Abstract
Survival of ungulates can vary seasonally due to changing environmental conditions, e.g. weather or predation pressure. The wild forest reindeer (Rangifer tarandus fennicus, “WFR”) migrate between calving and wintering grounds. The annual cycle of female WFR includes four main seasons (wintering, calving, rutting and autumn migration) during which they are subject to different conditions, but almost nothing is known about their survival or mortality patterns. We measured survival in 305 GPS-tagged female WFR in two subpopulations in Finland (2010–2022). Data were analysed for inter-annual and seasonal patterns in survival and cause-specific mortality (predation, traffic, accidents and unknown causes) with known-fate models. Inter-annual survival in Suomenselkä (mean 0.90) showed an increasing trend during the study and was higher than in Kainuu (mean 0.84) which showed a declining trend. Seasonal variation in survival was population dependent. Survival was lowest in Kainuu during the winter while in Suomenselkä, it was lowest during autumn migration. Concerning cause-specific mortality, seasonal variation mirrored variation in predation that was the primary cause of mortality (55% of cases). Wolves (Canis lupus) killed the majority of predated individuals (58%). Predation rates were two times higher in Kainuu where the wolf density was higher. We provide the first adult survival estimates for WFR and show that the seasonal variation in survival is clearly different between these two northern ungulate populations. This indicates that seasonal variation in survival is determined by site-dependent factors and cannot be generalised across populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adult survival has often the largest contribution to the population growth rate compared to other demographic rates, and inter-annual variation in adult survival is often limited especially in long-lived species (Gaillard et al. 2000). Importantly, seasonal variation in environmental factors can cause variation in adult mortality during the annual cycle. For example, the season of highest mortality is summer for caribou (Rangifer tarandus caribou, Seip 1992), autumn for mule deer (Odocoileus hemionus, Schuyler et al. 2019) and winter for white-tailed deer (Odocoileus virginianus, Kautz et al. 2020) or mule deer (Odocoileus hemionus, Jackson et al. 2021). Identifying the seasons of lowest adult survival increases understanding of the causes of mortality and the impacts of environmental factors on survival (Giavi et al. 2014; Rockwell et al. 2017).
Ungulate species vary from resident to partially migrant and fully migratory species that move between reproductive and wintering areas (Kauffman et al. 2021). Movement and the seasonally varying environments underscore the need for research on survival in the separate phases of the annual cycle among ungulates (Bolger et al. 2008). Adult survival of ungulates is affected by a multitude of factors, but density via resource limitation, predators and their interactions is often among the main causes of mortality (Gaillard et al. 2000; Bolger et al. 2008). Their effects can vary seasonally, but seasons of highest mortality may vary across species and habitats (e.g. Seip 1992; Kautz et al. 2020). In the north, ungulate survival is often commonly lowest during the winter because demanding weather and snow conditions affect food availability and predation (Tveraa et al. 2003; Kautz et al. 2020; Schmelzer et al. 2020). However, due to migration, the presence of predators at various seasons of the annual cycle may vary and consequently create different seasonal variations in survival (e.g. Seip 1992).
The reindeer (Rangifer tarandus) are migratory ungulates with a circumpolar distribution in the northern hemisphere and different subspecies living in forest and tundra biomes (Gunn 2016). Many wild populations are currently decreasing, and thus, reindeer are categorised as vulnerable by the IUCN red list (Vors and Boyce 2009; Gunn 2016; see also Gunn and Russell 2022). The Eurasian subspecies, the wild forest reindeer (R. t. fennicus, Lönn. 1909, hereafter “WFR”) has declined in the last decades: in Karelia from app. 7500 to 2300 individuals between the 1980s and mid-2010s (Danilov et al. 2018) and in Kainuu from the app. 1700 to 750 individuals between 2001 and 2015 (Paasivaara et al. 2018). Because ungulates are long-lived species, reductions in adult survival can cause population declines as has been observed for North American caribou populations (Wittmer et al. 2005; Hervieux et al. 2013). At present, nothing is known about long-term changes or annual, seasonal and spatial variation in adult survival of female WFR. Furthermore, while WFR are known to be significant prey of wolves (Canis lupus, L. 1758) and other large carnivores in Finland (Heikura 1997; Kojola et al. 2004, 2009; Gurarie et al. 2011, 2022), there is little published data on mortality causes of adult WFR (e.g. Heikura 1997; Kojola et al. 2004, 2009). As wolf populations have recovered and expanded their distributions in the last three decades (Kojola and Määttä 2004 [in Finnish]; Kojola et al. 2006 [in English]; see also Chapron et al. 2014), predation by large carnivores can be the main cause of adult female WFR mortality, and hence, variation in survival and especially mortality caused by predation may be linked to wolf densities in the distribution of the WFR.
Here, we study the survival of adult WFR females in two distinct subpopulations living in Finland (Kainuu and Suomenselkä) using tracking data from 305 females that were marked at various times during 2009–022 and followed using GPS transmitters. We determine proximate causes of mortality (predation, traffic, accidents and unknown causes) and evaluate their relative importance as sources of mortality. We test for inter-annual variation in survival and cause-specific mortalities. During the study years 2009–2022, the subpopulation in Kainuu was stable or declined slightly whereas the subpopulation in Suomenselkä grew (Fig. 1a; Paasivaara et al. 2018; Luke 2022). Hence, we also test for temporal trends to assess the potential role of adult survival in these observed changes. We further test for seasonal variation in survival and cause-specific mortalities. The annual cycle of WFR is determined by movement between wintering, calving and rutting areas, and it can be divided into four main periods: wintering, calving, rutting and autumn migration (Pulliainen et al. 1986). On the basis of environmental variation (e.g. weather and snow conditions), we predict that survival is lowest during winter. We test whether survival and cause-specific mortalities differ between the subpopulations. During 2009–2022, wolf densities in the WFR distribution were approximately 3.4 times higher, and spatial overlap between the total distribution of WFR and wolf territories was stronger in Kainuu compared to Suomenselkä (Fig. 1b; Luke 2009–2022; Online Resource 1). Hence, we predict that survival is lower, and mortality caused by predation is higher in Kainuu compared to Suomenselkä.
a The number of WFR individuals of Kainuu and Suomenselkä subpopulations from 2009 to 2022 (Luke 2022). Population growth rates (λ) calculated from these year specific population sizes (Nt) (as Nt1/Nt = λ) indicate a slightly declining Kainuu population (mean across years, λ = 0.994) and an increasing Suomenselkä population (mean across years, λ = 1.049); b the density of wolves/km2 in WFR distributions of Kainuu and Suomenselkä subpopulations from 2009 to 2022 (Luke 2009–2022; about counting methods, see Kojola et al. 2014, 2018; Mäntyniemi et al. 2022; see also Online Resource 1); c the number of tagged individuals in the different years of the study in Kainuu and Suomenselkä. Note that the survival analyses were done using data from 2010 onwards
Material and methods
Study populations
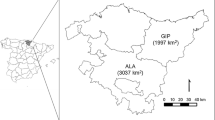
Our two study populations are situated in Central Finland in Suomenselkä and Kainuu which lie within the boreal vegetation zone (Fig. 2). This taiga forest zone is characterised by Norwegian spruce- (Picea abies) and Scots pine- (Pinus sylvestris) dominated coniferous forests (Ahti et al. 1968). These landscapes are relatively flat (Kainuu: 160–307 MSL; Suomenselkä 40–220 MSL) and frequently interspersed by mires and lakes. The prominent type of land use is forestry. Over 121–year-old forests comprised 11% and 6.7% of the total wood production area in Kainuu and Suomenselkä, respectively (Luke 2015). Areas in Suomenselkä include more fields and large mires (Eurola et al. 1984). An average permanent snow cover begins on 1 December and lasts until 25 April in Suomenselkä but is a month longer in Kainuu, where equivalent dates are 10 November and 5 May, respectively (FMI 2020; the dates are approximations, interpolated from the map). The mean snow depth on 15 March and an average precipitation during winter are lower in Suomenselkä than in Kainuu (40–60 cm, 110–120 mm; 60–80 cm, 120–140 mm, respectively; FMI 2020).
Migration occurs in early May between wintering grounds and calving sites, and vice versa after the rutting season from late October to December. This behaviour can be characterised as mixed or partial migration (Kauffman et al. 2021). Wintering from January until late April takes place in relatively small areas, on lakes or open mires, where animals are better protected by the herd’s vigilance (Pulliainen et al. 1983). The foraging sites need to provide the required amount of food with high nutritional value (caloric content), especially Cladina sp. lichens (i.e. “reindeer lichen”) that WFR can reach by digging through the snow. Wintering sites in Suomenselkä are located in Southern and Central Ostrobothnia and in the Kainuu region in Kuhmo, Sotkamo and Ristijärvi municipalities (Fig. 2).
The timing of spring migration varies between years and individuals but usually occurs at the turn of the month from April to May. Moreover, it happens in a shorter time frame compared to autumn migration (Pulliainen et al. 1986). Calving occurs briefly after settling in the calving range and even though there are no published data, the dropping of the calves happens approximately between 15 May and 15 June, based on accounts of field technicians working at the Natural Resource Institute Finland (e.g. Pulliainen et al. 1986). Nevertheless, females and calves spend time in these summer pasture areas until August. These areas are characterised by a mosaic of lakes, mires and forests that provide diverse nutrition for the calf and lactating female; and are spread across larger areas than winter grounds (Helle 1980, Fig. 2). The mean distances between wintering grounds and summer pastures were 96 km in Suomenselkä and 57 km in Kainuu, measured from the mean location of each individual in February and June (i.e. distance of spring migration) during 2010–2022 (Pöllänen et al. unpublished data). In Kainuu, dispersal northwards is restricted by the fenced reindeer management area. Rutting herds are formed during September and the rutting itself happens approximately between 20 September and 27 October (Pulliainen et al. 1986) This is followed by the long autumn migration period during which they move rather slowly towards the wintering areas.
Four large carnivores are known to hunt adult Rangifer (wolf: Kojola et al. 2009; brown bear, Ursus arctos, L. 1758: Åhman et al. 2022; lynx, Lynx lynx, L. 1758: Nybakk et al. 2002; wolverine, Gulo gulo, L. 1758: Landa et al. 1997). According to Heikkinen et al. (2022), the Finnish wolf population has shown strong fluctuation in the twenty-first century. Nevertheless, the number of wolves has steadily increased since 2017 and has doubled in two decades being 135–145 individuals in 2002 (Kojola and Määttä 2004) and 290 in March 2022 (Heikkinen et al. 2022, see also Mäntyniemi et al. 2022). Similar to the wolf, brown bears (from app. 1200 to 2670–2800 individuals between 2004 and 2020, Heikkinen et al. 2021), lynx (from app. 1100 to 2155–2280 individuals between 2004 and 2021, Holmala et al. 2020) and wolverines (from app. 100 to 390–400 individuals between 2005 and 2021, Kojola et al. 2021) have increased in Finland.
Data collection
Starting from January 2009, we marked female WFR and followed them with vectronic-type GPS that was mounted with collars. According to permit criteria of the Finnish National Animal Experiment Board (Decisions on the granting of the licence of team Ella, ESAVI: 6336/04, 587/04. and 23666/2018), we searched for mature females using a snowmobile or a helicopter and anaesthetised them before marking. Their age was determined on the basis of their teeth (i.e. by the structure and the tooth wear). All animals were adults between ages 2 and 14. The number of collars varied annually between 14 and 108 (mean = 70; Fig. 1c). Since 2009, we collared 198 females in Kainuu and 110 in Suomenselkä.
GPS collars updated their location every fourth hour. If the animal was stationary for at least four locating events, we considered it to be deceased after which we identified the cause of death. The cause of death was carefully examined at the location. Proximal causes of death were classified as predation; traffic; accidents; poaching; death at parturition, caused by complications; legal hunting in Suomenselkä; and disease. When there was not enough evidence to determine the cause of death, it was classified as unknown. These cases included carcasses that were consumed to the extent by different predators or scavengers that the cause of death could not be recognised and cases where collars were found in Russia without the expertise of a researcher to identify the cause of death. The cause of death was determined by professionals that understand predator behaviour and are skilled at reading predator tracks and signs. Predation was identified from the secondary presence of carnivores by using multiple sources of information. This was done using GPS information, signs of struggle at the death site, tracks of carnivores and their faeces, and with the awareness that the scavengers can make the identification less unambiguous. Dogs were often used as help. The species of a predator was identified based on bite marks on the carcass and associated signs at the kill site, such as snow tracks, scats or other leftovers (Norberg et al. 2010) to one of the four large carnivore species existing in WFR distribution: the brown bear, the Eurasian lynx, the wolverine and the grey wolf. The predator identification can be considered very reliable due to the short period of time between the killing occasion and checking the carcass (in Kainuu usually within 2 days, in Suomenselkä within 5 days) when no other large carnivore than one responsible could have left distracting signs. In addition, given that intraguild interactions between large predators (e.g. predation avoidance of wolverine from wolves: Ballard et al. 2003; van Dijk et al. 2008; wolves’ avoidance of brown bear kills: Ordiz et al. 2020) reduce confusion in the killing site, majority of causes of death by a predator can be identified to species level. However, in the analysis of predation mortality, we jointly analyse mortality from all predators. Importantly, when the cause of death by other causes could not be ruled out, mortality was included in the class of unknown causes. We considered the collar missing if it did not send the location and the animal was nowhere to be found.
Data analysis
We analysed the data using “known-fate” models (Cooch and White 2021). Known-fate models are based on the “Kaplan–Meier” method (Kaplan and Meier 1958) and originally created for a mark-recapture data. Survival can be estimated for the observation period (e.g. a month) in question when the fates of all collared individuals are known in that period. Continuity and assurance of the animal’s fate in data collection allows to assume an encounter probability of 1.
We used the program MARK (White and Burnham 1999) to fit known-fate models to our data. We coded the data into a binary code. The whole encounter history of an individual contained monthly periods where (1) “10” represented a collaring month and months when the animal survived, (2) “11” meant that the animal died in that month and (3) “00” represented a month when either the animal was not collared yet or the animal had disappeared due to low charge of the battery or breaking of the collar. In the latter, the encounter history ended a month before with “10”. We estimated mortality rates from survival estimates as 1—survival.
We ran five different analyses. In the first analysis, we analysed survival and included all causes of mortality. In the remaining four analyses, we analysed cause-specific mortality rates and focussed on the main causes of death, e.g. predation, traffic, unknown causes and accidents. In these analyses, we censored the month of death from the encounter history when an animal died from other mortality causes than the mortality cause in question. Unknown reasons and accidents were included in analyses due to their considerable contribution to total mortality. The total encounter history ranged from January 2010 till April 2022 and comprised 305 female individuals (195 in Kainuu, 110 in Suomenselkä).
Model fitting should be based on a priori considerations of the biological processes and structure in question (Burnham and Anderson 1998; Johnson and Omland 2004). Therefore, we fitted a priori models in each analysis which aimed to test for temporal variation in survival/mortality by comparing the following models: (1) the “Constant” model that included only the intercept and did not constrain any variation, (2) “Year” annual variation, (3) “Trend” linear change in survival/mortality during the study years and (4) “Season” described temporal variation between four periods of WFR female’s annual life cycle, formed according to the location data as follows: wintering (Jan–Apr), calving (May–Aug), rutting (Sep–Oct) and autumn migration (Nov–Dec). Here, the wintering season also included spring migration because we were unable to separate it from the winter months due to its rapid occurrence. We also included “Population” (Kainuu 1; Suomenselkä 0) as a factor to test for population differences in survival/mortality. Furthermore, we tested for an interaction between temporal variation (year, trend, season) and “Population” to test for differences in temporal variation between the two reindeer subpopulations.
In the model selection, we used the information theoretic approach by Burnham and Anderson (2002), which is an integrated model selection procedure in the program MARK. The Akaike’s information criterion (AIC) with the correction of small sample size (AICc) was used to evaluate the relative support for different models (Burnham and Anderson 2002). The selection of the best approximating model(s) was based on the ΔAICc values calculated as the differences between the current and minimum observed values of AIC (i.e. AIC, minimum AIC). Models with ΔAICc > 2 were considered not to fit the data (Burnham and Anderson 2002). We also calculated Akaike weights (wi) for all models; models with larger wi better approximate the data (see Burnham and Anderson 2002). Due to the model selection uncertainty, we calculated the model-averaged estimates of survival following Burnham and Anderson (2002) by including models within ΔAICc < 2. We also examined coefficients of factors in the most supported models and their confidence to assess their reliability as predictors.
Results
During the study, 107 females were found dead. The majority were killed by predators (55%). Most other mortalities were caused by traffic and accidents, while poaching, death at parturition, hunting and disease were less common (Table 1). Accidents included two cases of complications after collaring the animal; three WFR falling through the ice; four animals were killed by blunt force trauma (cause of death determined with autopsy at Finnish Food Authority); a few incidents where WFR were killed by a domestic dog, tangling to the fence and choking to death due to panic reaction. For one animal, the cause of death was categorised as a disease due to permanent illness or malnutrition from osteoarthritis, severe tooth wear and multiple bacterial infections (e.g. Echinococcus canadensis). The cause of death could not be determined in 13 cases. Most females were killed by wolves (58%) whereas other deaths were divided rather evenly between wolverine, brown bear and Eurasian lynx. In six cases, the predator could not be determined. Recorded deaths of females varied across the months occurring least often during June and July of the calving season (Fig. 3).
When modelling inter-annual survival, two models were within 2 AICc units. The first model included an interaction between population and season (comparison to model population + season ΔAICc = 6.30 in Table 2) with the model coefficients indicating a difference in winter survival between Kainuu and Suomenselkä (Table 3). Survival was higher in Suomenselkä during the winter (0.996, 95% CI 0.990–0.999) than in Kainuu (0.981, 95% CI 0.973–0.986) while the opposite occurred for survival in the autumn migration period (Fig. 4a). The second model included an interaction between population and trend (comparison to model population + trend ΔAICc = 4.80 in Table 2) and predicted declining inter-annual survival in Kainuu and increasing inter-annual survival in Suomenselkä (Fig. 5). Inter-annual survival was on average 0.844 (95% CI 0.808–0.874) in Kainuu and 0.896 (95% CI 0.854–0.927) in Suomenselkä. Temporal inter-annual variation (year model) was not supported (Table 2).
a Seasonal monthly survival (± 95% CI) of female wild forest reindeer separately for subpopulations in Kainuu and Suomenselkä when all mortality causes are considered. b Annual mortality (with 95% CI) of female wild forest reindeer caused by predation, traffic, unknown causes and accidents separately in Kainuu and Suomenselkä. c Seasonal monthly mortality from predation (± 95% CI) of female wild forest reindeer separately for subpopulations in Kainuu and Suomenselkä
Trends in inter-annual adult survival in Kainuu and Suomenselkä as predicted from model population*trend in Table 2
Mortality from predation was higher in Kainuu, annual mortality from predation was twice as high in Kainuu (0.102, 95 CI 0.078–0.134) compared to Suomenselkä (0.048, 95% CI 0.028–0.081; model population vs. model constant ΔAICc = 5.14 in Table 2; Fig. 4b). Importantly, the best model for predation included a clear interaction between population and season (ΔAICc = 4.3 in Table 2; Fig. 4c). In Kainuu, monthly mortality from predation was clearly highest during the winter (Jan–Apr) and the rut months (Sep–Oct) whereas it was lower during the autumn migration (Nov–Dec) and calving periods (May–Aug; Fig. 4c). In Suomenselkä, predation was lowest in the winter and highest in the autumn migration period (Fig. 4c). When translated to mortality for the whole periods, the mortalities were 5.5% and 0.7% during winter, 2.3% and 1.5% during calving, 2.0% and 0.8% during rut and 0.7% and 1.9% during autumn migration periods, respectively for Kainuu and Suomenselkä. Temporal annual variation and trends in predation mortality were not supported (Table 1).
When analysing mortality from traffic, the constant model was the best model and none of the variables received support (Table 2). Model averaged estimates of annual mortality from traffic were 0.016 (CI 0.006–0.026) for Kainuu and 0.016 (CI 0.005–0.027) for Suomenselkä. When analysing mortality from unknown causes, the best model included an interaction between population and season, but this model was not better than the constant model that was only 0.7 AICc units away suggesting none of the variables received support (Table 2). Model averaged estimates of annual mortality from unknown causes were 0.016 (CI 0.005–0.027) for Kainuu and 0.019 (CI 0.005–0.033) for Suomenselkä. When analysing mortality from accidents, the most supported model included an interaction between population and trend (Tables 2 and 3). The model predicted that accident mortalities have increased in Kainuu and decreased in Suomenselkä. Seasonal variation was not clear as the model without season was within 2 AICc units. Model averaged annual accident mortality was 0.026 (CI 0.010–0.041) in Kainuu and 0.011 (CI 0.000–0.026) in Suomenselkä. Annual predation mortality rates were 4.0–6.4 and 2.5–4.3 times higher than mortality due to traffic, unknown causes and accidents in Kainuu and Suomenselkä, respectively (Fig. 4b).
Discussion
We found no strong inter-annual variation in the survival of a reindeer living in the taiga forests of Finland. While our results follow theoretical expectations for long-lived herbivores which predict that adult survival of ungulates is canalised against temporal variation (Gaillard et al. 1998, 2000), they contrast findings from Arctic reindeer populations that may suffer severe mortality in certain years with extreme conditions (e.g. rain-on-snow) or high densities (Kohler and Aanes 2004; Tyler 2010; Hansen et al. 2019). We note, however, that our ability to find annual variation was reduced in the first years of our study due to a lower amount of data (see Fig. 1c). Nevertheless, a lack of strong annual variation has been described also for other boreal Rangifer populations (e.g. Schmelzer et al. 2020). Limited support for annual variation in survival suggests that boreal forests are, as an ecosystem, more stable than Arctic environments, and extreme conditions that create annual variation survival are rare. Boreal habitats in Finland also provide alternative food resources such as arboreal lichens when ground-lying lichen becomes inaccessible due to ice, and an opportunity for reindeer to change foraging sites when food availability is low (Heikura 1998).
We found population-specific seasonal variation in survival. In Kainuu, survival was lowest during the winter and highest during the calving season whereas in Suomenselkä survival was lowest during autumn migration and highest during the winter. Seasonal variation in survival was mostly caused by predation, which was the main cause of mortality being 3–6 times larger than any of the other mortality causes (traffic, accident and unknown causes) that did not show clear seasonal variation. In Kainuu, predation mortality was highest during the winter (the season of lowest survival) during which mortality from predation was 1.4–3.7 times higher than in other seasons. In Suomenselkä predation mortality was 2.3–5.2 higher in the autumn migration season (the season of lowest survival) compared to other seasons. These seasonal predation mortality patterns contradict the general pattern described among North American woodland caribou in which predation rates of adults are generally lowest in the winter and highest in the summer (Seip 1992; Wittmer et al. 2005) suggesting that seasonal predation is strongly dependent on local conditions. Further research is needed to confirm the mechanisms affecting the seasonal predation of WFR populations in Finland. The lower predation rate of adults during the calving season may, in part, reflects the availability of more easily accessible food sources for predators. For example, WFR calves suffer high predation mortality during the first months after parturition (Kojola et al. 2009).
Mortality caused by predation was two times higher in Kainuu compared to Suomenselkä. This is in line with the higher wolf density in Kainuu compared to Suomenselkä (Fig. 1b). This difference translated to differences in inter-annual adult survival rates which were lower in Kainuu (0.84) than in Suomenselkä (0.90). These survival rates are comparable to those reported for woodland caribou inhabiting similar forest conditions in Alberta, Canada (0.85; Hervieux et al. 2013) and three populations in Quebec, Canada (0.78–0.89; Schmelzer et al. 2020). This rate of annual survival is insufficient in keeping these caribou populations stable (Hervieux et al. 2013; Schmelzer et al. 2020), whereas the increasing “Fortymile” caribou herd in Alaska had higher survival (0.91; Boertje et al. 2017). Out of our study populations, the Suomenselkä subpopulation grew during the study whereas the subpopulation in Kainuu was stable or slowly declining (Paasivaara et al. 2018; Fig. 1a). As these changes in population size are in line with the trends observed in inter-annual survival, and because adult survival has a strong contribution to the population growth rate in long-lived species (Gaillard and Yoccoz 2003), it is possible that the past population trends reflect differences and changes in adult survival.
To conclude, we show that (1) adult survival of females differed in two northern forest–dwelling migratory WFR populations being lower in Kainuu than in Suomenselkä and that (2) these populations showed opposite temporal trends in survival and (3) clearly different seasonal variations, but (4) there was no strong inter-annual variation. Importantly, we further show that (5) the observed population and seasonal variation in inter-annual survival mirrored predation which was the main proximate mortality cause for adult females. (6) Predation-caused mortality varied between subpopulations and (7) seasons being highest during the winter in Kainuu and during the autumn migration in Suomenselkä. Our results warrant further studies on potential mechanisms affecting the survival and mortality of the Kainuu population, including climatic conditions and habitat features such as habitat fragmentation and forestry that may affect across the winter, a particularly demanding seasons for WFR and other Rangifer.
References
Åhman B, Rasmus S, Risvoll C, Eilertsen SM, Norberg H (2022) Large predators and their impact on reindeer husbandry. In: Horstkotte T, Holand Ø, Kumpula J, Moen J (ed) Reindeer Husbandry and Global Environmental Change 118–130. Routledge
Ahti T, Hämet-Ahti L, Jalas J (1968) Vegetation zones and their sections in northwestern Europe. Ann Bot Fenn 5:169–211. https://www.jstor.org/stable/23724233. Accessed 23 Apr 2023
Ballard WB, Carbyn LN, Smith DW (2003) Wolf interactions with non-prey. In: Mech D, Boitani L (ed) Wolves: behaviour, ecology and conservation, The University of Chicago Press, Chicago. 259–271. URL: https://digitalcommons.unl.edu/usgsnpwrc/325/. Accessed 23 Apr 2023
Boertje RD, Gardner CL, Ellis MM, Bentzen TW, Gross JA (2017) Demography of an increasing caribou herd with restricted wolf control. J Wildlife Manage 81:429–448. https://doi.org/10.1002/jwmg.21209
Bolger DT, Newmark WD, Morrison TA, Doak DF (2008) The need for integrative approaches to understand and conserve migratory ungulates. Ecol Lett 11:63–77. https://doi.org/10.1111/j.1461-0248.2007.01109.x
Burnham KP, Anderson DR (1998) Practical use of the information-theoretic approach. Model selection and inference. Springer, New York, pp 75–117
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Chapron G, Kaczensky P, Linnell JD, von Arx M, Huber D, Andrén H et al (2014) Recovery of large carnivores in Europe’s modern human-dominated landscapes. Science 346:1517–1519. https://doi.org/10.1126/science.1257553
Cooch EG, White GC (2021) Known-fate models. In: Cooch EG (ed) Program MARK – A Gentle Introduction, 19th edn. Chapter 16
Danilov PI, Panchenko DV, Tirronen KF, Fyodorov FV, Belkin VV (2018) Changes in mammal fauna of the northern palearctic and living range dynamics of the component species. Biol Bull. https://doi.org/10.1134/S1062359018030032
Eurola S, Hicks S, Kaakinen E (1984) Key to Finnish mire types. In: Moore PD (ed) European mires, Academic press, pp 11–117
FMI (2020) Snow statistics. Finnish Meteorological Institute. https://en.ilmatieteenlaitos.fi/snow-statistics. Accessed 17 Jan 2022
Gaillard J, Yoccoz NG (2003) Temporal variation in survival of mammals: a case of environmental canalization? Ecology 84:3294–3306. https://doi.org/10.1890/02-0409
Gaillard JM, Festa-Bianchet M, Yoccoz NG (1998) Population dynamics of large herbivores: variable recruitment with constant adult survival. Trends Ecol Evol 13:58–63. https://doi.org/10.1016/S0169-5347(97)01237-8
Gaillard JM, Festa-Bianchet M, Yoccoz NG, Loison A, Toigo C (2000) Temporal variation in fitness components and population dynamics of large herbivores. Annu Rev Ecol Syst 31:367–393. https://doi.org/10.1146/annurev.ecolsys.31.1.367
Giavi S, Moretti M, Bontadina F, Zambelli N, Schaub M (2014) Seasonal survival probabilities suggest low migration mortality in migrating bats. PLoS ONE 9:e85628. https://doi.org/10.1371/journal.pone.0085628
Gunn A (2016) Rangifer tarandus. The IUCN Red List of Threatened Species 2016: e.T29742A22167140. https://doi.org/10.2305/IUCN.UK.2016-1.RLTS.T29742A22167140.en
Gunn A, Russell D (2022) Update on the global status of wild reindeer and caribou. IUCN SSC Deer Specialist Group. URL: https://www.deerspecialistgroup.org/article/1060/. Accessed 23 Apr 2023
Gurarie E, Bracis C, Brilliantova A, Kojola I, Suutarinen J, Ovaskainen O et al (2022) Spatial memory drives foraging strategies of wolves, but in highly individual ways. Front Ecol Environ. https://doi.org/10.3389/fevo.2022.768478
Gurarie E, Suutarinen J, Kojola I, Ovaskainen O (2011) Summer movements, predation and habitat use of wolves in human modified boreal forests. Oecologia 165:891–903. https://doi.org/10.1007/s00442-010-1883-y
Hansen BB, Pedersen ÅØ, Peeters B, Le Moullec M, Albon SD, Herfindal I et al (2019) Spatial heterogeneity in climate change effects decouples the long-term dynamics of wild reindeer populations in the high Arctic. Global Change Biol 25:3656–3668. https://doi.org/10.1111/gcb.14761
Heikkinen S, Kojola I, Mäntyniemi S (2021) Karhukanta Suomessa 2020 [in English: the brown bear population in Finland in 2020]. Luonnonvara- ja biotalouden tutkimus 20/2021. Luonnonvarakeskus, Helsinki: 13. In Finnish. URN: http://urn.fi/URN:ISBN:978-952-380-177-6. Accessed 23 Apr 2023
Heikkinen S, Valtonen M, Härkälä A, Johansson H, Harmoinen J, Helle I, Mäntyniemi S, Kojola I (2022) Susikanta Suomessa maaliskuussa 2022 [in English: Finland’s wolf population on March 2022]. Luonnonvara- ja biotalouden tutkimus 59/2022, Luonnonvarakeskus, Helsinki: 139. In Finnish. URN: http://urn.fi/URN:ISBN:978-952-380-470-8. Accessed 23 Apr 2023
Heikura K (1997) Some aspects on the recent changes in the Kuhmo-Lake Kiitehenjärvi subpopulation of the wild forest reindeer (Rangifer tarandus fennicus, Lönnb.). In: Lindholm T, Heikkilä R, Heikkilä M (ed) Ecosystems, fauna and flora of the Finnish-Russian Nature Reserve Friendship, The Finnish Environment 124:203–221
Heikura K (1998) The lichen resources, their use, and the wintering grounds of the wild forest reindeer (Rangifer tarandus fennicus, Lönnb.) in the Kuhmo-Kamennojeozero subpopulation. In: Danilov PI (ed) Dynamics of game animal populations in northern Europe, Russian Academy on Science, Karelian Research Center, Petrozavodsk, Russia, 2nd Int. Symp 27–32
Helle T (1980) Sex segregation during calving and summer period in wild forest reindeer (Rangifer tarandus fennicus Lönn.) in Eastern Finland with special reference to habitat requirements and dietary preferences. Proceedings of the 2nd Reindeer/Caribou Symposium. Direktoratet for vilt og ferskvannfisk: 508–518
Hervieux D, Hebblewhite M, DeCesare NJ, Russell M, Smith K, Robertson S, Boutin S (2013) Widespread declines in woodland caribou (Rangifer tarandus caribou) continue in Alberta. Can J Zool 91:872–882. https://doi.org/10.1139/cjz-2013-0123
Holmala K, Valtonen M, Herrero A (2020) Ilveskanta Suomessa 2021 [in English: the Eurasian lynx population in Finland in 2021]. Luonnonvara- ja biotalouden tutkimus 70/2021. Luonnonvarakeskus, Helsinki: 29. In Finnish. URN: http://urn.fi/URN:ISBN:978-952-380-285-8. Accessed 23 Apr 2023
Jackson NJ, Stewart KM, Wisdom MJ, Clark DA, Rowland MM (2021) Demographic performance of a large herbivore: effects of winter nutrition and weather. Ecosphere 12(1):e03328. https://doi.org/10.1002/ecs2.3328
Johnson JB, Omland KS (2004) Model selection in ecology and evolution. Trends Ecol Evol 19:101–108. https://doi.org/10.1016/j.tree.2003.10.013
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481. https://doi.org/10.1080/01621459.1958.10501452
Kauffman MJ, Aikens EO, Esmaeili S, Kaczensky P, Middleton A, Monteith KL, Morrison TA, Mueller T, Sawyer H, Goheen JR (2021) Causes, consequences, and conservation of ungulate migration. Annu Rev Ecol Evol S 52:453–478. https://doi.org/10.1146/annurev-ecolsys-012021-011516
Kautz TM, Belant JL, Beyer DE Jr, Strickland BK, Duquette JF (2020) Influence of body mass and environmental conditions on winter mortality risk of a northern ungulate: evidence for a late-winter survival bottleneck. Ecol Evol 10:1666–1677. https://doi.org/10.1002/ece3.6026
Kohler J, Aanes R (2004) Effect of winter snow and ground-icing on a Svalbard reindeer population: results of a simple snowpack model. Arct Antarct Alp Res 36:333–341. https://doi.org/10.1657/1523-0430(2004)036[0333:EOWSAG]2.0.CO;2
Kojola I, Aspi J, Hakala A, Heikkinen S, Ilmoni C, Ronkainen S (2006) Dispersal in an expanding wolf population in Finland. J Mammal 87:281–286. https://doi.org/10.1644/05-MAMM-A-061R2.1
Kojola I, Heikkinen S, Holmala K (2018) Balancing costs and confidence: volunteer-provided point observations, GPS telemetry and genetic monitoring of Finland’s wolves. Mammal Res 63:415–423. https://doi.org/10.1007/s13364-018-0371-3
Kojola I, Heikkinen S, Mäntyniemi S, Ollila T (2021) Ahmakanta Suomessa 2021 [in English: the wolverine population in Finland in 2021]. Luonnonvara- ja biotalouden tutkimus 88/2021. Luonnonvarakeskus. Helsinki: 11. In Finnish. URN: http://urn.fi/URN:ISBN:978-952-380-329-9. Accessed 23 Apr 2023
Kojola I, Helle P, Heikkinen S, Linden H, Paasivaara A, Wikman M (2014) Tracks in snow and population size estimation: the wolf in Finland. Wildl Biol 20:279–284. https://doi.org/10.2981/wlb.00042
Kojola I, Huitu O, Toppinen K, Heikura K, Heikkinen S, Ronkainen S (2004) Predation on European wild forest reindeer (Rangifer tarandus) by wolves (Canis lupus) in Finland. J Zool 263:229–235. https://doi.org/10.1017/S0952836904005084
Kojola I, Määttä E (2004) Suurpetojen lukumäärä ja lisääntyminen vuonna 2003 [in English: [Large carnivores in Finland in 2003]. Riistantutkimuksen Tiedote, 194:1–7. In Finnish
Kojola I, Tuomivaara J, Heikkinen S, Heikura K, Kilpeläinen K, Keränen J, Paasivaara A, Ruusila V (2009) European wild forest reindeer and wolves: endangered prey and predators. Ann Zool Fenn 46:416–422. https://doi.org/10.5735/086.046.0602
Landa A, Strand O, Swenson JE, Skogland T (1997) Wolverines and their prey in southern Norway. Can J Zool 75:1292–1299. https://doi.org/10.1139/z97-153
Luke (2009–2022) Reports of wolf population in Finland (in Finnish). Jukuri, open repository of the Natural Resources Institute Finland (Luke). jukuri.luke.fi. Accessed 23 Apr 2023
Luke (2015) MELA summary reports - NFI11. Finnish Natural Resource Institute. http://mela2.metla.fi/mela/tupa/index.php. Accessed 13 Jan 2022
Luke (2022) Metsäpeurojen määrä Suomenselällä yhä kasvussa (in English: the number of wild forest reindeer still increases in Suomenselkä). Published 4 March 2022. https://www.luke.fi/fi/uutiset/metsapeurojen-maara-suomenselalla-yha-kasvussa. Accessed 23 Apr 2023
Mäntyniemi S, Helle I, Kojola I (2022) Assessment of the residential Finnish wolf population combines DNA captures, citizen observations and mortality data using a Bayesian state-space model. Eur J Wildlife Res 68:1–16. https://doi.org/10.1007/s10344-022-01615-5
Norberg H, Kojola I, Härkönen S (2010) Petovahinkojen tunnistamisopas [in English: identification guide to Finnish predators] Metsästäjäin Keskusjärjestö, Keski-Suomen Painotuote Oy, Äänekoski. In Finnish. https://riista.fi/wp-content/uploads/2013/03/Petovahinko-opas_fi.pdf. Accessed 23 Apr 2023
Nybakk K, Kjelvik O, Kvam T, Overskaug K, Sunde P (2002) Mortality of semi-domestic reindeer Rangifer tarandus in central Norway. Wildlife Biol 8:63–68. https://doi.org/10.2981/wlb.2002.009
Ordiz A, Milleret C, Uzal A, Zimmermann B, Wabakken P, Wikenros C, Kindberg J et al (2020) Individual variation in predatory behavior, scavenging and seasonal prey availability as potential drivers of coexistence between wolves and bears. Diversity 12:356. https://doi.org/10.3390/d12090356
Paasivaara A, Gavrilov M, Juntunen A, Kokko S, Korhonen L, Ovaskainen R, Timonen P (2018) Suomen metsäpeurakanta 2017 [in English: the wild forest reindeer population in 2017]. In: Helle P (ed) Riistakannat 2017, Luonnonvarakeskus, Luonnonvara- ja biotalouden tutkimus, 15/2018, pp 18–23. In Finnish. URN: http://urn.fi/URN:ISBN:978-952-326-554-7. Accessed 23 Apr 2023
Pulliainen E, Danilov PI, Heikura K, Erkinaro E, Sulkava S, Lindgren E (1986) The familiar area hypothesis and movement patterns of wild forest reindeer in Karelia, Northern Europe. Rangifer. https://doi.org/10.7557/2.6.2.652
Pulliainen E, Sulkava S, Erkinaro E, Heikura K, Lindgren E (1983) Seasonal movements of the wild forest reindeer (Rangifer tarandus fennicus) in eastern Finland. Acta Zool Fenn 175:15–16
Rockwell SM, Wunderle JM, Sillett TS, Bocetti CI, Ewert DN, Currie D, White JD, Marra PP (2017) Seasonal survival estimation for a long-distance migratory bird and the influence of winter precipitation. Oecologia 183:715–726. https://doi.org/10.1007/s00442-016-3788-x
Schmelzer I, Lewis KP, Jacobs JD, McCarthy SC (2020) Boreal caribou survival in a warming climate, Labrador, Canada 1996–2014. Glob Ecol Conserv 23:e01038. https://doi.org/10.1016/j.gecco.2020.e01038
Schuyler EM, Dugger KM, Jackson DH (2019) Effects of distribution, behavior, and climate on mule deer survival. J Wildlife Manage 83:89–99. https://doi.org/10.1002/jwmg.21558
Seip DR (1992) Factors limiting woodland caribou populations and their interrelationships with wolves and moose in southeastern British Columbia. Can J Zool 70:1494–1503. https://doi.org/10.1139/z92-206
Tveraa T, Fauchald P, Henaug C, Yoccoz NG (2003) An examination of a compensatory relationship between food limitation and predation in semi-domestic reindeer. Oecologia 137:370–376. https://doi.org/10.1007/s00442-003-1373-6
Tyler NJ (2010) Climate, snow, ice, crashes, and declines in populations of reindeer and caribou (Rangifer tarandus L.). Ecol Monogr 80:197–219. https://doi.org/10.1890/09-1070.1
van Dijk J, Andersen T, May R, Andersen R, Andersen R, Landa A (2008) Foraging strategies of wolverines within a predator guild. Can J Zool 86:966–975. https://doi.org/10.1139/Z08-073
Vors LS, Boyce MS (2009) Global declines of caribou and reindeer. Global Change Biol 15:2626–2633. https://doi.org/10.1111/j.1365-2486.2009.01974.x
White GC, Burnham KP (1999) Program MARK: survival estimation from populations of marked animals. Bird Study 46:120–139. https://doi.org/10.1080/00063659909477239
Wittmer HU, McLellan BN, Seip DR, Young JA, Kinley TA, Watts GS, Hamilton D (2005) Population dynamics of the endangered mountain ecotype of woodland caribou (Rangifer tarandus caribou) in British Columbia, Canada. Can J Zool 83:407–418. https://doi.org/10.1139/z05-034
Acknowledgements
We thank The Finnish Cultural Foundation—Kainuu Regional Fund for supporting this study (to ATP), the Natural Resource Institute Finland (Luke), the Ministry of Agriculture and Forestry of Finland and the WildForestReindeerLIFE project (LIFE15 NAT/FI/000881) for enabling effective data collection. We want to thank Arto Juntunen (deceased), Marja Hyvärinen, Petri Timonen, Sanna Kokko-Timonen, Markku Gavrilov, Leo Korhonen, Reima Ovaskainen, Esa Leinonen, Markus Suominen (deceased), Pekka and Kauko Kilpeläinen. Without their exceptional professional expertise in the field, this research could not have been possible. We thank Samuli Heikkinen for kindly providing us with the digital wolf territory data for Online Resource 1. We also thank the Finnish Forest and Park Services, Finnish Wildlife Agency and local hunter associations in Kainuu and Suomenselkä for successful co-operation. We thank Robert Serrouya, Torkild Tveraa, Anssi Vainikka and three anonymous referees for valuable comments.
Funding
Open Access funding provided by University of Oulu including Oulu University Hospital. This study was funded by The Finnish Cultural Foundation—Kainuu Regional Fund, WildForestReindeerLife project of European Union and partially by The Finnish Natural Resources Institute.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection and analysis were performed by Antti Pöllänen, Veli-Matti Pakanen and Antti Paasivaara. The first draft of the manuscript was written by Antti Pöllänen, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Data were collected by field staff of the Natural Resources Institute. Unnecessary pain and stress to the study animals are prevented by careful planning of capturing, handling and sampling of the animals. Administration of anaesthetic, marking and handling of wild animals is done under permission from the Finnish National Animal Experient Board (Decisions on the granting of the licence of team Ella, ESAVI: 6336/04.10.03/2012, 587/04.10.07/2016 and 23666/2018)). The study is conducted by using respectfully Responsible Conduct of Research (RCR) by the Finnish National Board of Research Integrity. According to RCR, the study will carefully, honestly and with detail exercise recording, presenting and detecting results and also act openly in presenting methodology.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pöllänen, A.T., Pakanen, VM. & Paasivaara, A. Survival and cause-specific mortality in adult females of a northern migratory ungulate. Eur J Wildl Res 69, 60 (2023). https://doi.org/10.1007/s10344-023-01686-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-023-01686-y