Abstract
The Forest Thrush (FT), Turdus lherminieri, is a secretive, ground-dwelling forest bird species of conservation concern, endemic to only four Caribbean islands. Factors influencing habitat selection and abundance by FT have been seldom documented so far. We assessed variation in the presence and abundance of FT in various forested habitats in Guadeloupe. To that end, we deployed 5-camera-trap arrays over 14 days on 24 different survey stations resulting in 1680 trap days. We observed FT more frequently at camera trap stations where rainforest dominated, with local abundance declining with increasing canopy openness. Furthermore, temperature was the most important factor affecting the presence of FT at our study sites. FT was essentially diurnal, with some activity at dawn and dusk. We document for the first-time spatial co-occurrence between FT and potential mammal predators. FT co-occurred positively with rats and negatively with cats. Although FT is globally listed as near threatened by IUCN, the species the species appeared to be relatively abundant in Guadeloupe, possibly as a consequence of the suspension of hunting since 2014 and/or the almost total protection of the Guadeloupe tropical rainforest. We recommend the use of camera traps to improve knowledge for the conservation status of the species in other part of its area of distribution and to provide additional information on the potential impact of exotic predatory mammals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extensive habitat destruction is a major source of biodiversity loss worldwide (Pimm et al. 2006; Brook et al. 2008). This phenomenon mainly results from human activities such as logging, agricultural expansion, or human settlement (Wilcove et al. 2013; Dávalos et al. 2016). In this respect, the conservation of tropical forests is of prime importance, as they support more than two-thirds of the World’s biodiversity despite covering less than 10% of the Earth’s land surface. However, they are particularly exposed to unabated deforestation and forest fragmentation (Bradshaw et al. 2009; Hansen et al. 2013; Giam 2017). Consequently, many tropical forest species are declining, particularly in restricted areas, such as oceanic islands (Alroy 2017). This is particularly true of birds, with more than 600 globally threatened species (Stattersfield et al. 1998; Ricketts et al. 2005) and more than 90% of species extinctions having occurred on islands (Johnson and Stattersfield 1990; Banko et al. 2013).
The Caribbean islands constitute one of the 25 hotspots of biodiversity on Earth (Myers et al. 2000; Pimm et al. 2014), with a high diversity of bird species and high levels of avian endemism (Vázquez-Miranda et al. 2007; Catanach et al. 2021). However, the small extension of this hotspot makes it one of the most vulnerable areas of conservation importance (Brooks et al. 2002). Deforestation is in particular widespread in the insular Caribbean due to human exploitation (Tole 2001; Dolisca et al. 2007; Hedges et al. 2018) and natural disasters (Wiley and Wunderle 1993; Eppinga and Pucko 2018). This phenomenon is particularly affecting the avifauna, with several Caribbean endemic bird species being declining (Wunderle 2008; Arendt et al. 2013; Lloyd et al. 2016; Devenish-Nelson et al. 2019; Akresh et al. 2021). In addition, scientific attention given to Caribbean forest-dependent varies markedly among Caribbean islands and family groups (Devenish-Nelson et al. 2019).
In this context, we studied the Caribbean-endemic Forest Thrush (hereafter FT), Turdus lherminieri, typically found in various forest habitat types (Benito-Espinal and Hautcastel 2003; Arnoux 2012; Parashuram et al. 2015). The species' distribution area is restricted to four islands in the Lesser Antilles, Montserrat, Guadeloupe, Dominica, and St Lucia (Benito-Espinal and Hautcastel 2003; Eraud et al. 2012). Currently, FT is globally listed as near threatened by IUCN, considering the impact of anthropogenic deforestation and introduced predators. The Montserrat population declined abruptly following the 1995–1997 volcanic eruptions but appears to have recovered since then (Dalsgaard et al. 2007). A small, possibly declining, population exists in Dominica (Durand and Baptiste 2008), whereas the St Lucia population might be limited to a few individuals, if not extinct (Toussaint et al. 2009; Arnoux et al. 2014). The larger Guadeloupe FT population appears to be stable, according to data from audio-count surveys and mist netting (Eraud et al. 2013; Guillemot et al. 2020). The four populations have been considered as distinct subspecies according to plumage patterns (Clement and Hathway 2000; Zuccon 2011). Accordingly, Arnoux et al. (2013, 2014) provided some evidence for morphological and genetic differentiation between Montserrat, Guadeloupe, and Dominica, as well as within Guadeloupe, possibly as a result of habitat fragmentation.
At a more local scale, Parashuram et al. (2015) studied the influence of local habitat structure on FT abundance in Montserrat, relying on visual and acoustic detection during point count surveys. They concluded that FT prefers mature mesic and wet forests at mid elevations. However, the performance of methods relying on visual and auditory cues to survey tropical forest birds may vary according to habitat type, in direct relation to habitat characteristics and sound attenuation (Waide and Narins 1988; Anderson et al. 2015), possibly affecting conclusions about habitat selection. In addition, auditory point count method has maximal efficiency only when conducted during the peak of calling activity (Levesque and Lartiges 2000; Rivera-Milán et al. 2022) and/or when broadcasting the call of the target species (Cambrone et al. 2021). A potential alternative survey method consists in relying on camera traps. In comparison with point count surveys, the use of camera traps allows the collection of various and valuable ecological information such as the presence of other species and diel activity. The method is particularly suitable for studying discrete and recluse bird species such as FT (O’Connell et al. 2011; Suwanrat et al. 2015; Smith et al. 2017; Cook et al. 2020; Jean-Pierre et al. 2022). Finally, species distribution coupled with environmental variables can easily be modelled from camera-trap data (Maseko et al. 2017; Smith et al. 2017).
We therefore relied on camera traps to [1] estimate FT local abundance, detection and occupancy in Guadeloupe forests, [2] understand the influence of environmental variables on occupancy, detection and abundance, and [3] assess spatiotemporal co-occurrence between FT and potential mammal predators, including humans.
Material and methods
Study area
Field work took place on Basse-Terre and Grande-Terre, the two main islands of the Guadeloupe archipelago, French West Indies, which are separated by a narrow sea channel. The western island of Basse-Terre is mainly mountainous, with a maximum height of 1467 m (Gadalia et al. 1988), whereas the eastern island of Grande-Terre is lower and flatter, with a maximum height of 177 m, and is of coral-limestone formation (Lasserre 1961). The elevation gradient translates into a high diversity of habitats, with tropical forest dominating over tropical dry forest and tropical wet coastal forest (Rousteau 1996a).
Data collection
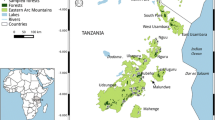
Data on the presence of FT and that of potential mammalian predators were collected during a study initially aimed at documenting the presence and abundance of two quail-dove species (Jean-Pierre et al. 2022). We relied on passive infrared camera traps (Moultrie© M-40i, with a 125° angle of view) to document the presence and assess the abundance of FT and other species. To that end, we surveyed 24 stations (each station consisting of an array of 5 camera traps) over 2 separate 7-day periods between February and May 2019, including 6 tropical dry forest stations, 6 tropical wet coastal forest stations and 12 tropical rainforest stations (Fig. 1, see Jean-Pierre et al. 2022 for additional information).
Locations of the 24 camera trap stations investigated in Guadeloupe between February and May 2019. This includes 6 tropical dry forest stations, 6 tropical wet coastal forest stations and 12 tropical rainforest stations. Each station had five cameras that were monitored for two 7-day periods. The distance between these stations ranged from 2.82 km to 9.81 km
Although Guadeloupe has a high diversity of forest habitats, steep landscape and extensive urban areas are particularly present. Consequently, stations were set to be representative of the islands’ high diversity of forest habitats, along the altitudinal gradient (Smith 2004; Talvitie et al. 2006; Louppe et al. 2021; Jean-Pierre et al. 2022). Stations were set at elevations ranging from 0.76 m to 768.65 m.
At each station, we set up five camera traps along a straight line, with a 200-m distance between adjacent cameras. Each camera-trap location was surveyed twice for a period of seven consecutive days, between February and March 2019 and again between April and May 2019, resulting in 1680 days of trapping. At each camera-trap location, we attached a single camera to a robust tree at a height of between 20 and 30 cm, according to the small body size of the targeted bird species (O’Connell et al. 2011). We selected trees at locations where the vegetation was not too dense to allow an optimal range of camera sensor in closed habitats. Cameras were active 24 h d−1. In order to avoid multiple photographs of the same individual over short periods of time, camera-traps were set to take three pictures each time a movement was detected, with a 30-s delay between pictures (Smith et al. 2017). We assessed habitat type, forest structuration, canopy openness and temperature at each camera-trap location. Following (Rousteau 1996b), we considered four forest habitat types (tropical dry forest, tropical rainforest, tropical wet coastal forest) based on direct observations in the field. We visually estimated the forest structuration as dense (≥ 66% tree cover) or sparse (≤ 66% tree cover), with a corresponding score of 1 or -1, respectively. At each station, we calculated an average value for the five camera-traps, thus resulting in a continuous forest structuration index ranging from -1 to 1. We visually estimated canopy openness at each camera-trap location as open (≥ 66% open), partially open (33–66 open), or closed (0–33% open), with a corresponding score of 1, 0.5 or 0, respectively, (see Jean-Pierre et al. 2022). We then calculated an average for the five camera-traps to obtain a continuous canopy openness index ranging from 0 to 1, for each station (Cook et al. 2020). We collected elevation data and ambient temperature at each camera-trap location using a Garmin Dakota 20 GPS and camera-trap records, respectively. On each FT detection, ambient temperature was automatically recoded by the camera. Otherwise, we estimated average daily temperature using other diurnal species captured by the camera traps. According to the pattern of diel activity of FT (see results), we only took into account captures at morning, noon and afternoon.
Statistical analysis
We used the total number of individuals (captures) of each species at each camera trap location to assess local abundance (Preston 1948). Then, we calculated the total abundance per station by combining the local abundances of the five camera traps at each location. We used a Wilcoxon signed-rank test for paired data (Rosner et al. 2006) to compare estimates of local abundance obtained at each station between the two sampling periods, as the data did not follow a normal distribution ( Shapiro–Wilk normality test p < 0.01; Mohd Razali and Bee Wah 2011).
To compare the median number of more than two groups, we used a Kruskal–Wallis analysis of variance by ranks, followed by Dunn's tests to perform subsequent pairwise comparisons between groups. We calculated naïve occupancy for each species by dividing the number of camera-trap locations where at least one individual was photographed, by the total number of camera-trap locations. For each species, we also calculated capture per unit effort (camera-trapping sampling occasion) by dividing the number of photographed individuals by 100 trap-days.
The influence of environmental variables on detection and abundance was then assessed using Royle and Nichols' heterogeneity model (Royle and Nichols 2003), which links abundance and heterogeneous detection probabilities. This method is especially recommended for assessing site occupancy at different spatial scales in the case of almost undetectable populations of unmarked individuals (Royle and Nichols 2003; Besnard and Salles 2010). Using data from 7-day trapping sessions, detection was considered as a binary variable (1 = detection, 0 = non-detection, correcting for occasional camera malfunction) at each station considering all identifiable species. Species detection (1) and non-detection (0) were extracted for each camera-trap day (i.e., 24 h period) at each station, with a 10 min buffer time between detections of the same species. Thus, abundance modelling was only concerned with species binary detection history under specific habitat conditions, not with species abundance history. According to Royle and Nichols (2003), the most important source of heterogeneity in site-specific detection probabilities is variation in animal abundance, Ni. The model assumes that all individuals of site i during sample j, have the same detection probabilities, rij, and that detections are independent. If at least one individual is found within the site, the species is recorded. Thus, the probability of detecting a species is linked to the probability of detecting an individual by:
To maximize observation independence, we analysed data at the station level. In addition, we relied on occupancy-detection models to evaluate the influence of environmental variables on FT distribution. We used the R package « unmarked» v0.13–0 (Fiske and Chandler 2011) to build abundance-detection models and occupancy-detection models.
We modelled abundance (λ) and occupancy (ψ) based on temperature, elevation, canopy openness, and forest type, and probability of detection (p) based on forest structure and temperature. We first ran a model in which both detection probability and abundance (or occupancy) were independent of covariate influence, e.g. p(.),λ(.) or p(.),ψ(.). We did not include all environmental variables influencing abundance (or occupancy) and detection in a global model because several of them were highly correlated (Spearman rank correlation test; canopy openness and temperature: rs = 0.67; elevation and temperature: rs = -0.82; p < 0.01 in both cases). However, we chose to keep all the environmental variables for modelling occupancy and abundance, as model selection allowed us to separately consider highly correlated variables. Accordingly, we modelled the influence of each environmental variable on p while holding λ (or ψ) constant, and vice versa, e.g., [p(covariate1), λ(.)] and [p(covariate1), ψ(.)] or [p(.), λ(covariate1)] and [p(.),ψ(covariate1)]. Finally, each environmental variable was tested on p, without or with additive effects, e.g., [p(covariate1 + covariate2), ψ(covariate1)] or [p(covariate1 + covariate2), λ(covariate1)] or [p(covariate1), ψ(covariate1 + covariate2)] and [p(covariate1), λ(covariate1 + covariate2)]. Only covariates that were not correlated between themselves were included in additive effects.
We used Akaike's information criterion to rank models, and models with AICc < 2 were considered to assess the significance of covariates (Burnham et al. 2011). The ratio of AIC weights was used to calculate evidence for the best model in comparison to other models. Using 10,000 parametric bootstraps, we checked for goodness-of-fit and mean dispersion parameter ĉ for all valid models (Burnham and Anderson 1998; MacKenzie and Bailey 2004).
Following Jean-Pierre et al. (2022), we examined spatial and temporal overlap between FT and potential predators, including: domestic cats (Felis catus Linnaeus, 1758), domestic dogs (Canis familiaris Linnaeus, 1758), rodents (pooling all species), northern raccoons (Procyon lotor, 1758), small Indian mongooses (Urva auropunctata, 1836), and humans. We then considered four different time periods of unequal length to assess diel activity (Lucherini et al. 2009; Gerber et al. 2012; Monterroso et al. 2014): night (from 1 h after sunset to 1 h before sunrise), dawn (from 1 h prior to 1 h after sunrise), day (from 1 h after sunrise to 1 h before sunset), and dusk (from 1 h prior to 1 h after sunset). We considered variation in daylength between the two trapping periods by defining average sunrise and sunset dates for each of them. We first used Fisher's exact test (Fisher 1922, 1992; Agresti 1992) to compare the proportion of birds caught during different periods of the diel cycle (night, dawn, day and dusk) between the two trapping periods. We then used Jacobs Selectivity Index (JSI, Jacobs 1974) to assess selectivity in diel rhythm activity, as described by Monterroso et al. (2014). The JSI scale runs from -1 to 1, with -1 representing total avoidance, 0 representing no preference, and 1 representing total preference. We employed bootstrap resampling (500 replicates) and recalculated the JSI for each bootstrap sample to determine the average JSI index and 95 percent confidence intervals for each period and species. When the 95 percent CI of the JSI was positive (or negative) and did not overlap zero, the four periods were considered positively (or negatively) selected (i.e., used as expected by chance). A paired-sample Wilcoxon test was used to compare the JSI index between the two sampling periods for each species.
We examined spatial co-occurrence of FT with predators using the probabilistic model proposed by Veech (2013), included in the R package "cooccur" v1.0 (Griffith et al. 2016). This model determines to what extent the frequency of co-occurrence of two species differs from what would be expected under the null hypothesis of independent distributions.
In addition, to explore temporal activity and overlap, we calculated kernel density estimates of diel activity (Meredith and Ridout 2016), using the R "Overlap" v1.1 package (Niedballa et al. 2016). We favoured ∆4 over ∆1 as a non-parametric estimator of the coefficient of overlapping as our sample size was larger than 75 (Schmid and Schmidt 2006), as recommended by Ridout and Linkie (2009). We calculated an average value from 10,000 bootstrap samples for each pair of species to estimate the precision of overlap coefficients.
Results
Out of a total of 9450 detection-events, 330 corresponded to FT, 1056 to rodents, 483 to small Indian mongooses, 107 to dogs, 72 to domestic cats, and 101 to racoons, (see Jean-Pierre et al. 2022).
Forest Thrush naïve occupancy and local abundance
As FT local abundance at each trapping station did not differ between the two sessions (Wilcoxon’s test, P = 0.26), we pooled the two data sets for further analysis. Overall, FT was detected on 16 of the 24 camera trapping stations, corresponding to a naïve occupancy rate of 0.67, and a camera trapping rate (number of individual detections/100 trap-days) of 18. Moreover, FT was found in all forest types, although more frequently in survey stations located in the tropical rainforest [Ntropical forest = 11/12, Ntropical wet coastal forest = 3/6, Ntropical dry forest = 2/6; Fisher’s exact test, p = 0.02; Fig. 2].
Similarly, FT local abundance differed significantly between the three forest habitats, (Kruskal Wallis analysis of variance, X2 = 9.50, p = 0.009). However, this was mainly due to FT abundance being significantly higher at tropical rainforest stations than at tropical wet coastal or tropical dry forest stations (post-hoc Dunn’s test, p = 0.031 and p = 0.005, respectively; Fig. 3), whereas FT abundance does not differ between stations located in tropical dry forests and tropical wet coastal forests (p = 0.574).
Modelling occupancy and detection
We used a subset of our camera-trap records for modelling purposes (see methods). After considering a buffer time of 10 min between detections of the same species, we retained 297 captures of FT, resulting in 83 detections in model matrices. According to the best model (M1), temperature was the only environmental variable affecting detection (β = -2.27, SE = 0.49, p < 0.01), with decreasing detection with increasing temperature (Fig. 4).
In addition, the best model suggested that canopy openness influenced FT occupancy, with decreasing FT occupancy with increasing canopy openness (β = -1.58, SE = 0.69, p < 0.03; Table 1).
Mean dispersion parameters ĉ obtained using MacKenzie and Bailey’s goodness-of-fit test for the four abundance-detection best models were: ĉM1 = 0.70, ĉM2 = 0.69, ĉM3 = 0.60, ĉM4 = 0.66 and for the occupancy-detection best model was: ĉM1 = 0.78.
The four best models (ΔAICc < 2) retained temperature as the only informative environmental variable affecting detection (β = -2.17, SE = 0.59, CI: -3.33,-1, Table 1), with decreasing level of detection with increasing temperature (Fig. 4). Indeed, although forest structure was retained in model M4, it did not significantly affect FT detection (ψ = 0.60, SE = 0.46, p > 0.05). We therefore retained canopy openness as the only informative environmental variable affecting abundance (β = -0.58, SE = 0.31; Fig. 4). Indeed, although temperature was retained in the M2 model, it did not significantly affect FT abundance (λ = -0.53, SE = 0.31, p > 0.05).
Pattern of diel activity and spatial and temporal co-occurrence with potential predators
As the proportions of FT captured during night, dawn, day, and dusk did not differ between the two sampling periods analysis (Fisher exact test, p = 1), data were pooled for subsequent analyses. FT was essentially diurnal, with however some activity at dawn and dusk (Table 2).
Domestic dogs, mongooses, humans, were essentially diurnal, whereas rodents, raccoons and domestic cats were essentially nocturnal (see Jean-Pierre et al. 2022). FT positively co-occurred spatially with rodents and negatively with domestic cats (Table 3).
However, rodents were essentially nocturnal (see Jean-Pierre et al. 2022), such that FT activity (Table 2) and rodent activity showed little overlap (Table 4).
Discussion
Forest Thrush local abundance, detection and occupancy in Guadeloupe forests
From previous studies conducted in Guadeloupe (Eraud et al. 2012; Arnoux et al. 2013; Levesque et al. 2020), FT was known to occur mainly in areas of continuous tropical rainforest, from 100 to 1,400 m above sea level and tropical wet coastal forests. On Monserrat, Parashuram et al. (2015) concluded from auditory and visual point counts that FT prefers mature mesic forest, with birds being more abundant at mid-elevations under closed canopies. We therefore expected to obtained similar results, since tropical rainforests and tropical wet coastal forests have not been much altered by fragmentation, thanks to effective protection by local authorities. Our results confirm the global pattern. We detected FT predominantly in the tropical rainforest habitat and, to a lesser extent, in the tropical wet coastal forest habitat. In addition, their abundance decreased with canopy openness. Comparing to previous studies, however, we also detected the FT in the tropical dry forest habitat, albeit at lower apparent abundance. This is particularly noticeable, as in Guadeloupe this forest ecosystem has been severely degraded by human pressure (Magnin 2018). For example, we detected the presence of FT at Deshaies, a touristic town in Northwestern Basse-Terre. This area, bordering the beaches of Guadeloupe, is particularly subject to human pressure, with high forest fragmentation and intense human frequentation. We therefore suggest monitoring this FT population over the long term in order to estimate demographic trends and connectivity with populations in other habitats, possibly through a capture-mark-recapture study and molecular tools.
In addition, our results provide new information about FT ecology and conservation status in Guadeloupe. First, our data indicate that forest thrushes are relatively abundant in Guadeloupe. Indeed, detection rates and estimates of naïve occupancy obtained in the present study are among the highest values reported so far for ground-dwelling bird species using the same methodology (Supplementary materials Table S1). This might be explained by a combination of factors. First, hunting has been suspended since 2014 in Guadeloupe. More precisely, before the hunting ban, the Guadeloupe FT population was estimated at 46,900—49,500 pairs (93,800—99,000 mature individuals; Eraud et al. 2012). However, the estimation has not been updated since then. On the other hand, from 2015 to 2017, the FT population trend was estimated from 10 sites in Guadeloupe (Guillemot et al. 2020). Although the number of recaptured birds was relatively stable, FT frequency of occurrence increased from 2015 to 2017, possibly as a consequence of the hunting ban. Second, a large proportion of the tropical rainforest habitat is under protection in Guadeloupe (Magnin 2018). Unfortunately, there are no studies comparing FT population trends before and after the protection of its main natural habitat. We then suggest comparing FT population trends between protected and unprotected tropical rainforests to better assess the importance of habitat protection for conservation.
However, a multiyear survey of the Guadeloupe FT population is necessary to ascertain population trends. In particular, comparison of adult survival and breeding success between habitat types would be quite valuable for future management plans. This could be achieved through combining the use of camera-traps with capture that of combinations of colour rings allowing subsequent individual identification of marked individuals in the field from photographic records (Toy et al. 2017; Brides et al. 2018; Santangeli et al. 2020).
Second, an important outcome from model selection is that temperature, rather than elevation per se, was the most important factor influencing the presence of forest thrushes at our study sites. Obviously, the two variables are highly correlated between themselves as the species is predominantly found in the tropical rainforest, characterized by its rainy and cold climate due to the higher elevation in the southern half of Basse-Terre (Grubb 1971; Laere et al. 2016). Interestingly, forest type was not retained in the best model, indicating that difference in suitability between habitats might be related to a large extent to difference in ambient temperature. Indeed, due to marked difference in elevations, Basse-Terre, which is home to the tropical rainforest, has generally higher temperatures than Grande-Terre, which is home to a large part of the tropical dry forest and the tropical wet coastal forest (Rousteau 1996a, b; MDDEE 2012; Laere et al. 2016).
The importance of temperature is particularly relevant in the context of current global warming (Crick 2004; Descamps et al. 2017). Our findings are consistent with other studies, showing that rising temperatures may directly affect bird species, positively or negatively, via their limited thermal niche (Şekercioĝlu et al. 2012), particularly in rainforest montane birds (de la Fuente et al. 2023). However, the effect of increased mean temperature and variability may vary between species depending on their ecology, with forest specialists and insectivores being less able to benefit from increased temperature along an elevational gradient (Dulle et al. 2016). More precisely, comparative evidence suggests that ground-dwelling tropical species with limited dispersal ability, as is the case for FT (Arnoux et al. 2014), might be more affected by climatic instability than arboreal ones (Scheffers et al. 2017). The use of data loggers placed on FT and in their different forested habitats (see Jirinec et al. 2022) in the future may provide a better understanding microhabitat selection in FT in relation to light and thermal niches.
Finally, temperature is likely to co-vary with other variables such as, for instance, soil moisture that was not measured in the present study. Spatiotemporal variation in moisture can have direct influence on arthropods diversity and abundance and, in turn, influence bird distribution in forest habitats (Petit et al. 1985; Smith et al. 2010). Parashuram et al. (2015) suggested that variations of local abundance of FT in different areas may reflect foraging habitat quality. In addition, Stanley et al. (2021) provided strong evidence for an effect of moisture on food abundance and space-use strategies of wood thrushes, Hylocichla mustelina, wintering in Belize. In particular, individuals in drier habitats experienced lower food abundance and were in lower body condition.
As other woodland-dwelling thrush species, FT is adapted to forage terrestrially in leaf-litter. Both moisture and a dense canopy could then provide optimal foraging conditions for FT in Guadeloupe, and, possibly, other islands. Future studies should then benefit from assessing to what extent FT spatial distribution and local abundance reflects spatial variation in food availability in leaf-litter, and how this could be affected by climate change.
The use of camera-traps allowed us to document for the first-time spatial co-occurrence between FT and potential mammal predators. FT co-occurred with rats and negatively with cats. Analysis of diel activity indicated that rodents were essentially nocturnal (see Jean-Pierre et al. 2022) whereas FT were essentially diurnal. Rodents may however, prey upon FT eggs since nests are placed at relatively low height from the ground (Benito-Espinal and Hautcastel 2003). The observed spatial segregation between FT and cats may result from active avoidance by FT of areas where cats are particularly abundant or may reflect the local impact of predation by cats on FT. Although, overall, cats were mostly active at night, some individuals were recorded during the day, and it is possible that different individuals have different diel patterns of activity (Hertel et al. 2017), particularly if they specialize on different preys (Dickman and Newsome 2015). Although predation by domestic and feral cats is a major cause of bird mortality in many areas (Loss et al. 2015; Marra and Santella 2016), including islands (Blackburn and Duncan 2007; Medina et al. 2011; Nogales et al. 2013; Doherty et al. 2016), its importance in Caribbean islands has not been documented so far. In the absence of any data on the diet of domestic and feral cats in forested habitats in Guadeloupe, their potential impact on FT populations is difficult to assess. More to the point, the interactions between cats, rats and FT might be particularly complex, particularly through what is known as the "mesopredator release effect" (Courchamp et al. 1999; Ballari et al. 2016; Takimoto and Nishijima 2022). Indeed, superpredators such as feral domestic cats may contribute to control mesopredators such as rats, such that their overall impact on FT is difficult to assess.
The use of camera traps allowed us to obtain valuable ecological information on FT spatial occupancy, abundance, diel activity, spatial co-occurrence with potential mammal predators, and their underlying factors. Similarly, several studies successfully used camera traps to study elusive ground-dwelling avian species (O’Brien and Kinnaird 2008; Ramesh and Downs 2014; Suwanrat et al. 2015; Smith et al. 2017). However, the use of camera-traps comes with some limitations (reviewed in Meek et al. 2015; Cordier et al. 2022). In particular, performance is likely to vary between camera trap models and settings (reviewed in Palencia et al. 2022), such that comparisons between studies should be done with caution. More importantly, camera traps may influence the behaviour of animals through associated mechanical noise, odor, and emitted light, although evidence is mainly limited to mammals (Caravaggi et al. 2020). We therefore used infrared-flash cameras, thus providing back and white pictures, to document the presence of nocturnal potential predators, to avoid potential biases associated with the use of white flash cameras (Schipper 2007; but see Taggart et al. 2019 for counter evidence in domestic cats). A wise use of camera traps should therefore be based on a balance between the benefit of obtaining high quality data (i.e., color photographs for improved species and/or individual identification) and the risk of interference with the behaviour of targeted species. Faced with all these pitfalls, we suggest that researchers plan a processing time for the captures obtained. In addition, visiting camera trap locations is necessary to verify that the camera traps are working properly.
Conclusions and recommendations
Overall, our study shows that the use of unbaited camera-traps can be a reliable tool to assess spatial occupancy and local abundance of FT, as previously reported in the literature for other ground-dwelling and secretive forest bird species (Suwanrat et al. 2015; Smith et al. 2017; Murphy et al. 2018; Pérez-Irineo and Santos-Moreno 2021; Jean-Pierre et al. 2022). Contrary to auditory point count and auditory distance sampling, the method can be used with the same efficiency independently of seasonal variation in FT vocal activity or meteorological conditions. However, future survey of FT in Montserrat, Guadeloupe, Dominica, and St Lucia, as well as other forest ground-dwelling birds in the insular Caribbean (see Jean-Pierre et al. 2022), may benefit from combining the use of camera-traps with that of acoustic recorders (Buxton et al. 2018). We also suggest to further investigate the potential impact of exotic mammal predators on FT and other ground-dwelling forest birds, possibly through collecting faeces in the wild (Carrión and Valle 2018) or from direct analysis of stomach content (Balestrieri et al. 2011) following control operations.
Data availability
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.
References
Agresti A (1992) A survey of exact inference for contingency tables. Stat Sci 7:131–153. https://doi.org/10.1214/SS/1177011454
Akresh ME, Askins RA, King DI et al (2021) Resilience in the aftermath of hurricanes: Fluctuations in a Critically Endangered population of West Indian Woodpeckers Melanerpes superciliaris nyeanus over two decades. Bird Conserv Int 31:185–205. https://doi.org/10.1017/S0959270920000386
Alroy J (2017) Effects of habitat disturbance on tropical forest biodiversity. Proc Natl Acad Sci USA 114:6056–6061. https://doi.org/10.1073/PNAS.1611855114/SUPPL_FILE/PNAS.201611855SI.PDF
Anderson AS, Marques TA, Shoo LP, Williams SE (2015) Detectability in audio-visual surveys of tropical rainforest birds: The influence of species, weather and habitat characteristics. PLoS One 10:e0128464. https://doi.org/10.1371/JOURNAL.PONE.0128464
Arendt WJ, Qian SS, Mineard KA (2013) Population decline of the Elfin-woods Warbler Setophaga angelae in eastern Puerto Rico. Bird Conserv Int 23:136–146. https://doi.org/10.1017/S0959270913000166
Arnoux E (2012) Variabilités phénotypique et génétique chez la Grive à pieds jaunes, Turdus lherminieri, à différentes échelles. PhD thesis, University of Bourgogne. https://tel.archives-ouvertes.fr/tel-01002901.
Arnoux E, Eraud C, Navarro N et al (2014) (2014) Morphology and genetics reveal an intriguing pattern of differentiation at a very small geographic scale in a bird species, the forest thrush Turdus lherminieri. Hered 1136(113):514–525. https://doi.org/10.1038/hdy.2014.56
Arnoux E, Eraud C, Thomas A et al (2013) Phenotypic variation of Forest Thrushes Turdus lherminieri in Guadeloupe: Evidence for geographic differentiation at fine spatial scale. J Ornithol 154:977–985. https://doi.org/10.1007/S10336-013-0965-0/TABLES/3
Balestrieri A, Remonti L, Prigioni C (2011) Assessing carnivore diet by faecal samples and stomach contents: A case study with Alpine red foxes. Cent Eur J Biol 6:283–292. https://doi.org/10.2478/S11535-010-0106-1/MACHINEREADABLECITATION/RIS
Ballari SA, Kuebbing SE, Nuñez MA (2016) Potential problems of removing one invasive species at a time: a meta-analysis of the interactions between invasive vertebrates and unexpected effects of removal programs. PeerJ 4:e2029. https://doi.org/10.7717/peerj.2029
Banko PC, Camp RJ, Farmer C et al (2013) Response of palila and other subalpine Hawaiian forest bird species to prolonged drought and habitat degradation by feral ungulates. Biol Conserv 157:70–77. https://doi.org/10.1016/J.BIOCON.2012.07.013
Benito-Espinal E, Hautcastel P (2003) Les oiseaux des Antilles et leur nid. PLB éd., Les Abymes, Guadeloupe
Besnard A, Salles JM (2010) Suivi scientifique d’espèces animales. Aspects méthodologiques essentiels pour l'élaboration de protocoles de suivis. Note méthodologique à l'usage des gestionnaires de sites Natura 2000. Rapport DREAL PACA, pôle Natura 62:169. https://www.paca.developpement-durable.gouv.fr/IMG/pdf/NT_MethodoSuiviBio_Faune_final2_cle658bab.pdf
Blackburn TM, Duncan RP (2007) Causes of avian extinction on islands. Anim Conserv 10:149–150. https://doi.org/10.1111/j.1469-1795.2007.00110.x
Bradshaw CJA, Sodhi NS, Brook BW (2009) Tropical turmoil: a biodiversity tragedy in progress. Front Ecol Environ 7:79–87. https://doi.org/10.1890/070193
Brides K, Middleton J, Leighton K, Grogan A (2018) The use of camera traps to identify individual colour-marked geese at a moulting site. Ringing Migr 33:19–22. https://doi.org/10.1080/03078698.2018.1525194
Brook BW, Sodhi NS, Bradshaw CJA (2008) Synergies among extinction drivers under global change. Trends Ecol Evol 23:453–460. https://doi.org/10.1016/J.TREE.2008.03.011
Brooks TM, Mittermeier RA, Mittermeier CG et al (2002) Habitat Loss and Extinction in the Hotspots of Biodiversity. Conserv Biol 16:909–923. https://doi.org/10.1046/J.1523-1739.2002.00530.X
Burnham KP, Anderson DR (1998) Practical use of the information-theoretic approach. Model Sel Inference 75–117. https://doi.org/10.1007/978-1-4757-2917-7_3
Burnham KP, Anderson DR, Huyvaert KP (2011) AIC model selection and multimodel inference in behavioral ecology: Some background, observations, and comparisons. Behav Ecol Sociobiol 65:23–35. https://doi.org/10.1007/S00265-010-1029-6/FIGURES/3
Buxton RT, Lendrum PE, Crooks KR, Wittemyer G (2018) Pairing camera traps and acoustic recorders to monitor the ecological impact of human disturbance. Glob Ecol Conserv 16:e00493. https://doi.org/10.1016/J.GECCO.2018.E00493
Cambrone C, Bezault E, Cézilly F (2021) Efficiency of the call-broadcast method for detecting two Caribbean-endemic columbid game species. Eur J Wildl Res 67:1–18. https://doi.org/10.1007/S10344-021-01507-0/TABLES/5
Caravaggi A, Cole Burton A, Clark DA (2020) A review of factors to consider when using camera traps to study animal behavior to inform wildlife ecology and conservation. Conserv Sci Pract 2:e239. https://doi.org/10.1111/csp2.239
Carrión PL, Valle CA (2018) The diet of introduced cats on San Cristobal Island, Galapagos: cat feces as a proxy for cat predation. Mamm Biol 90:74–77. https://doi.org/10.1016/J.MAMBIO.2018.02.004
Catanach TA, Halley MR, Allen JM et al (2021) Systematics and conservation of an endemic radiation of Accipiter hawks in the Caribbean islands. Ornithology 138:3. https://doi.org/10.1093/ORNITHOLOGY/UKAB041
Clement P, Hathway R (2000) Thrushes. Helm identification guides. London Christopher Helm 463
Cook RN, Ramirez-Parada T, Browne L et al (2020) Environmental correlates of richness, community composition, and functional traits of terrestrial birds and mammals in a fragmented tropical landscape. Landsc Ecol 35:2825–2841. https://doi.org/10.1007/S10980-020-01123-4/TABLES/3
Cordier CP, Smith DAE, Smith, YE et al (2022) Camera trap research in Africa: A systematic review to show trends in wildlife monitoring and its value as a research tool. Global Ecol Conserv 40:e02326. https://doi.org/10.1016/j.gecco.2022.e02326
Courchamp F, Langlais M, Sugihara G (1999) Cats protecting birds: modelling the mesopredator release effect. J Anim Ecol 68:282–292. https://doi.org/10.1046/J.1365-2656.1999.00285.X
Crick HQP (2004) The impact of climate change on birds. Ibis (Lond 1859) 146:48–56. https://doi.org/10.1111/J.1474-919X.2004.00327.X
Dalsgaard B, Hilton GM, Gray GAL et al (2007) Impacts of a volcanic eruption on the forest bird community of Montserrat, Lesser Antilles. Ibis (Lond 1859) 149:298–312. https://doi.org/10.1111/J.1474-919X.2006.00631.X
Dávalos LM, Sanchez KM, Armenteras D (2016) Deforestation and coca cultivation rooted in twentieth-century development projects. Bioscience 66:974–982. https://doi.org/10.1093/BIOSCI/BIW118
Davies HF, Rioli W, Puruntatameri J et al (2019) Estimating site occupancy and detectability of the threatened partridge pigeon (Geophaps smithii) using camera traps. Austral Ecol 44:868–879. https://doi.org/10.1111/AEC.12755
de la Fuente A, Navarro A, Williams SE (2023) The climatic drivers of long-term population changes in rainforest montane birds. Glob Change Biol 29:2132–2140. https://doi.org/10.1111/gcb.16608
Descamps S, Aars J, Fuglei E et al (2017) Climate change impacts on wildlife in a High Arctic archipelago – Svalbard, Norway. Glob Chang Biol 23:490–502. https://doi.org/10.1111/GCB.13381
Devenish-Nelson ES, Weidemann D, Townsend J, Nelson HP (2019) Patterns in island endemic forest-dependent bird research: the Caribbean as a case-study. Biodivers Conserv 28:1885–1904. https://doi.org/10.1007/S10531-019-01768-X/FIGURES/4
Dickman CR, Newsome TM (2015) Individual hunting behaviour and prey specialisation in the house cat Felis catus: Implications for conservation and management. Appl Anim Behav Sci 173:76–87. https://doi.org/10.1016/J.APPLANIM.2014.09.021
Doherty TS, Glen AS, Nimmo DG et al (2016) Invasive predators and global biodiversity loss. Proc Natl Acad Sci U S A 113:11261–11265. https://doi.org/10.1073/PNAS.1602480113/SUPPL_FILE/PNAS.1602480113.SD03.RTF
Dolisca F, McDaniel JM, Teeter LD, Jolly CM (2007) Land tenure, population pressure, and deforestation in Haiti: The case of Forêt des Pins Reserve. J for Econ 13:277–289. https://doi.org/10.1016/J.JFE.2007.02.006
Duclos TR, DeLuca WV, King DI (2019) Direct and indirect effects of climate on bird abundance along elevation gradients in the Northern Appalachian mountains. Divers Distrib 25:1670–1683. https://doi.org/10.1111/DDI.12968
Dulle HI, Ferger SW, Cordeiro NJ et al (2016) Changes in abundances of forest understorey birds on Africa’s highest mountain suggest subtle effects of climate change. Diversity Distrib 22:288–299
Durand S, Baptiste BJ (2008) Important bird areas in the Caribbean: key sites for conservation: Dominican Republic. BirdLife International Conservation Series 15, Cambridge, U.K
Ehlers Smith DA, Ehlers Smith YC, Ramesh T, Downs CT (2017) Camera-trap data elucidate habitat requirements and conservation threats to an endangered forest specialist, the spotted ground thrush (Zoothera guttata). For Ecol Manage 400:523–530. https://doi.org/10.1016/J.FORECO.2017.06.045
Eppinga MB, Pucko CA (2018) The impact of hurricanes Irma and Maria on the forest ecosystems of Saba and St. Eustatius, northern Caribbean. Biotropica 50:723–728. https://doi.org/10.1111/BTP.12600
Eraud C, Levesque A, Van Laere G, Magnin H (2013) La Grive à pieds jaunes (Turdus lherminieri) en Guadeloupe: État des connaissances sur l’importance et la répartition des effectifs. Unpublished report, ONCFS-Parc National Guadeloupe. https://www.guadeloupe.developpement-durable.gouv.fr/IMG/pdf/eraud-levesque-van_laere-magnin_2013-png_oncfs.pdf
Eraud C, Magnin H, Van Laere G, Arnoux E, Levesque A (2012) Biologie des populations et statut de conservation des oiseaux endémiques des Antilles en Guadeloupe. Rapport d’étude ONCFS-Parc National Guadeloupe. https://side.developpement-durable.gouv.fr/CENT/doc/SYRACUSE/226502/biologie-des-populations-et-statut-de-conservation-des-oiseaux-endemiques-des-antilles-en-guadeloupe
Fisher RA (1992) Statistical methods for research workers. Springer 66–70. https://doi.org/10.1007/978-1-4612-4380-9_6
Fisher RA (1922) On the interpretation of χ 2 from contingency tables, and the calculation of P. J R Stat Soc 85:87. https://doi.org/10.2307/2340521
Fiske IJ, Chandler RB (2011) unmarked: An R package for fitting hierarchical models of wildlife occurrence and abundance. J Stat Softw 43:1–23. https://doi.org/10.18637/JSS.V043.I10
Gadalia A, Gstalter N, Westercamp D (1988) La chaîne volcanique de Bouillante, Basse-Terre de Guadeloupe, (Petites Antilles). Identité pétrographique, volcanologique et géodynamique. Géologie France 2–3:101–130
Gerber BD, Karpanty SM, Kelly MJ (2012) Evaluating the potential biases in carnivore capture-recapture studies associated with the use of lure and varying density estimation techniques using photographic-sampling data of the Malagasy civet. Popul Ecol 54:43–54. https://doi.org/10.1007/S10144-011-0276-3/FIGURES/2
Giam X (2017) Global biodiversity loss from tropical deforestation. Proc Natl Acad Sci USA 114:5775–5777. https://doi.org/10.1073/PNAS.1706264114/ASSET/DF89A525-6B04-4F73-BB34-D9FBA18AE3A2/ASSETS/PNAS.1706264114.FP.PNG
Griffith DM, Veech JA, Marsh CJ (2016) Cooccur: probabilistic species co-occurrence analysis in R. J Stat Softw 69:1–17. https://doi.org/10.18637/JSS.V069.C02
Grubb PJ (1971) (1971) Interpretation of the ‘Massenerhebung’ effect on tropical mountains. Nat 2295279(229):44–45. https://doi.org/10.1038/229044a0
Guillemot B, Rozet D, Coquelet P et al (2020) Evolution de l’abondance de la Grive à pieds jaunes (Turdus lherminieri) en Guadeloupe. Unpublished report, Office Français de la Biodiversité, Unité Territoriale Connaissance Antilles, Guadeloupe. https://www.guadeloupe.developpement-durable.gouv.fr/IMG/pdf/2015-2019_rapportsuivi_gpj_vf.pdf.
Hallett MT, Collins F, Li MF et al (2021) Medium and large bodied mammals and ground birds of the Barima Mora Passage, Region 1, Guyana. https://www.guyanamarineconservation.org/HALLETT-Barima-Mora-report.pdf.
Hansen MC, Potapov PV, Moore R et al (2013) High-resolution global maps of 21st-century forest cover change. Science 342:850–853. https://doi.org/10.1126/SCIENCE.1244693/SUPPL_FILE/HANSEN.SM.PDF
Hedges SB, Cohen WB, Timyan J, Yang Z (2018) Haiti’s biodiversity threatened by nearly complete loss of primary forest. Proc Natl Acad Sci U S A 115:11850–11855. https://doi.org/10.1073/PNAS.1809753115
Hertel AG, Swenson JE, Bischof R (2017) A case for considering individual variation in diel activity patterns. Behav Ecol 28:1524–1531. https://doi.org/10.1093/BEHECO/ARX122
Jacobs J (1974) Quantitative measurement of food selection. Oecologia 14:413–417. https://doi.org/10.1007/BF00384581
Jean-Pierre A, Loranger-Merciris G, Cézilly F (2022) Spatial Occupancy, Local Abundance and Activity Rhythm of Three Ground Dwelling Columbid Species in the Forests of Guadeloupe in Relation to Environmental Factors. Divers 14:480. https://doi.org/10.3390/D14060480
Jirinec V, Rodiguez PF, Amaral BR et al (2022). Light and thermal niches of ground-foraging Amazonian insectivorous birds. Ecology 103:e3645. https://doi.org/10.1002/ecy.3645
Johnson TH, Stattersfield AJ (1990) A global review of island endemic birds. Ibis (Lond 1859) 132:167–180. https://doi.org/10.1111/J.1474-919X.1990.TB01036.X
Laere G, Gall Y, Maurice A et al (2016) The Forest Ecosystems Observatory in Guadeloupe (FWI). Caribbean Naturalist, Eagle Hill Intitute 108–115
Lasserre G (1961) La Guadeloupe: étude géographique. Union française d’impression 2
Levesque A, Eraud C, Villers A et al (2020) Bilans 2014–2019 du programme Stoc-Guadeloupe. https://www.amazona-guadeloupe.com/rapports-study-reports.html.
Levesque A, Lartiges A (2000) Colombidés Antillais. Biologie–Ecologie–Méthodes d’études. Analyse Bibliographique. Direction Régionale de l’Environnement de la Guadeloupe and Office National de la Chasse et de la Faune Sauvage. https://side.developpement-durable.gouv.fr/ACCIDR/doc/SYRACUSE/203417/colombides-antillais-biologie-ecologie-methodes-d-etudes-analyse-bibliographique.
Lloyd JD, Rimmer CC, McFarland KP (2016) Assessing conservation status of resident and migrant birds on Hispaniola with mist-netting. PeerJ 2016:e1541. https://doi.org/10.7717/PEERJ.1541/FIG-5
Loss SR, Will T, Marra PP (2015) Direct mortality of birds from anthropogenic causes. Annu Rev Ecol Evol Syst 46:99–120. https://doi.org/10.1146/annurev-ecolsys-112414-054133
Louppe V, Herrel A, Pisanu B et al (2021) Assessing occupancy and activity of two invasive carnivores in two Caribbean islands: implications for insular ecosystems. J Zool 313:182–194. https://doi.org/10.1111/JZO.12845
Lucherini M, Reppucci JI, Walker RS et al (2009) Activity pattern segregation of carnivores in the high andes. J Mammal 90:1404–1409. https://doi.org/10.1644/09-MAMM-A-002R.1/2/JMAMMAL-90-6-1404-FIG3.JPEG
MacKenzie DI, Bailey LL (2004) Assessing the fit of site-occupancy models. J Agric Biol Environ Stat 93(9):300–318. https://doi.org/10.1198/108571104X3361
Magnin H (2018) Le Parc national de la Guadeloupe: un territoire insulaire unique dédié à la protection de la biodiversité. Études caribéennes 41. https://journals.openedition.org/etudescaribeennes/13187.
Marra PP, Santella C (2016) Cat Wars. Cat Wars. https://doi.org/10.1515/9781400882878/HTML
Maseko MST, Ramesh T, Kalle R, Downs CT (2017) Response of Crested Guinea-fowl (Guttera edouardi), a forest specialist, to spatial variation in land use in iSimangaliso Wetland Park, South Africa. J Ornithol 158:469–477. https://doi.org/10.1007/S10336-016-1406-7/FIGURES/4
MDDEE (2012) Profil environnemental régional de la Guadeloupe 2011. Direction de l’environnement, de l’aménagement et du logement. Guadeloupe. https://www.guadeloupe.developpement-durable.gouv.fr/IMG/pdf/4_PER__Guadeloupe_VF5.pdf
Medina FM, Bonnaud E, Vidal E et al (2011) A global review of the impacts of invasive cats on island endangered vertebrates. Glob Chang Biol 17:3503–3510. https://doi.org/10.1111/J.1365-2486.2011.02464.X
Meek PD, Ballard, GA, Fleming, PJS (2015). The pitfalls of wildlife camera trapping as a survey tool in Australia. Austral Mammal 37:13–22
Meredith M, Ridout M (2016) Overview of the overlap package. https://cran.r-project.org/web/packages/overlap/vignettes/overlap.pdf
Mohd Razali N, Bee Wah Y (2011) Power comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Lilliefors and Anderson-Darling tests. J Stat Model Anal 2:13–14
Monterroso P, Alves PC, Ferreras P (2014) Plasticity in circadian activity patterns of mesocarnivores in Southwestern Europe: Implications for species coexistence. Behav Ecol Sociobiol 68:1403–1417. https://doi.org/10.1007/S00265-014-1748-1/TABLES/3
Murphy AJ, Farris ZJ, Karpanty S et al (2018) Using camera traps to examine distribution and occupancy trends of ground-dwelling rainforest birds in north-eastern Madagascar. Bird Conserv Int 28:567–580. https://doi.org/10.1017/S0959270917000107
Myers N, Mittermeler RA, Mittermeler CG et al (2000) (2000) Biodiversity hotspots for conservation priorities. Nat 4036772(403):853–858. https://doi.org/10.1038/35002501
Niedballa J, Sollmann R, Courtiol A, Wilting A (2016) camtrapR: an R package for efficient camera trap data management. Methods Ecol Evol 7:1457–1462. https://doi.org/10.1111/2041-210X.12600
Nogales M, Vidal E, FélM M et al (2013) Feral cats and biodiversity conservation: The urgent prioritization of island management. Bioscience 63:804–810. https://doi.org/10.1525/BIO.2013.63.10.7
O’Brien TG, Kinnaird MF (2008) A picture is worth a thousand words: the application of camera trapping to the study of birds. Bird Conserv Int 18:S144–S162. https://doi.org/10.1017/S0959270908000348
O’Connell AF, Nichols JD, Karanth KU (2011) Camera traps in animal ecology: Methods and analyses, camera traps in animal ecology: Methods and Analyses. Springer Japan. https://doi.org/10.1007/978-4-431-99495-4
Palencia P, Vicente J, Soriguer RC (2022) Towards a best-practices guide for camera trapping: assessing differences among camera trap models and settings under field conditions. J Zool 316:197–208. https://doi.org/10.1111/jzo.12945
Parashuram D, Oppel S, Fenton C et al (2015) The Forest Thrush Turdus lherminieri prefers mature mesic forest with dense canopy. Bird Conserv Int 25:503–513. https://doi.org/10.1017/S0959270914000495
Pérez-Irineo G, Santos-Moreno A (2021) Bird activity patterns in the understorey of an evergreen forest in Oaxaca, Mexico. Neotrop Biol Conserv 16:1–10. https://doi.org/10.3897/neotropical.16.e59360
Petit DR, Petite KE, Grugg TC Jr (1985) On atmospheric moisture as a factor influencing distribution of breeding birds in temperate deciduous forest. Wilson Bulletin 97:88–96
Pimm SL, Raven P, Peterson A et al (2006) Human impacts on the rates of recent, present, and future bird extinctions. Proc Natl Acad Sci U S A 103:10941–10946. https://doi.org/10.1073/PNAS.0604181103
Pimm SL, Jenkins CN, Abell R et al (2014) The biodiversity of species and their rates of extinction, distribution, and protection. Science. 344. https://doi.org/10.1126/SCIENCE.1246752/SUPPL_FILE/PIMM.SM.PDF
Preston FW (1948) The commonness, and rarity, of species. Ecology 29:254–283. https://doi.org/10.2307/1930989
Ramesh T, Downs CT (2014) Land use factors determining occurrence of Red-necked Spurfowl (Pternistis afer) in the Drakensberg Midlands, South Africa. J Ornithol 155:471–480. https://doi.org/10.1007/S10336-013-1028-2/TABLES/4
Ricketts TH, Dinerstein E, Boucher T et al (2005) Pinpointing and preventing imminent extinctions. Proc Natl Acad Sci U S A 102:18497–18501. https://doi.org/10.1073/PNAS.0509060102/SUPPL_FILE/INDEX.HTML
Ridout MS, Linkie M (2009) Estimating overlap of daily activity patterns from camera trap data. J Agric Biol Environ Stat 143(14):322–337. https://doi.org/10.1198/JABES.2009.08038
Rivera-Milán FF, Martínez AJ, Matos A et al (2022) Puerto Rico plain pigeon, scaly-naped pigeon and red-tailed hawk: population dynamics and association patterns before and after hurricanes. Endanger Species Res 47:75–89. https://doi.org/10.3354/ESR01166
Rosner B, Glynn RJ, Lee MLT (2006) The wilcoxon signed rank test for paired comparisons of clustered data. Biometrics 62:185–192. https://doi.org/10.1111/J.1541-0420.2005.00389.X
Rousteau A (1996a) Structures, flores, dynamiques: réponses des forêts pluviales des petites antilles aux milieux montagnards. Phytogeographie tropicale: realites et perspectives. ORSTOM editions, Paris 308–321
Rousteau A (1996b) Ecological map of Guadeloupe. University of Antilles Guyane, National Park of Guadeloupe, National Forestry Service
Royle JA, Nichols JD (2003) Estimating abundance from repeated presence–absence data or point counts. Ecology 84:777–790. https://doi.org/10.1890/0012-9658
Santangeli A, Pakanen VM, Bridgeford P et al (2020) The relative contribution of camera trap technology and citizen science for estimating survival of an endangered African vulture. Biol Conserv 246:108593. https://doi.org/10.1016/J.BIOCON.2020.108593
Scheffers BR, Shoo L, Phillips B et al (2017) Vertical (arboreality) and horizontal (dispersal) movement increase the resilience of vertebrates to climatic instability. Global Ecol Biogeogr 26:787–798. https://doi.org/10.1111/geb.12585
Schipper J (2007) Camera-trap avoidance by kinkajous Potos flavus: rethinking the ‘“non-invasive”’ paradigm. Small Carn Conserv 36:38–41
Schmid F, Schmidt A (2006) Nonparametric estimation of the coefficient of overlapping—theory and empirical application. Comput Stat Data Anal 50:1583–1596. https://doi.org/10.1016/J.CSDA.2005.01.014
Şekercioĝlu çaĝan H, Primack RB, Wormworth J, (2012) The effects of climate change on tropical birds. Biol Conserv 148:1–18. https://doi.org/10.1016/J.BIOCON.2011.10.019
Si X, Kays R, Ding P (2014) How long is enough to detect terrestrial animals? Estimating the minimum trapping effort on camera traps. PeerJ e374. https://doi.org/10.7717/PEERJ.374/SUPP-2.
Smith DAE, Smith YCE, Downs CT (2017) Seasonal habitat requirements of Lemon Dove (Aplopelia larvata) in coastal forest: camera-trap surveys of a reclusive species. African Zool 52:199–207. https://doi.org/10.1080/15627020.2017.1388193
Smith DR (2004) Application of adaptive cluster sampling to biological populations. Sampl Rare Elus Species 75–122
Smith JAM, Reitsma LR, Marra PP (2010) Moisture as a determinant of habitat quality for a nonbreeding Neotropical migratory songbird. Ecology 91:2874–2882. https://doi.org/10.1890/09-2212.1
Stanley CQ, Dudash MR, Ryder TB et al (2021) Variable tropical moisture and food availability underlie mixed winter space-use strategies in a migratory songbird. Proc R Soc B 288. https://doi.org/10.1098/RSPB.2021.1220
Stattersfield AJ, Crosby MJ, Long AJ, Wege DC (1998) Endemic bird areas of the world: Priorities for biodiversity conservation (Birdlife International, Cambridge, UK)
Suwanrat S, Ngoprasert D, Sutherland C et al (2015) Estimating density of secretive terrestrial birds (Siamese Fireback) in pristine and degraded forest using camera traps and distance sampling. Glob Ecol Conserv 3:596–606. https://doi.org/10.1016/J.GECCO.2015.01.010
Taggart PL, Peacock DE, Fancourt BA (2019) Camera trap flash-type does not influence the behaviour of feral cats (Felis catus). Austral Mammal 42:220–222. https://doi.org/10.1071/AM18056
Takimoto G, Nishijima S (2022) A simple theory for the mesopredator release effect: when does an apex predator protect their shared prey from a mesopredator? Oikos 2022:e09021. https://doi.org/10.1111/OIK.09021
Talvitie M, Leino O, Holopainen M (2006) Inventory of sparse forest populations using adaptive cluster sampling. Silva Fenn 40:101
Tole L (2001) (2001) Jamaica’s Disappearing Forests: Physical and human aspects. Environ Manag 284(28):455–467. https://doi.org/10.1007/S002670010236
Toussaint A, John L, Morton M (2009) The status and conservation of Saint Lucia’s forest birds. Technical Report No. 12 to the National Forest Demarcation and Bio-Physical Resource Inventory Project, FCG International Ltd, Helsinki, Finland
Toy DL, Roche EA, Dovichin CM (2017) Small high-definition video cameras as a tool to resight uniquely marked interior least terns (Sternula antillarum athalassos). 40:180–186. https://doi.org/10.1675/063.040.0211
Vázquez-Miranda H, Navarro-Sigüenza AG, Morrone JJ (2007) Biogeographical patterns of the avifaunas of the Caribbean Basin Islands: a parsimony perspective. Cladistics 23:180–200. https://doi.org/10.1111/J.1096-0031.2006.00133.X
Veech JA (2013) A probabilistic model for analysing species co-occurrence. Glob Ecol Biogeogr 22:252–260. https://doi.org/10.1111/J.1466-8238.2012.00789.X
Waide RB, Narins PM (1988) Tropical forest bird counts and the effect of sound attenuation. Auk 105:296–302. https://doi.org/10.2307/4087493
Wilcove DS, Giam X, Edwards DP et al (2013) Navjot’s nightmare revisited: logging, agriculture, and biodiversity in Southeast Asia. https://doi.org/10.1016/j.tree.2013.04.005
Wiley JW, Wunderle JM (1993) The effects of hurricanes on birds, with special reference to Caribbean islands. Bird Conserv Int 3:319–349. https://doi.org/10.1017/S0959270900002598
Wunderle JM (2008) From the past to the globalized future for Caribbean birds. J Caribb Ornithol 21:79
Zuccon D (2011) A new name for the Montserrat Forest thrush. Bull Brit Ornithol Club 131:199–200
Acknowledgements
The authors thank Christopher Cambrone and Etienne Bezault for their support in 2019. We sincerely acknowledge Anatòli Bec-Canet, Lilian Clerembault, Loïc Jason, Taz Ramsay, Ronyl Narsey, Véronique Holstein, and Ludovic Pruneau for help in data collection.
Funding
This work was funded by Caribaea Initiative, the Office Français de la Biodiversité (OFB), and a doctoral grant from the French Ministry of Research and Higher Education to AJP.
Author information
Authors and Affiliations
Contributions
AJP: Investigation, Methodology, Data collection, Statistical analysis, Writing – original draft, Writing – review and editing. GL: Project administration, Conceptualization, Methodology, Review and editing. LJSL: Data collection, Statistical analysis, Writing – original draft. FC: Project administration, Funding acquisition, Conceptualization, Methodology, Statistical analysis, Writing – review and editing.
Corresponding author
Ethics declarations
Declaration of Competing Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10344_2023_1698_MOESM1_ESM.docx
Table S1 Estimates of detection rate and naïve occupancy obtained from camera-traps surveys of ground-dwelling forest bird species of various conservation status (LC = Least Concern; NT = Near Threatened; VU = Vulnerable) (DOCX 20 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jean-Pierre, A., Loranger-Merciris, G., Saint-Louis, L.J. et al. Factors affecting spatial occupancy and local abundance of the Forest Thrush, Turdus lherminieri, in Guadeloupe forests. Eur J Wildl Res 69, 76 (2023). https://doi.org/10.1007/s10344-023-01698-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-023-01698-8