Abstract
Collection from jugular vein (JV) or intrathoracic cavity is the traditional procedure for blood sampling dead wildlife, but it is often source of preanalytical biases. Cavernous sinus of dura mater (CS) is an alternative site for withdrawal that may help to solve these issues but it has been used only for serology, while no data are available for biochemistry. This work compares metabolic profiles from CS and JV blood in red deer Cervus elaphus, to evaluate the usability of CS for metabolic profiling. A total of 134 deer were sampled in the frame of a culling program within the Stelvio National Park (Central Italian Alps). Hemolysis was statistically lower in CS than JV with 88% vs 44% of samples suitable for biochemistry. Thirty-six couples of poorly hemolytic sera were used for biochemical analysis: total protein, albumin, urea, triglycerides, total cholesterol, non-esterified fatty acids (NEFA), betahydroxybutyrate (BOHB), calcium, and phosphorus. Statistical differences were found for protein, albumin, cholesterol, and calcium; agreement was found for urea, NEFA, BOHB, and phosphorus while for total protein, albumin, cholesterol, and calcium, CS showed lower concentrations with both constant and proportional biases. These results may be related to a higher impact of water dilution in CS. Although the lower protein concentration in CS cannot significantly influence the serological results, caution is required for potential false negative results when antibody levels are close to cut-off values. CS leads to a higher percentage of samples suitable for metabolic profiles but results should be compared with adequate reference intervals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Health surveillance of wildlife faces several field and logistic issues. Laboratory analysis may be useful and objective indicators of health status of a wild population but the difficulty of sampling is one of the most important limitations for monitoring programs in free-ranging animals, especially in the case of blood, a very delicate matrix.
Metabolic profiles are screening blood tests conducted on groups of animals, with the aim to assess the metabolic status of a population, thus to compare the welfare conditions of different populations and indirectly assess ecosystem health (Maceda-Veiga et al. 2015). They include a customized series of hemato-biochemical analysis aimed to investigate specific metabolic conditions (energetic, lipidic, proteic, or mineral metabolisms) or to monitor the metabolic effects of some environmental factors (infectious diseases, severe weather conditions, starvation, prolonged stressors, intoxication). Metabolic profiles have been successfully used in livestock to predict peripartum diseases and fertility, to diagnose metabolic diseases, and to assess nutritional status (Ingraham and Kappel 1988); however, their use in wild ruminants is more limited (English and Lepherd 1981; Johnson et al. 2010; Severin et al. 2012; Zele and Vengust 2012; Kuker et al. 2015).This is partly due to the fact that hemato-biochemical tests are strongly affected by several preanalytical factors that may bias the results and prevent an adequate comparison among groups (Braun et al. 2015). In particular, acute stress, induced in living wild animals by capture, immobilization, and sampling procedures, may be very intense and leads to several clinico-pathological alterations confounding the effects of several environmental and pathological variables (Marco and Lavin 1999; Evans 2008). The use of blood samples taken from culled wild animals may be a good alternative to minimize the effects of capture stress if culled animals are killed with a single shot when they are calm and minimally alerted. Relevant increases in plasma stress hormone concentration require at least 3–5 min from stress event (Romero and Wingfield 2015) and much more time is needed to find relevant alterations of hematochemical parameters, if animals rapidly encounter death. Under certain circumstances, to investigate hemato-biochemical tests, blood samples from culled animals may be a better representation of the real condition of wildlife than those from live captures.
Field conditions can often affect the availability and quality of samples and this is an issue that needs to be focused on (Schilling et al. 2022). When working with free-living wild ungulates, difficulties are related, for example, to the possibility of taking samples only after culling, adverse weather conditions, field characteristics, difficulties in performing withdrawal, interoperator skill to withdraw, and shipping conditions of samples to the lab. All these factors may be difficult to manage, predict, and quantify, resulting in heterogeneous quality and reliability of the collected samples. In this sense, concerning blood sampling, some problems emerge in obtaining suitable sera for laboratory investigations, rather than the presence of damage of anatomical structures that may lead to the contamination of samples making them unusable or unsuitable for analysis. In addition, it may occur that the volume of the sample available for laboratory investigations is not sufficient to perform all the planned analyses.
Hunters usually eviscerate and bleed the carcass directly in the field to ensure the best quality of meat. The appropriate and complete bleeding not only prevent later blood sampling, but at the same time can represent an opportunity. For these reasons, hunters are often required, after appropriate training, to collect blood from hunted animal for scientific purposes. Sampling can take place through collection from the jugular vein (JV) or intrathoracic cavity puncture during the bleeding of carcasses, but this sampling method may affect quality or quantity of sera samples. Although hunters receive a training on withdrawal methodology, the compliance on its application can be heterogeneous, due to hunting practice, species, area, hunter experience, and traditions. Thus, blood taken from the thoracic cavity may result in coagulation; furthermore, this method of sampling predisposes to matrix contamination with the risk of obtaining autolytic, cytotoxic, and/or haemolytic sera (Boadella and Gortázar 2011; Arenas-Montes et al. 2013). These alterations may be also severe if samples are taken with a delay of a few hours after the death of the animal. As these alterations lead to a low sample size/lack of samples and/or to low volume/heterogeneous quality/suitability of samples, it is necessary to define alternative sampling methods to solve these diagnostic concerns.
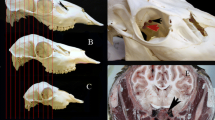
Cavernous sinus (CS) of dura mater encephali has been proposed as an alternative site for blood collection in dead wild ungulates. In addition to its applicability in hunting, this type of sampling could also be useful in cases where dead animals are found and blood cannot be obtained through traditional methods. The technique has been previously proposed in hunted wild boar (Sus scrofa) (Arenas-Montes et al. 2013) and then positively applied also to red deer, fallow deer (Dama dama), Pyrenean chamois (Rupicapra rupicapra pyrenaica), and European mouflon (Ovis aries musimon) (Jiménez-Ruiz et al. 2016). Compared with traditional intrathoracic site, CS provides a higher blood volume, serum/blood ratio, and a lower degree of hemolysis. To date, however, serum from CS has been used only for serological detection of antibodies for some infectious diseases, with better diagnostic performance than results obtained from intrathoracic puncture (Jiménez-Ruiz et al. 2016). No data nor validation about the possible use of CS blood for other tests such as metabolic profiles is currently available.
The aims of the present work are (1) to describe the concentrations of major biochemical variables in serum from CS and (2) to compare metabolic profiles from CS and JV blood in culled red deer, in order to evaluate the possible use of CS as a feasible sampling site also for metabolic status evaluation in red deer and to assess if the two sampling sites may be used alternatively.
Materials and methods
Sampling
Blood samples were collected from 134 red deer of different sex and age classes during a culling plan conducted in the red deer wintering site (ca. 5000 ha) in the Stelvio National Park, This park is located in the Province of Sondrio (10°250 N, 46°270 E), and the habitat is typical of Central Italian Alps characterized by step valleys from 700 to 3900 m above sea level (m.a.s.l.). The culling plan was legally authorized by ISPRA, the Italian Ministry of Environment (Prot. 48,585/T-A25-Ispra), and conducted by professional hunters under the supervision of the Park Authority. Culling occurred between late October and early February and no restriction was imposed in terms of age or female lactation status.
According to the depopulation plan, the culled animals are delivered to an authorized game control centre where bleeding and evisceration are performed, morphobiometric data (age, sex, and shooting area) are registered, and various biological samples, including blood, are collected. The timing of delivery of carcasses varied approximately between 2 and 8 h after culling depending on the area of the Park where the hunt was carried out which could be influenced by the roughness of the terrain, snowy and ice winter conditions, and possibility to be reached by vehicles.
Blood samples were collected using two methods, by taking them directly from the JV (Vengušt et al. 2020) after serving the large vessels of the neck during the bleeding process, and through the sampling technique designed by Arenas-Montes et al. (2013). In particular, a well-trained technician sampled blood from the intracavernous venous sinus inserting an 80-mm needle into the medial ocular canthus at a 45° angle ventro-medio-caudal to the median plane of the head. Jiménez-Ruiz et al. (2016) applied in deer the same method described in wild boar (Arenas-Montes et al. 2013), confirming the same inclination of needle and adjusting the depth according to the age of the animal. Using a 10-mL syringe, it was possible to take a sample equivalent to the entire capacity of the syringe.
Respectively, 127 blood samples were withdrawn from JV and 104 from CS. On a few occasions, logistical difficulties related to the lack of trained personnel at the game control centre limited the number of samples taken from CS. Blood samples were collected in sterile plain tubes, immediately centrifuged (2500 × g for 10 min), and supernatant was separated and kept frozen at − 20 °C until execution of tests.
Samples from both sampling sites matched for 100 animals, and were thus retained for the purposes of the present research.
Laboratory tests
Hemolysis was blindly evaluated by a single evaluator (SG) using a visual colorimetric 7-tiers scale and samples equal or less than the value of 4 (corresponding to 100 mg/dL hemoglobin) were considered suitable for analysis (Dugan et al. 2005) and processed using an automated spectrophotometer (BTC3500vet, Biotecnica Instruments Spa) and instrument-specific reagents for the measurement of the following biochemical analytes: total protein, albumin, urea, triglycerides, total cholesterol, non-esterified fatty acids (NEFA), betahydroxybutyrate, total calcium, and inorganic phosphorus. These parameters were chosen as representative of proteic (total protein, albumin, urea), energetic (NEFA, betahydroxybutyrate), lipidic (triglycerides, total cholesterol), and mineral (total calcium and inorganic phosphorus) metabolisms (Stockham and Scott 2008).
Statistical analysis
To assess whether the two sampling sites were comparable in terms of biochemical analytes, the multiple steps recommended for clinical pathology were applied (Jensen and Kjelgaard-Hansen 2006). In particular, biochemical values from the two sampling sites were tested for statistical differences; next, the correlation between the results of the two groups was tested; finally, the agreement was tested to check if constant or proportional errors are present. Before performing these analyses, data were checked for normality using the Shapiro–Wilk test.
Difference in the hemolysis index was tested in all 104 samples from CS and 127 from JV using Wilcoxon–Mann–Whitney U test.
To test for difference in the concentration/level of each biochemical analyte in two sampling sites the T-test for paired data, if normally distributed, or Wilcoxon–Mann–Whitney, for not normally distributed data, was used. Secondly, the association was assessed through correlations between values of the two different sample sites through Pearson’s and Spearman’s tests (respectively for normally and not normally distributed data).
Finally, the agreement was assessed by plotting data using Bland–Altman and Passing-Bablok plots (Jensen and Kjelgaard-Hansen 2006). The Bland–Altman plot is a useful graphical representation of the agreement between the two tests. The graph is plotted on the XY axis where Y represents the difference of the two measurements, and the X-axis shows the mean of the two measurements. Agreement was interpreted as good or poor based on intercept and slope values from Passing-Bablok analysis: when the 95% confidence interval (CI) included intercept = 0 and slope = 1, respectively, agreement was considered good. If one or both of the 95% CI did not include the 0 and 1 values, a constant (intercept different from 0) or proportional (slope different from 1) error was present, and agreement was considered poor. Finally, concentration of different analytes was also checked using Pearson/Spearman’s test for detecting any possible correlation among them.
For each test, significance was fixed at p < 0.05. Statistical analysis was performed using the application Analyse-it for Microsoft Excel (www.analyse-it.com).
Results
Hemolysis
Hemolysis indexes were statistically lower in CS than in JV samples (Fig. 1, Mann–Whitney U, p < 0.001). After removal of samples with hemolysis index higher than 4, ninety-two (88%) samples from CS were considered suitable for further biochemical tests vs 56 (44%) samples from JV. A total of 36 animals with both types of samples of adequate quality remained and they were used for comparison between the two sampling sites for the biochemical tests.
Clinical biochemistry
Results of descriptive statistics in JV and CS are summarized in Table 1.
Statistical differences between the concentration of hematochemical analytes from JV and CS blood were found for total protein, albumin, total cholesterol, and calcium, whereas correlation between the two sampling sites was found for urea, triglycerides, total cholesterol, NEFA, betahydroxybutyrate, and total calcium.
The agreement analysis on 36 samples obtained from both the sampling sites showed a good agreement between the two sampling sites only for urea, NEFA, betahydroxybutyrate, and inorganic phosphorus (Figs. 2, 3, 4, and 5). For the other variables, agreement was considered poor with a proportional bias for triglycerides (Fig. 6) and both constant and proportional biases for total protein, albumin, cholesterol, and calcium and a trend to lower concentrations in CS samples (Figs. 7, 8, 9, and 10).
Betahydroxybutyrate. Bland–Altman difference plots on the left (mean and 95% confidence interval) and Passing-Bablok fit on the right (mean and 95% confidence interval) for betahydroxybutyrate (BOH) in blood of red deer sampled from cavernous sinus (CS) and jugular vein (JV). Results from the two sites appear in agreement
Inorganic phosphorus. Bland–Altman difference plots on the left (mean and 95% confidence interval) and Passing-Bablok fit on the right (mean and 95% confidence interval) for inorganic phosphorus (P) in blood of red deer sampled from cavernous sinus (CS) and jugular vein (JV). Results from the two sites appear in agreement
Triglycerides. Bland–Altman difference plots on the left (mean and 95% confidence interval) and Passing-Bablok fit on the right (mean and 95% confidence interval) for triglycerides (TRIGL) in blood of red deer sampled from cavernous sinus (CS) and jugular vein (JV). Results from the two sites show a proportional bias
Total protein. Bland–Altman difference plots on the left (mean and 95% confidence interval) and Passing-Bablok fit on the right (mean and 95% confidence interval) for total protein (PROT) in blood of red deer sampled from cavernous sinus (CS) and jugular vein (JV). Results from the two sites show both constant and proportional biases
Albumin. Bland–Altman difference plots on the left (mean and 95% confidence interval) and Passing-Bablok fit on the right (mean and 95% confidence interval) for albumin (ALB) in blood of red deer sampled from cavernous sinus (CS) and jugular vein (JV). Results from the two sites show both constant and proportional biases
Total cholesterol. Bland–Altman difference plots on the left (mean and 95% confidence interval) and Passing-Bablok fit on the right (mean and 95% confidence interval) for total cholesterol (CHOL) in blood of red deer sampled from cavernous sinus (CS) and jugular vein (JV). Results from the two sites show both constant and proportional biases
Total Calcium. Bland–Altman difference plots on the left (mean and 95% confidence interval) and Passing-Bablok fit on the right (mean and 95% confidence interval) for total calcium (CA) in blood of red deer sampled from cavernous sinus (CS) and jugular vein (JV). Results from the two sites show both constant and proportional biases
From the analysis of correlation between different analytes, the concentrations of total proteins and albumin resulted highly positively correlated (p < 0.001, r = 0.860) and they both were highly correlated also to total cholesterol (p < 0.001 r = 0.567 and p < 0.001 r = 0.670 respectively) and total calcium (p < 0.001 r = 0.700 and p < 0.001 r = 0.791 respectively). The other analytes were not correlated among each other or without a biological meaning.
Discussion
Metabolic profile is a series of specific analytical tests run in combination and used as a population-based rather than individual-based diagnostic, with particular emphasis placed on monitoring subclinical rather than clinical diseases (Ingraham and Kappel 1988). Ideal analytes measured in a metabolic profile should reflect nutritional status; they are not generally tightly controlled by hormonal or other physiological factors and should be characterized by a relatively small analytical variation in comparison to changes related to nutritional or metabolic status (Stockham and Scott 2008). The choice of the analytes to be included in a metabolic profile may be customized based on the problem to be investigated. In the present research, the choice of the tests investigated is based on the most diffuse analytes, carrying the above-mentioned characteristics, that are generally used to investigate energy balance (NEFA, betahydroxybutyrate), protein metabolism (total protein, albumin, urea), lipid metabolism (triglyceride, total cholesterol), and mineral balance (calcium and inorganic phosphate).
Despite the huge potential, studies using metabolic profiles in wild animals are scarce because they often suffer from different biases. The issue of preanalytical artefacts is one of the major factors affecting laboratory results and this may be particularly heavy for wild animals sampled in field and/or by non-professional phlebotomists (such as hunters). Blood from CS appears to be an adequate source of abundant and minimally hemolytic samples for studies on hunted ungulates but, in order to avoid preanalytical biases, researchers involved should be adequately trained to obtain good quality samples. Among preanalytical factors, hemolysis is the most important and it may consistently alter the quality of serum samples preventing the execution of many biochemical tests or leading to severe interferences. This may be a crucial point when dealing with wild animals in which resampling is not possible and caseloads are generally limited (Schilling et al. 2022). Several hemato-biochemical tests are strongly influenced by hemolysis (Braun et al. 2015); thus, for the evaluation of metabolic profile, many samples have to be discarded if hemolytic and this affects the statistical significance of results. Intrathoracic/intracardiac puncture and collection from JV are the techniques usually performed for blood withdrawal from dead ungulates. In the present research, we used a visual colorimetric method to compare the quality of sera and we introduced a strict inclusion criterion by keeping only samples with absent or minimal hemolysis (less than 100 mg/dL). This cut-off value is similar to that of 150 mg/dL suggested Clinical and Laboratory Standards Institute (CLIA) (Smitt et al. 2012). The use of strict inclusion criteria for hemolysis is indicated for the purposes of the present study since we wanted to exclude that the differences in results were related to the effects of hemolysis. In fact, it is noteworthy to underline that no hematochemical variable was statistically correlated to the degree of hemolysis in our sample. Sera from CS blood were significantly less hemolytic than those from JV and the percentage of samples considered suitable for hematochemical profile was twice as much greater in CS than in JV. This confirms the results obtained by Jiménez-Ruiz et al. (2016) who reported that serum samples from CS are more abundant and less hemolytic than intrathoracic ones.
Before applying a new method (for instance CS sampling), it is crucial its validation through its comparison with an established method (such as JV sample), to assess whether the new measurements are comparable with the existing ones. This procedure, often defined as method comparison, is based on a multistep process including evaluation of differences between paired results (i.e. if the results of the two groups are overlapping or not), assessment of association of the paired results (i.e. if results are correlated), and if the results are in agreement (i.e. if constant or proportional errors are present) (Jensen and Kjelgaard-Hansen 2006). In case this procedure fails to show agreement, the two methods cannot be used alternatively and results must be matched just with those produced with the same method (in this case, the same sampling site) and compared with specific reference intervals. By comparing results of hematochemical variables between the two sampling sites, differences were found only for the concentration of total protein, albumin, total cholesterol, and total calcium. This is mainly due by an inconsistent decrease of protein and albumin, likely due to a higher water content in the CS samples rather than a selective decrease of specific proteins. To confirm this hypothesis, we performed serum protein electrophoresis on some paired samples with great differences in protein concentrations and overlapping results in protein composition were found (data not shown), thus supporting a non-selective lower protein content in CS. Total cholesterol and total calcium likely follow the same trend since they are massively linked to transport proteins (HDL and LDL lipoproteins for cholesterol and albumin for calcium) in plasma; thus, their concentration is directly influenced by the protidemia. This is also confirmed by the high direct correlation between total calcium and total cholesterol with protein and albumin concentrations. In contrast, small molecules such as urea, betahydroxybutyrate, NEFA, and inorganic phosphorus are in agreement between the two different sample sites, thus suggesting a passive diffusion as small solutes among all body barriers.
The different water (and small solutes) content between blood samples taken from the two different sites is difficult to explain although supported also by the study of Jiménez-Ruiz et al. (2016) which reported a significantly higher serum/blood ratio in samples from CS compared to those from intrathoracic sampling site (64.30 vs 37.57%). One possible hypothesis is that the drainage of fluid from peripheral tissues is different in different body sites thus leading to different content of plasma solutes, but we cannot exclude a partial contribution of other fluids with low protein content (such as cerebrospinal fluid -CSF) to the composition of blood taken from CS. No specific studies about the anatomy of dural venous sinuses in ruminants and specifically in deer are available but, in humans, dural venous sinuses are a series of channels that drain venous blood from the cranial cavity and they also participate in the cerebrospinal fluid circulation and return after resorption by the arachnoid granulations (Bayot et al. 2022). A similar function is also likely in deer and this aspect might contribute to a different composition of venous blood from CS, leading to a dilution of many blood analytes (since CSF is composed almost entirely by water and few small molecules, with less than 1% of protein content than in plasma).
The poor agreement found for some variables between blood from CS and JV does not prevent the possible use of CS for evaluation of metabolic profiles but it just indicates that the two types of samples are not interchangeable and that specific reference intervals are not overlapping. Besides protein and albumin, most hematochemical variables are correlated between the two sites and, among those who are not correlated, protein and albumin are likely linked to the water content (as previously discussed). Thus, it is likely that, with adequate reference intervals calculated for CS blood, they could share the same biological meaning.
To date, blood from CS has been proposed as a possible source for serological tests only. The only published paper on red deer (Jiménez-Ruiz et al. 2016) indicated an overall good agreement among serological results in CS and intrathoracic blood but a slight lower percentage of seropositivity (for instance, for Blue Tongue) was found in CS samples (60% vs 75%). The authors suggested that this discrepancy could be associated to the degradation of the sera collected by the traditional method extraction. The results of the present studies suggest a possible alternative explanation for the lower sensitivity of serological test run on CS blood in red deer, being supported by the lower protein concentrations demonstrated by our data. In the present study, we found that differences in protein concentration between the two sites are generally limited and it is unlikely to cause a significant decrease of qualitative serologic results in most cases. However, caution should be taken in the interpretation of antibody titers and, when necessary, cut-off values should be shifted down for CS samples to improve test sensitivity.
The present research has some limitations; first of all, the sample size. Indeed, since we aimed to represent field conditions, the difficult alpine context leads to blood samples that can be affected by various factors. In this sense, the major difference that emerged was the lower haemolysis that CS samples show. Our decision to apply a more rigorous and restrictive approach is aimed to avoid the effect of hemolysis and resulted in a considerable reduction in paired samples for later analyses. Although the number obtained was limited, the confidence intervals that emerged returned meaningful values for making inferences from a hematological-pathological point of view. Moreover, limited sample size affects primarily error II type (i.e. the probability of erroneously accepting the absence of difference) and in the present study, we identified differences between CS and JV in four out of nine parameters. Even if the number of differences would be greater, these results already suggest that direct comparisons of metabolic profiles obtained from CS and JV should not be carried and specific reference intervals should be calculated. Unfortunately, while our sample size is enough to conclude the impossibility of making comparisons prevents us from calculating reference intervals with adequate representativeness. The results of different hematochemical variables obtained in CS likely suffered for the low number of cases but pave the way for a further study on a higher caseload aimed also to calculate reference intervals for each parameter in different sampling sites.
Another limit of the present research is the absence of data on the ratio between serum and total blood in samples from the two different sites. This could have confirmed the hypothesis of a different water content suggested by protein quantitation and by previously published papers.
Finally, in the present caseload, although the carcasses did not show apparent pathological pictures, no deepen data on possible pathological conditions affecting deer are available; thus, it is not possible to check if the analytes measured in the two different sites tend to provide the same biological information in clinical conditions.
In conclusion, the present study confirms the possible use of blood from CS of dura mater also for the evaluation of metabolic profile in red deer, although results should be compared with samples taken from the same sites and with adequate specific reference intervals for each analyte. Results of serology could be minimally affected in most clinical conditions even though attention should be paid for low antibody levels and possibly, decreased cut-off values should be considered for CS blood in order to increase sensitivity and avoid false negative (type II) error. A more robust study on this aspect is needed including a higher number of animals and species and different serological methods. In addition, further investigations are necessary to establish the appropriate reference intervals for hematochemical variables in the two different types of samples using an adequate number of samples and to compare results of metabolic profiles with biological data in order to define the possible meaning and usefulness to monitor welfare in wild red deer populations.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Arenas-Montes A, García-Bocanegra I, Paniagua J, Franco JJ, Miró F, Fernández-Morente M, Carbonero A, Arenas A (2013) Blood sampling by puncture in the cavernous sinus from hunted wild boar. Eur J Wildl Res 59:299–303. https://doi.org/10.1007/s10344-013-0701-3
Bayot ML, Reddy V, Zabel MK (2022) Neuroanatomy, dural venous sinuses. In: StatPearls. Treasure Island (FL): StatPearls Publishing
Boadella M, Gortázar C (2011) Effect of haemolysis and repeated freeze-thawing cycles on wild boar serum antibody testing by ELISA. BMC Res Notes 4:498. https://doi.org/10.1186/1756-0500-4-498
Braun JP, Bourgès-Abella N, Geffré A, Concordet D, Trumel C (2015) The preanalytic phase in veterinary clinical pathology. Vet Clin Pathol 44(1):8–25. https://doi.org/10.1111/vcp.12206
Dugan L, Leech L, Speroni KG, Corriher J (2005) Factors affecting hemolysis rates in blood samples drawn from newly placed IV sites in the emergency department. J Emerg Nurs 31(4):338–334. https://doi.org/10.1016/j.jen.2005.05.004
English AW, Lepherd EE (1981) The hematology and serum biochemistry of wild fallow deer (Dama dama) in New South Wales. J Wildl Dis 17:289–295. https://doi.org/10.7589/0090-3558-17.2.289
Evans GO (2008) Animal Hematotoxicology: A practical guide for toxicologists and biomedical researchers. CRC Press
Ingraham RH, Kappel LC (1988) Metabolic profile testing. Vet Clin North Am Food Anim Pract 4(2):391–411. https://doi.org/10.1016/s0749-0720(15)31056-2
Jensen AL, Kjelgaard-Hansen M (2006) Method comparison in the clinical laboratory. Vet Clin Pathol 35(3):276–286. https://doi.org/10.1111/j.1939-165x.2006.tb00131.x
Jiménez-Ruiz S, Arenas-Montes A, Cano-Terriza D, Paniagua J, Pujols J, Mirò F, Fernandez-Aguilar X, Gonzalez MA, Franco JJ, García-Bocanegra I (2016) Blood extraction method by endocranial venous sinuses puncture in hunted wild ruminants. Eur J Wild Res 62(6):775–780. https://doi.org/10.1007/s10344-016-1056-3
Johnson D, Harms NJ, Larter NC, Elkin BT, Tabel H, Wei G (2010) Serum biochemistry, serology and parasitology of boreal caribou (Rangifer tarandus caribou) in the Northwest territories, Canada. J Wildl Dis 46:1096–1117. https://doi.org/10.7589/0090-3558-46.4.1096
Kuker S, Huber M, Evans A, Kjellander P, Bergvall UA, Jones KL (2015) Hematology, serum chemistry and serum protein electrophoresis ranges for free ranging roe deer (Capreolus capreolus) in Sweden. J Wildl Dis 51(1):269–273. https://doi.org/10.7589/2014-02-025
Maceda-Veiga A, Figuerola J, Martínez-Silvestre A, Viscor G, Ferrari N, Pacheco M (2015) Inside the Redbox: applications of haematology in wildlife monitoring and ecosystem health assessment. Sci Total Environ 514(2015):322–332. https://doi.org/10.1016/j.scitotenv.2015.02.004
Marco I, Lavin S (1999) Effect of the method of capture on the hematology and blood chemistry of red deer (Cervus elaphus). Res Vet Sci 66:81–84. https://doi.org/10.1053/rvsc.1998.0248
Romero ML, Wingfield JC (2015) Tempests, poxes, predators, and people: stress in wild animals and how they cope. Oxford University Press
Schilling AK, Mazzamuto MV, Romeo C (2022) A review of non-invasive sampling in wildlife disease and health research: what’s new? Animals 12(13):13. https://doi.org/10.3390/ani12131719
Severin K, Masek TJ, Konjevci D, Slavica A, Marinculic A, Martinkovi F, Vegust G, Dzaja P (2012) Liver enzymes and blood metabolites in a population of free-ranging red deer (Cervus elaphus) naturally affected with Fascioloides magna. J Helminthol 86:19–196. https://doi.org/10.1017/S0022149X1100023X
Smitt MB, Chan YW, Dolci A, Kellogg MD, McCudden CR, McLean M, Miller JJ (2012) Hemolysis, icterus, and lipemia/turbidity indices as indicators of interference in clinical laboratory analysis; Approved Guideline. 2012; ISBN 1562387995
Stockham SL, Scott ME (2008) Fundamentals of veterinary clinical pathology, 2 ed Blackwell Publishing
Vengušt G, Žele Venguš D, Toplak I, Rihtarič D, Kuhar U (2020) Post-epidemic investigation of Schmallenberg virus in wild ruminants in Slovenia. Transboundary & Emerg Dis 67(4):1708–1715
Zele D, Vengust G (2012) Biochemical indicators in serum of free-ranging roe deer (Capreolus capreolus) in Slovenia. Acta Vet Brno 81:377–381. https://doi.org/10.2754/avb201281040377
Funding
Open access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement. This research was supported by the University of Milan Piano di Sostegno alla Ricerca, Linea 2–2017 (Nicola Ferrari).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by S Comazzi, A Giordano, N Formenti, and T Trogu. The first draft of the manuscript was written by S Guanziroli and S Comazzi and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Comazzi, S., Guanziroli, S., Giordano, A. et al. Comparison between cavernous sinus and jugular vein as post mortem sampling sites for blood metabolic profiles in wild red deer (Cervus elaphus). Eur J Wildl Res 69, 82 (2023). https://doi.org/10.1007/s10344-023-01715-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-023-01715-w