study, our objective was to pinpoint the main urban environmental factors that explain the nesting preferences of the yellow-legged gull inhabiting the highly dense city of Barcelona (northeastern Spain). To achieve this, we conducted an integrated analysis, combining micro-scale assessments of factors (e.g. position, substrate, height or roof type) associated with urban nesting sites with a macro-scale assessment of spatial variables derived from GPS tracking of the movements of breeding yellow-legged gulls. Moreover, as litter has been reported as an important part of the urban nests of the yellow-legged gull (Galimany et al. 2023), we analysed the type and abundance of litter in nests and main factors related to this. This study, aside from improving our understanding of the urban ecology of this species, is also useful for the implementation of management strategies aimed to mitigate the nest presence in Barcelona and other urban populations where this (or ecologically similar) gull species are present and improve citizen coexistence.
Materials and methods
Study area and fieldwork procedures
This study was conducted in the city of Barcelona (Fig. 1A), a coastal city with a Mediterranean climate and presents a population of around 1,600,000 inhabitants (Idescat 2019). Barcelona is an urban coastal ecosystem surrounded by ports, waste dumps, agricultural areas and green areas (Carmona et al. 2021; Galimany et al. 2023). This situation provides a diverse availability of trophic resources that can be exploited by an urban yellow-legged gull population of between 200 and 300 breeding pairs (Antón et al. 2017; Vez-Garzón et al. 2023). Moreover, Barcelona is geographically confined by the sea, a mountain and two rivers. These constraints the urban expansion and force the construction of high-rise buildings to address housing demands.
(a) Location of Barcelona city in Europe (orange star point). Black points represent other cities with a population greater than 500,000 habitants and pink shadow represent the distribution of yellow-legged gull during the breeding period. (b) Location of the 148 nest (green circles) and 148 generated random points (orange triangles) used for the study. (c) Nest density (number the nests·km− 2) of the 148 nests present in this study
During the breeding season of the year 2019 (between April and May), a total of 148 urban nests were identified and visited throughout the city (Fig. 1B) by the Public Health Agency of Barcelona (ASPB). Nest identification by the ASPB was based on reports from residents of Barcelona (but do not correspond to the total amount of nests in the city). From each nest, we recorded its GPS position, the altitude above sea level, and took a set from one to three pictures depicting the substrate where the nest was placed as well as any urban litter present.
To identify the main macro-scale variables that could be associated to the nesting, we identified the main habitats used by urban yellow-legged gulls by equipping 14 breeding adult (six females and eight males) yellow-legged gulls with GPS units during 2018 and 2019 (9 individuals were tagged with GPS-WIMBISF-25 from Wimbitek SL, and 5 individuals were tagged with GPS-CatLog from Perthold Engineering LLC). All gulls were captured at the beginning of the breeding period using a baited fish trap within a metropolitan park in Barcelona. The GPS units were attached using a conventional Teflon harness (Thaxter et al. 2014). GPS units recorded the GPS positions of each bird at intervals of every 5 min for CatLog and 25–30 min for Wimbitek. All GPS data were regularized to 30 min to obtain the same time interval between fixes through the r package adehabitatLT (Calenge 2019). GPS devices and harness weighed less than 1.8% of the body mass of the birds (20 g for the GPS versus 1062 ± 120 g [mean ± SD] for the tracked gulls), less than the 3 to 5% threshold suggested for seabirds (Passos et al. 2010).
Nest data characterization
Four categorical variables were used to characterize each nest at the micro-scale (Fig. 2). Specifically, we categorized each nest based on the following criteria: (1) the nest’s location (nest located on the main roof, nest positioned above a structure on the main roof or nest located at the ground level); (2) the roof’s slope (flat or inclined); (3) the nest’s position (corner placement, center placement, positioning between machinery or pipes, placement within a pot, placement within a gutter or other variations), and (4) the type of substrate (gravel, smooth ground, soil or other substrates).
Examples of different nesting typologies of yellow-legged gulls in the city of Barcelona during the 2019 breeding period: (a) Nest in the corner of a flat roof with gravel flooring, (b) Nest on a flat roof, between machines, on top of a metal grate (substrate = other). (c) Nest in a cement gutter (smooth) on a sloping roof with tiles. All pictures were made by ASPB staff
Additionally, the type and abundance of litter in each nest was estimated using the pictures available for each nest. To be consistent between nests, we only took in account the litter associated to the nest (within the nest structure). The type of litter was characterized following the classification method established by Galimany et al. (2023) in a study that characterized the litter present in the nests of yellow-legged gull nests from Barcelona (Table S1). This classification (plastic, paper, metal, and others) was based on the Master List category of items from the European Marine Strategy Framework Directive (Galgani et al. 2013). In the case of the abundance of litter in each nest, we adapted the score of amount of litter from Ryan (2020) for each nest in four categories: (1) no litter observed; (2) between 1 and 10% of litter observed; (3) between 11 and 50% litter observed, and (4) more than 50% litter observed (see Figure S1). The same person (Joana Domingo) carried both the identification and abundance scoring to mitigate any potential biases.
Habitat use based on GPS-tracking data
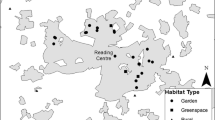
We identified the habitats used by the GPS-tracked yellow-legged gulls. For this, we estimated the percentage of GPS positions in each habitat type for each GPS-tracked individual during the breeding period (April-May) (Fig. 3A and B). Habitat type was determined by overlapping the GPS positions with land-cover data using the function over from sp (Pebesma and Bivand 2005). Land-cover data was aggregated into seven categories, according to previous foraging and diet information for this yellow-legged population (Gimeno et al. 2023; Méndez et al. 2020; Vez-Garzón et al. 2023; Galimany et al. 2023): urban, fishing port, sea, green areas, garbage dump, river, industrial and others (e.g. agriculture).
Macro-scale variables for nesting selection analyses
Based on the habitat use information obtained with the GPS tracking data, we considered eight variables (for both presence and absence) that could be associated to the nest selection of yellow-legged gull at macro-scale. Specifically, we calculated the minimum distance between each nesting location and each of the main habitats used by the yellow-legged gulls according to GPS data. Each of these different habitats is expected to be associated with availability of different resources. For example, we considered the minimum distance to the sea (which is expected to be related to the availability of marine resources), the minimum distance to the river (availability of freshwater resources), the minimum distance to landfills (availability of organic and inorganic waste), the minimum distance to the Barcelona fishing port (availability of fishing discards), and the minimum distance to green areas (availability of urban birds). Minimum distances were calculated using the sf_distance function in R (Pebesma 2018). Furthermore, since urban was the most important habitat used by GPS-tagged yellow-legged gulls (see Fig. 3A), we also considered human population density (number of censed persons per neighbourhood) and number of urban litter containers (proxy of garbage and litter availability within the city) within a 200 m radius of each nest. Population density, number of urban litter containers and building height were provided by the Public Health Agency of Barcelona (ASPB). Height distribution of the buildings in Barcelona was on average 11.5 m ± 9.7 s.d.
(a) GPS movements (coloured by habitat use) of yellow-legged gull within Barcelona city in 2018 and 2019 during the breeding period (April and May). (b) Percentage of habitat use (with standard mean error) associated to the GPS data from 2018 and 2019. The image of the yellow-legged gull was made by Martí Franch
Statistical analyses
We carried out first a descriptive analysis of the relative and absolute percentage of each of the four categorical variables associated to each nest. We considered the 148 nests characterized as presence of nest and we generated the same number of random locations within the urban area of Barcelona to be used as absences (Fig. 1B). Random locations were generated using the Create Random Points tool in ArcMap 10.8 over the rooftops layer from Barcelona city to exclude surfaces at ground level. For each random location, we calculated the same four categorical variables (location, slope, position, and substrate) as for presences using Google Earth (Mutanga and Kumar 2019). We also calculated macro-scale variables using the same methodology as for nests. We also performed a heat map density point through the Point Density tool in QGIS 3.26.1 with a search radius of 100 m and a previous coordinate transformation to UTM (Fig. 1C).
Then, to test if there was a nest site selection by the urban yellow-legged gulls, we carried out a binomial Generalized Linear Models (GLMs) with a logit link considering presence/absence of nest as a dependent variable. Prior to the GLM analyses, we standardized all the macro-scale variables and calculated the degree of correlation between different variables through the corrplot function from the R package (Figure S2). Distance to the river, distance to the sea, distance to the Barcelona port, and distance to the landfill presented significant correlation indices between them. Based on this, we only considered the variables distance to the Barcelona port and density of containers due to their importance as feeding points for the species in this urban population. To assess the best combinations of predictors affecting nesting habitat selection we used a model comparison approach and used AICc for model selection of main effects (Barton and Barton 2015). All potential combination of variables were considered using the function dredge from MuMIn in R. We considered a linear response for all variables. Additionally, when biologically relevant and significant, we also considered quadratic responses (building height). We included as predictor variables for nest presence the following: (1) building height, (2) squared building height, (3) population density, (4) roof type, (5) nest position, (6) substrate type and (7) port distance. To analyse factors affecting explaining the amount of litter present in the nests (four categories), we applied an ordinal logistic regression model and used a model comparison approach based on AICc, as above, for model selection. For analyses on the amount of litter we only considered variables related to litter sources (density of containers, distance to green areas, distance to dumps, population density, and distance to fishing port).
Results
Descriptive characterization of the yellow-legged nests
We found nests along all the urban area of Barcelona, with a more density of nests in the southwestern area of the city (Fig. 1C). Out of the 148 nests detected during 2019, 123 were found on flat roofs or above a structure on the main roof (83%), 73 nests were located at corner placement (53%) and 76 nests were situated on gravel substrate (51%) (Fig. 4). Whether on a roof or above a structure on the main roof, the nest was mainly placed in a flat surface (72% of nests on the roof and 94% of nests above a structure on the main roof) (Fig. 4). Moreover, the preferred place for nest placement was in a corner, both for nests on the roof (57%) or above a structure (69%). Lastly, the predominant substrate type found at nesting sites was gravel, accounting for 89% on roofs and 74% above a structure (Fig. 4).
Dichotomous classification of nests was determined based on four categorical variables, arranged from left to right: nest location, slope, position, and substrate type. Thicker lines represent the predominant categories within the analysed nests. In parentheses, the relative percentage and sample size of each category are provided
GPS tracking data
By using GPS tracking data from 14 breeding gulls, we were able to identify the main habitats covered by them and, thus, the identification of the main macro-scale variables associated to the nesting and the potential origin of the litter found in the nests. GPS-tracking data revealed that the main habitat used by yellow-legged gulls breeding in Barcelona was the urban environment (mean percentage ± standard deviation of the GPS positions = 75.63 ± 26.97%), followed by sea (8.98 ± 17.80%), fishing port (7.28 ± 14.29%), industrial (3.68 ± 7.18%), green areas (3.27 ± 3.94%), dump (0.45 ± 1.26%), and rivers (0.11 ± 0.31%) (Fig. 3B).
Nesting selection
Only one model was supported to best explain the nesting habitat selection of yellow-legged gulls (∆AIC < 2). The final GLM model retained the building height variable, both linear and squared responses, population density, distance to the port, and three categorical variables: roof type, nest position, and substrate type. Main effects from all variables retained by the model were significant, except for population density and squared height (Table 1; Table S3). The partial effects of height showed nest preferences for altitudes beyond 12 m (Fig. 5). There was a negative trend of port distance in nest selection (Fig. 5; Table 1). The frequency of random points for roof type concentrated on the roof, while in the presence of nests there was no difference in selection between the main roof and above a structure on the main roof (Figs. 4 and 5). The predominant substrate type for random points was smooth ground; while in the presence nests, it was mainly on gravel substrate (Figs. 4 and 5). The corner of the roofs was predominantly selected by the yellow-legged gulls, which markedly differed from random points, where more than 90% were in the center of the roof (Figs. 4 and 5).
Presence, abundance and type of litter in the nests
The presence of litter was detected in more than 80% nests, with 42% of them classified as containing between 1 and 10% of litter, followed by 22% of them classified as containing 11–50% or more than 50% of litter (Fig. 6A). Plastic was the main type of litter found in the nests, constituting 86% of the nests with litter, followed by paper (46%), wood (39%), metal (18%) and hygienic waste (11%) (Fig. 6B). Human population density showed a significant association to the amount of litter in the nests (Fig. 6C; Table 1). Density of containers, distance to dumps and distance to green areas only showed a marginal significant association (Fig. 6C; Table 1). Occurrence probabilities of nests with no litter or with 1–10% of litter increased with low population densities, while occurrence probabilities of nests with 11–50% or > 50% of litter increased with high population densities (Fig. 6C and D).
(a) Percentage of nests with litter presence classified based on the quantity of litter in four categories. (b) Frequency of nests found with each type of litter (“Others” includes textile items and rubber objects). (c) Partial effect of population abundance (persons per neighbourhood) around the nests that determined the presence of litter in the nests of yellow-legged gulls breeding at Barcelona. (d) Example of a nest containing > 50% of litter. The picture was made by ASPB staff
Discussion
In the present study, we identified and modelled various parameters related to the nesting habitat selection of the yellow-legged gull in an urban environment. Our results showed that yellow-legged gulls occupied nesting sites using habitat cues obtained at two complementary spatial scales, including the habitat associated to the nest, and other macro-scale variables associated to the distance from feeding habitats. By considering the presence of anthropogenic materials in the nests, we also provide evidence of the relevance of the use of urban waste in nest construction. These findings not only contribute to our understanding of the urban ecology of this species, they also have practical implications for implementing management strategies to mitigate potential disturbances caused by this species to humans in urban ecosystems.
Nesting selection
The results revealed a clear preference to nest on roofs, avoiding the ground level of the city, which differs from their behaviour in natural environments (Benussi and Fraissinet 2020; Delgado et al. 2021). This is consistent with the nesting behaviour of other urban gull species breeding in cities (Pais de Faria et al. 2022). In urban areas, there are many ground-level obstacles and a high risk of predation, making it an unsuitable nesting site. In relation to the roofs, yellow-legged gulls installed their nest mainly on the main roofs or above structures over the main roof of the buildings, often being elevator shafts or spaces occupied by antennas (Pallejà, 2021). Furthermore, the major part of nests were situated in the corners of the roof, a location that probably provide better protection for the eggs and small chicks, and a better visibility for adults to avoid potential risks (Benussi and Fraissinet 2020). This protection could also be related to the fact that nests on inclined roofs were mostly associated with positioning in a gutter or crevice, providing shelter and having a lower slope, thus reducing the probability of nest destruction due to weather conditions. The study by Dalla Pria et al. (2022) on urban populations of herring gulls in Ireland found similar results, observing that this ecological similar species to the yellow-legged gull nested on flat roofs and structures that offered protection, such as chimneys, pipes, or ventilation grilles. Regarding the type of substrate, we found a clear use of gravel substrates. Yellow-legged gulls might select this type of substrate in comparison to other substrates such smooth ground, which heat up and cool down more than gravel. This choice could ensure better microclimatic conditions during incubation and the period when chicks stay in the nesting area before fledging. In addition, the gravel is permeable to rain, reducing the possibility that a rain event could flood the nest (Guzman-Sanchez et al. 2018).
The nesting selection model emphasized the significance of building height where the nest is located, similar to the results obtained with herring gulls in Ireland (Dalla Pria et al. 2022). However, in our case, yellow-legged gulls exhibited a positive response concerning building height. Specifically, yellow-legged gulls showed a preference for buildings with intermediate heights beyond 12 m. In the case of very low buildings, yellow-legged gulls did not select them potentially to minimize contact with the human population (Arizaga 2023). Additionally, the model did not indicate a selection of buildings over 40 m, possibly because they are more exposed to weather conditions such as wind or higher solar radiation (Vermeulen et al. 2015) or also imposed flying constraints during the commuting movements between the nest and feeding grounds (Sage et al. 2019).
The distance to the fishing port is also a variable associated to the nesting site selection. Particularly, the spatial distribution of the nests in Barcelona showed a higher density close to the fishing port, as urban yellow-legged gulls use this habitat to feed on discarded fishing resources in both open sea and around the ports (Méndez et al. 2020; Vez-Garzón et al. 2023), although we cannot rule out the possibility that buildings around the port show more suitable characteristics for nest construction. The distance to landfills was negatively correlated with the distance to the port, meaning nests were located far from landfills. However, studies tracking the feeding movements of adult individuals in urban Barcelona have shown the use of some landfills near the city (Méndez et al. 2020). Nonetheless, the distance to landfills is greater than the distance to the port or the sea, and thus, the energy cost associated to foraging trips are higher than feeding in the port area.
Litter in nests
We found that 80% of the nests analysed in this study contained litter, being plastic the main litter detected. Plastic waste is a growing global problem with an urgent need to reduce its usage (UNEP, 2021). These materials are ubiquitous, durable, and considered by the United Nations Environment Programme as a critical issue for the environment (UNEP, 2021). Moreover, plastics constitute the largest and most persistent fraction of marine litter, accounting for at least 85% of total marine debris (UNEP, 2021). Breeding adults use urban litter to construct part of the nest, possibly mistaking them for natural materials, or the lack of natural materials drives them to collect urban materials as substitutes (Lopes et al. 2020; Galimany et al. 2023). Furthermore, many birds use anthropogenic materials as an indicator of nest quality (Sergio et al. 2011). The results showed that 42% of the nests had between 1% and 10% anthropogenic waste in the nest, while 22% had percentages exceeding 50% of anthropogenic materials in relation to natural materials. Human population density was positively correlated with the amount of litter in the nest. Human population density is often related to container and waste density, as areas with higher human population generate more waste. However, the consumption of organic waste from landfills and containers by the yellow-legged gull population in Barcelona has decreased over the years (Méndez et al. 2020; Vez-Garzón et al. 2023). Therefore, it is essential to deepen into the relationship between population density and the presence or quantity of anthropogenic waste found in nests. In this study, the analysis of garbage presence was conducted visually through photographs and thus, it might underestimate the presence of garbage in the nests. In fact, a study conducted in the city of Barcelona using nests collected in 2021 found anthropogenic waste in all examined nests (Galimany et al. 2023).
Conclusions
Understanding the ecology of yellow-legged gulls in urban areas has implications for urban population management to prevent nest establishment, not only in Barcelona but also in other similar cities where this or other ecological-similar species are present (Benussi and Fraissinet 2020; Lopes et al. 2021; Coccon et al. 2022; see also Fig. 1A). By analysing the main factors related to nest presence, interventions can be implemented to reduce conflicts between gulls and humans. Measures include reducing or changing the gravel substrate on roofs in order to make this substrate less attractive for nesting, cleaning these areas to avoid leaving elements that may contribute to nest establishment and carrying out good maintenance of roofs, covering corners with blocks making preferred nesting areas inaccessible, modifying the slope of small nesting surfaces such as antenna or lift sheds, and improving the efficiency of waste management. However, urban populations have an important role for balancing the population dynamics of natural populations of the species, which are declining. Therefore, management measures should take into account population dynamics estimations. Social and educational factors (e.g. feeding animals) may also provide a strong influence on human-gull interactions in cities. Future research could assess the relationship between nest presence and garbage content and compile a report on effective management measures.
Data availability
No datasets were generated or analysed during the current study.
References
Antón M, Herrando S, García D, Ferrer X, y, Cebrián R (2017) Atles dels ocells nidificants de Barcelona. Ajuntament de Barcelona/ICO/UB/Zoo, Barcelona, Spain
Arizaga J (2023) In: Martín P, Masero J, J. A. (eds) Gaviota patiamarilla – Larus michahellis En: Enciclopedia virtual de Los Vertebrados Españoles López. Museo Nacional de Ciencias Naturales, Madrid. http://www.vertebradosibericos.org/ – https://doi.org/10.20350/digitalCSIC/15460
Barton K, Barton MK (2015) Package ‘mumin’. Version 1(18):439
Belant JL (1997) Gulls in urban environments: Landscape-level management to reduce conflict. Landsc Urban Plann 38(3–4):245–258. https://doi.org/10.1016/S0169-2046(97)00037-6
Benussi E, Fraissinet M (2020) The colonization of the western yellow-legged Gull (Larus michahellis) in an Italian city: evolution and management of the phenomenon. Problematic Wildl II: New Conserv Manage Challenges Human-Wildlife Interact : 191–212
Calenge C (2019) Package History of the package adehabitatLT adehabitatLT
Carmona M, Aymí R, Navarro J (2021) Importance of predictable anthropogenic food subsidies for an opportunistic gull inhabiting urban ecosystems. Eur J Wildl Res 67: 9. https://doi.org/10.1007/s10344-020-01446-2
Chace JF, Walsh JJ (2006) Urban effects on native avifauna: a review. Landsc Urban Plann 74(1):46–69. https://doi.org/10.1016/j.landurbplan.2004.08.007
Coccon F, Vanni L, Dabalà C, Giunchi D (2022) The abundance of yellow-legged gulls Larus michahellis breeding in the historic centre of Venice, Italy and the initial effects of the new waste collection policy on the population. Urban Ecosyst 25(2):643–656. https://doi.org/10.1007/s11252-021-01175-7
Dalla Pria C, Cawkwell F, Newton S, Holloway P (2022) City living: nest-site selection preferences in urban herring gulls, Larus argentatus. Geographies 2(2):161–172. https://doi.org/10.3390/geographies2020011
De Pais J, Paiva VH, Veríssimo SN, Lopes CS, Soares R, Oliveira J, Ramos JA (2023) Plenty of rooftops with few neighbours occupied by young breeding yellow-legged Gulls (Larus michahellis): does this occur at the expense of their health condition? Ibis 165(1):312–321. https://doi.org/10.1111/ibi.13123
Delgado S, Zorrozua N, Arizaga J (2021) No evidence of habitat effect on clutch size, egg quality, and hatching success of the yellow-legged gull Larus michahellis at a micro-spatial scale. Mar Ornithol 49(2):241–246
Duhem C, Roche P, Vidal E, Tatoni T (2008) Effects of anthropogenic food resources on yellow-legged gull colony size on Mediterranean islands. Popul Ecol 50(1):91–100. https://doi.org/10.1007/s10144-007-0059-z
Galgani F, Hanke G, Werner S, Oosterbaan L, Nilsson P, Fleet D, Liebezeit G (2013) Monitoring guidance for marine litter in European seas. Centro Oceanográfico de Vigo, Vigo, Spain
Galimany E, Navarro J, Martino I, Aymí R, Cermeño P, Montalvo T (2023) Gulls as potential sentinels for urban litter: combining nest and GPS-tracking information. Environ Monit Assess 195: 5211–9. https://doi.org/10.1007/s10661-023-11133-9
Gimeno M, García JA, Afán I, Aymí R, Montalvo T, Navarro J (2023) Age-related differences in foraging behaviour at sea and interactions with fishing vessels in an opportunistic urban gull. ICES J Mar Sci 80:2405–2413. https://doi.org/10.1093/icesjms/fsac120
Goddard MA, Dougill AJ, Benton TG (2010) Scaling up from gardens: biodiversity conservation in urban environments. Trends Ecol Evol 25(2):90–98. https://doi.org/10.1016/j.tree.2009.07.016
Guzman-Sanchez S, Jato-Espino D, Lombillo I, Diaz-Sarachaga JM (2018) Assessment of the contributions of different flat roof types to achieving sustainable development. Build Environ 141:182–192. https://doi.org/10.1016/j.buildenv.2018.05.063
He Q, Silliman BR (2019) Climate Change, Human impacts, and Coastal ecosystems in the Anthropocene. Curr Biol 29(19):R1021–R1035. https://doi.org/10.1016/j.cub.2019.08.042
Idescat.2019. Anuari estadístic de catalunya. Pesca. Institut d'Estadística de Catalunya, Barcelona.https://www.idescat.cat/pub/?id=aec&n=468. WorldCat
Lopes CS, de Faria JP, Paiva VH, Ramos JA (2020) Characterization of anthropogenic materials on yellow-legged gull (Larus michahellis) nests breeding in natural and urban sites along the coast of Portugal. Environ Sci Pollut Res 27(29):36954–36969. https://doi.org/10.1007/s11356-020-09651-x
Lopes CS, Paiva VH, Vaz PT, Pais de Faria J, Calado JG, Pereira JM, Ramos JA (2021) Ingestion of anthropogenic materials by yellow-legged gulls (Larus michahellis) in natural, urban, and landfill sites along Portugal in relation to diet composition. Environ Sci Pollut Res 28(15):19046–19063. https://doi.org/10.1007/s11356-020-12161-5
Martín-Vélez V, Montalvo T, Afán I, Sánchez-Márquez A, Aymí R, Figuerola J, Navarro J (2022) Gulls living in cities as overlooked seed dispersers within and outside urban environments. Sci Total Environ 823: 153535. https://doi.org/10.1016/j.scitotenv.2022.153535
Martín-Vélez V, Navarro J, Figuerola J, Aymí R, Sabaté S, Planell R, Vila J, Montalvo T (2024) A spatial analysis of urban gulls contribution to the potential spread of zoonotic and antibiotic-resistant bacteria. Science of the Total Environment, 912: 168762. https://doi.org/10.1016/j.scitotenv.2023.168762
McCauley DJ, Pinsky ML, Palumbi SR, Estes JA, Joyce FH, Warner RR (2015) Marine defaunation: animal loss in the global ocean. Science 347(6219). https://doi.org/10.1126/science.1255641
McKinney ML (2006) Urbanization as a major cause of biotic homogenization. Biol Conserv 127(3):247–260. https://doi.org/10.1016/j.biocon.2005.09.005
McKinney ML, Lockwood JL (1999) Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends Ecol Evol 14(11):450–453. https://doi.org/10.1016/S0169-5347(99)01679-1
Méndez A, Montalvo T, Aymí R, Carmona M, Figuerola J, Navarro J (2020) Adapting to urban ecosystems: unravelling the foraging ecology of an opportunistic predator living in cities. Urban Ecosyst 23:1117–1126. https://doi.org/10.1007/s11252-020-00995-3
Mutanga O, Kumar L (2019) Google earth engine applications. Remote Sens 11(5):11–14. https://doi.org/10.3390/rs11050591
Navarro J, Grémillet D, Ramirez FJ, Afán I, Bouten W, Forero MG (2017) Shifting individual habitat specialization of a successful predator living in anthrop ogeniclandscapes. Mar Ecol Prog Ser 578:243–251. https://doi.org/10.3354/meps12124
Navarro J, Grémillet D, Afán I, Miranda F, Bouten W, Forero MG, Figuerola J (2019) Pathogen transmission risk by opportunistic gulls moving across human landscapes. Sci Rep 9: e10659. https://doi.org/10.1038/s41598-019-46326-1
Pais de faria J, Lopes CS, Kroc E, Blight LK, Nager RG, Ramos JA, Pereira L (2022) [eds.]. CRC, Boca Raton, FL. Doi: https://doi.org/10.1201/9781003047520
Pallejà Algueró Júlia (2021) Repensar els terrats de Barcelona: anàlisi estructural i constructiu dels espais semi-privats. BS thesis. Universitat Politècnica de Catalunya
Passos C, Navarro J, Giudici A, González-Soĺis J (2010) Effects of extra mass on the pelagic behavior of a seabird. Auk 127:100–107. https://doi.org/10.1525/auk.2009.09036
Pebesma E (2018) Simple features for R: standardized support for spatial vector data. R J 10(1):439–446. https://doi.org/10.32614/rj-2018-009
Pebesma E, Bivand RS (2005) S classes and methods for spatial data: the sp package. R news 5(2):9–13
Ramírez F, Afán I, Bouten W, Carrasco JL, Forero MG, Navarro J (2020) Humans shape the year-round distribution and habitat use of an opportunistic scavenger. Ecol Evol 10:4716–4725. https://doi.org/10.1002/ece3.6226
Rock P, Camphuysen CJ, Shamoun-Baranes J, Ross-Smith VH, Vaughan IP (2016) Results from the first GPS tracking of roof-nesting Herring Gulls Larus argentatus in the UK. Ringing Migration 31(1):47–62. https://doi.org/10.1080/03078698.2016.1197698
Ryan PG (2020) Using photographs to record plastic in seabird nests. Mar Pollut Bull 156:111262. https://doi.org/10.1016/j.marpolbul.2020.111262
Sage E, Bouten W, Hoekstra B, Camphuysen KCJ, Shamoun-Baranes J (2019) Orographic lift shapes flight routes of gulls in virtually flat landscapes. Sci Rep 9(1):1–10. https://doi.org/10.1038/s41598-019-46017-x
Senar JC, Domènech J, Arroyo L, Torre I, Gordo O (2016) An evaluation of monk parakeet damage to crops in the metropolitan area of Barcelona. Anim Biodivers Conserv 39(1):141–145. https://doi.org/10.32800/abc.2016.39.0141
Sergio F, Blas J, Blanco G, Tanferna A, López L, Lemus JA, Hiraldo F (2011) Raptor nest decorations are a reliable threat against conspecifics. Science 331(6015):327–330
Spelt A, Williamson C, Shamoun-Baranes J, Shepard E, Rock P, Windsor S (2019) Habitat use of urban-nesting lesser black-backed gulls during the breeding season. Sci Rep 9(1):1–11. https://doi.org/10.1038/s41598-019-46890-6
Thaxter CB, Ross-Smith VH, Clark JA, Clark NA, Conway GJ, Marsh M, Burton NHK (2014) A trial of three harness attachment methods and their suitability for long-term use on Lesser Black-backed Gulls and Great Skuas. Ringing Migration 29(2):65–76. https://doi.org/10.1080/03078698.2014.995546
United Nations Environment Programme (2021) From Pollution to Solution: A global assessment of marine litter and plastic pollution. Nairobi, Kenya.
Vergara A, Pitart C, Montalvo T, Roca I, Sabaté S, Hurtado JC, Planell R, Marco F, Ramírez B, Peracho V, De Simón M, y, Vila J (2017) Prevalence of extended-spectrum-β-lactamase- and/or carbapenemase-producing Escherichia coli isolated from yellow-legged gulls from Barcelona, Spain. Antimicrobial Agents and Chemotherapy, 61(2). https://doi.org/10.1128/AAC.02071-16
Vermeulen T, Knopf-Lenoir C, Villon P, Beckers B (2015) Urban layout optimization Framework to maximize direct solar irradiation. Comput Environ Urban Syst 51:1–12. https://doi.org/10.1016/j.compenvurbsys.2015.01.001
Vez-Garzón M, Giménez J, Sánchez-Márquez A, Montalvo T, Navarro J (2023) Changes in the feeding ecology of an opportunistic predator inhabiting urban environments in response to COVID-19 lockdown. Royal Soc Open Sci 10: 221639. https://doi.org/10.1098/rsos.221639
Vidal E, Medail F, Tatoni T (1998) Is the yellow-legged gull a superabundant bird species in the Mediterranean? Impact on fauna and flora, conservation measures and research priorities. Biodivers Conserv 7(8):1013–1026. https://doi.org/10.1023/A:1008805030578
Acknowledgements
Thanks to the ASPB and Zoo de Barcelona staffs for their help in the nest identification and GPS deployments, and to Pep Arcos, Joan Petrus and Santi Mañosa for their very useful feedback and suggestions in previous drafts. We acknowledge the accreditation of the “Severo Ochoa Center of Excellence” (CEX2019-000928-S). All the experimental procedures were conducted in accordance with the EU and Spanish scientific legislation.
Funding
This study is part of the projects BCN-Gulls and the Intramural CSIC Project “Opportunistic gulls as sentinel species to monitor urban marine ecosystems”. VMV was founded with a Margarita Salas grant 2022 from funding Next Generation EU and a Juan de la Cierva fellowship from the Spanish Government (JDC2022-049638-I).
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
JN to the study conception and design. Material preparation was performed by TM, data collection was performed by TM and JD; and analysis were performed by VMV, JD, LC and JN. The first draft of the manuscript was written by VMV and JD and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martín-Vélez, V., Domingo, J., Cardador, L. et al. Unravelling urban nesting site selection in an opportunistic gull: an integrated analysis of micro-spatial habitat and litter quantification. Eur J Wildl Res 70, 69 (2024). https://doi.org/10.1007/s10344-024-01822-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-024-01822-2