Abstract
Long-term studies of community composition and relative abundance are key tools in wildlife management and biodiversity conservation. However, few studies of this kind are available for Mediterranean carnivores, especially in the Iberian Peninsula, a hotspot of mammal biodiversity in Europe. We used 15 years of carnivore monitoring data from the Doñana National Park, one of the most representative areas for carnivores in Iberia, to obtain population trends for the main Mediterranean carnivore species. They were positive for red fox, stable for badger and Egyptian mongoose, and negative for common genet and Iberian lynx. The importance of long-term datasets and the implications of the results for the studied species at global level are discussed, above all for species whose population trends are less well known. This is the case of the Egyptian mongoose, for which we present novel information on its long-term population trend in Europe, and of the Iberian lynx, an endangered species with a clear negative trend in this well-protected area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Long-term studies of trends are an essential tool in conservation biology as they help to understand ecological processes such as predator–prey relationships (Karanth et al. 2004), detect threats to species and/or ecosystems (Morrison et al. 2018), unravel disease dynamics (Barroso et al. 2020), and assess the impact of management actions on populations (Carro et al. 2019). Therefore, trustworthy long-term research into trends is crucial in ecology and conservation studies (Sinclair et al. 2007; Lindenmayer et al. 2012; Kuebbing et al. 2018; Reinke et al. 2019). However, several inherent difficulties exist—above all, their continuity over time—that makes it rare to find datasets covering more than a decade (Magurran et al. 2010; Schradin and Hayes 2017).
In the case of carnivores, long-term studies have contributed notably, for example, to improving knowledge of social cooperation and behavior and their ecological functions (Schradin and Hayes 2017). Moreover, studies of this type are particularly important for carnivores, given that their elusive habits make them harder to detectable. It is a challenge to derive results on species’ conservation status from isolated or short-term studies (Smith et al. 2017), so datasets covering a decade or more are essential for filling gaps in our knowledge of the way carnivore populations react to conservation management and changes in the environment (Durant et al. 2007; Smith et al. 2017). Additionally, the social perception of carnivores is often negative because of traditional human-carnivore conflicts (Treves and Karanth 2003), which means that reliable data on carnivore relative abundances over the years can help understand and resolve conflicts (Treves et al. 2004; Delibes-Mateos et al. 2013). Therefore, it is important to establish and maintain well-planned long-term monitoring programs that will potentially answer key questions regarding the biology and conservation of mammalian carnivores.
Time-extensive relative abundance estimates of target species are needed to establish population trends. Noninvasive survey methods, including track counts (Smallwood and Fitzhugh 1995; Blaum et al. 2008; Winterbach et al. 2016), remote camera trapping (e.g., Fabiano et al. 2020), genetic sampling (e.g., Stansbury et al. 2014), and distance sampling (e.g., Durant et al. 2011), are the most frequently used methods when working with carnivores (Long et al. 2012). Comparisons have shown the high reliability of track counts whenever feasible (Balme et al. 2009), that is, where sandy or snowy substrates provide reliable estimates of carnivore abundance that can be used for monitoring population trends (Funston et al. 2010; O’Donoghe et al. 2022).
Few studies in Europe have ever estimated the population trends of carnivore communities or particular species as part of long-term research (e.g., Cazacu et al. 2014; Mueller et al. 2020; Cimatti et al. 2021). Such studies usually only focus on top predators, and long-term studies on Mediterranean carnivore communities, for example, are noticeably wanting. In the Iberian Peninsula, the situation is similar, and there are very few publications on trends in carnivore populations (Sobrino et al. 2009; Garrote et al. 2011; Torres and Fonseca 2016). The information that, as mentioned above, is so crucial for undertaking management actions and conservation policies and for understanding environmental and population changes is clearly lacking.
In Mediterranean Iberia, carnivore communities are represented above all by generalist mesocarnivores (Monterroso et al. 2015; Jiménez et al. 2017), given that local extinctions of top predators have occurred throughout much of this region, giving rise to increases in mesopredator populations in a process known as “mesopredator release” (Prugh et al. 2009; Jiménez et al. 2019). This is important because the popular idea that there has been a great increase in mesopredator abundance in recent years—despite the abovementioned lack of reliable information about mesocarnivores densities and trends—can lead to poorly effective management actions (Virgós and Travaini 2005; Curveira-Santos et al. 2019). Therefore, the study of relatively well-preserved carnivore communities can provide a crucial assessment for management actions aimed at improving conservation plans.
Therefore, the main purpose of this study was to provide reliable trends for Mediterranean carnivore communities using a low-cost and efficient method that has been demonstrated to be effective wherever it is feasible. We tested this methodology for five carnivore species: Iberian lynx (Lynx pardinus), Egyptian mongoose (Herpestes ichneumon), red fox (Vulpes vulpes), common genet (Genetta genetta), and European badger (Meles meles), and discuss possible causes and consequences of the observed trends, which, in the case of Herpestes ichneumon, are the first trends based on a long-term dataset ever obtained for this European carnivore.
Materials and methods
Study area
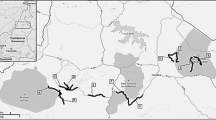
The study was carried out in Doñana National Park (DNP), SW Iberian Peninsula (37.100N, 6.230W; 54.252 ha), which enjoys a sub-humid Mediterranean climate in type with Atlantic influence. The area is characterized by marked annual variability in rainfall (García Barrón et al. 2011) (a yearly average of 580 mm) and hot and dry summers. As a result, the water balance is generally deficient except for 3–4 months of the year (Custodio et al. 2010). The main biotopes found in the national park are marshland, scrubland, and dunes (Valverde 1967; Rogers 1974), although the present study was undertaken mainly in scrubland and sand dune habitats, where carnivores are most abundant and can be detected all year by track counts.
DNP is exceptional in having no predator control plans except for some low-intensity actions carried out in certain areas. For example, as part of the Iberian Lynx Management Plan, foxes are captured to reduce their predation on rabbits and so lessen competition with the Iberian lynx. Captures are carried out by personnel from the DNP using a number of different methods. According to Palomares et al. (2011), fox control has had no effect on the abundance of this species, and these campaigns seem to have little or no impact on the DNP red fox population (Palomares et al. 2011). More importantly, small-game hunting is absent from the DNP, unlike 75% of Spanish territory (Arroyo et al. 2012). Few sites in the Iberian Peninsula—and even in Europe—have monitoring programs like those existing in DNP. The community structure of the main terrestrial carnivores in DNP has been studied in detail (Valverde 1967; Palomares et al. 2011; Soto-Navarro 2013), as have the population trends for carnivores like Iberian lynx (Garrote et al. 2011) and some of their prey species (Carro and Soriguer 2017; Carro et al. 2019). Likewise, mammal censuses are carried out annually by Doñana Biological Station Ecological Processes Monitoring Team (ESPN-ICTS-RBD-CSIC).
Dominance relationships between carnivore species have been studied in detail and show how intraguild predation by Lynx pardinus acts as an important driver of the distribution of some of the carnivore species, such as G. genetta and H. ichneumon, that populate this area.
Studied species
The studied species are almost all of the common carnivore species present in the area and include a top predator, Lynx pardinus (Temminck 1827), and four mesopredators, Vulpes vulpes (Linnaeus 1758), Meles meles (Linnaeus 1758), Herpestes ichneumon (Linnaeus 1758), and Genetta genetta (Linnaeus 1758). The remaining carnivore species in DNP, Lutra lutra (Linnaeus 1758), Mustela putorius (Linnaeus 1758), and Mustela nivalis (Linnaeus 1761), were not included because not enough data were available and/or the data-recording methods were not sufficiently reliable. Felis sylvestris (Schreber 1777) is also present but was not included due to the difficulties in distinguishing wildcat and feral cat tracks (Lozano and Urra 2014).
Carnivore track surveys
Track count censuses have been carried out discontinuously and annually in DNP since 1983. We selected the censuses from 2005 to 2019 because during this period, the monitoring program was consolidated and the current methodology began to be used. The censuses, carried out along the same 12 prefixed transects in DNP by ESPN-EBD-CSIC staff every year (Fig. 1), follow 2-km-long and 1.5-m-wide transects. Counts are performed from a 4 × 4-vehicle traveling at a constant speed (5–10 km/h) after the first autumn rains on three consecutive days to reduce the possible effect of non-biologic factors such as temperature or rain (Soto-Navarro 2013). Unfortunately, in recent years and due to climate change, the autumn rains may be delayed, and counts are made in the autumn–winter period. Transects are spaced over 1800 m apart to ensure that the whole area is well covered and to avoid detecting the same animals in different transects on the same night (Palomares et al. 2011). DNP is crossed by a large number of roads and firebreaks. Transects were chosen to cover the whole central sandy part of the DNP and not enter into the marshland, where animal tracks are not preserved. Habitats consist of scrub, grassland, and forest, all on a sandy substrate. The census taker records all carnivore tracks that cross the trail being followed and then disappear. Tracks that clearly belong to the same animal (i.e., tracks that follow the transect and then disappear) are counted only once. Tracks that run alongside the route until they disappear are recorded as belonging to a single individual. Tracks are cleared with a metal implement from the transect the day before the census, so that the tracks counted on the following day correspond to just one day of carnivore activity. Transects cover almost half the DNP, the rest of this protected area being covered by marshland that lies underwater during this part of the year and so is not suitable for all carnivore species.
Track identification was performed during transect counts by an expert, and data was recorded using Cybertracker (http://www.cybertracker.org/), an open-source software. For each track (a group of carnivore foot trails), the following information was recorded: waypoints (automatically generated GPS position), date, start, end, species identification, surveyors, driver, transect identity, and observations. All this information was automatically downloaded and transferred to a Microsoft Excel 2010 spreadsheet for further analysis.
KAIs (kilometric abundance indexes) were calculated for each species, transect, and year by estimating average track counts between same-year censuses and dividing by transect length (2 km). This index thus gives the relative abundance at a certain locality and time compared to the average for the study area (Crawford 1991; Conroy 1996; Engeman 2005).
Carnivore trends
TRIM software (trends and indices for monitoring data) (Pannekoek and van Strien 2005) was used to test long-term trends in the DNP carnivore community.
These models assume independent Poisson distributions for counts. Poisson distributions were checked for all samples. We used time-effects models and considered serial correlation and overdispersion. Goodness-of-fit was established by considering chi-square > 0.05 for imputed counts when overdispersion and serial correlation were, respectively, < 3 and < 0.4 following TRIM recommendations.
The overall slope for long-term trends was determined using multiplicative parameters. TRIM software classifies trends as one of six categories depending on whether the rate of change over the study period was more or less than 5% per year: a strong increase or decrease (> 5% per year); a moderate increase or decrease (< 5% per year); a stable trend (no significant increase or decline, and it is certain that trends are less than 5% per year); or, finally, an uncertain trend with large coefficients of interval (Pannekoek and van Strien 2005).
Results
KAIs were used to obtain carnivore population trends, which reveal great interspecific variation.
V. vulpes showed no fit for the time-effects model (χ2 = 296.10; p < 0.01), but the Wald-test (p = 0), overdispersion (2.014), and serial correlation (−0.086) parameters were adequate and gave a moderate positive trend (overall slope mode multiplicative (OSMM) = 1.042; sd = 0.009; p < 0.01) with an annual population increase of 4.2%. Several periods of population increase or decrease were detected by the data: there was population growth in 2005–2008 and again in 2010–2013, while 2008–2010 and 2014–2017 were periods of population decrease. The highest index of fox abundance corresponds to the 2018 count and the lowest to 2010, 2 years that were turning points in fox population trends (Table 1, Fig. 2).
The model for H. ichneumon showed good fit (χ2 = 112.19; p = 0.98), overdispersion (0.763), and serial correlation (−0.043), which implies a stable trend (OSMM = 1.014, sd = 0.017; p < 0.01) with a theoretical annual population increase of 1.4%. Noticeable peaks were registered in 2007, 2010, 2012 (maximum index of relative abundance), and 2017, with a constant population increase in 2014–2017. The minimum value for the relative abundance index was registered in 2014 (Table 1, Fig. 2).
The model for M. meles also had a good fit (χ2 = 124.02; p = 0.916; overdispersion = 0.844; serial correlation = 0.065) and, as in the case of the mongoose, gave a stable trend (OSMM = 1.014; sd = 0.016; p < 0.01), with a theoretical annual population increase of 1.4%. The maximum and minimum abundance indices correspond to the 2018 and 2013 counts, respectively; a period of constant population increase was registered in 2007–2012 (Table 1, Fig. 2).
G. genetta abundances did not show any clear trend and were established as Uncertain (OSMM = 0.957; sd = 0.024; p = 0.15) with good statistical support (χ2 = 71.70; p = 1; overdispersion = 0.539; serial correlation = 0.082). Theoretical population decrease would be 5.3% annually, attending to OSMM value. The highest index of relative abundance was detected in 2007, while the lowest value corresponds to 2017. The genet’s population dynamics were characterized by periods of greater relative abundance alternating with low population events in 2009, 2012, 2015, and 2017 (Table 1, Fig. 2).
Finally, the model for L. pardinus also had a good fit (χ2 = 84.88; p = 0.992; overdispersion = 0.713; serial correlation = −0.032) that shows a moderate decline (OSMM = 0.879; sd = 0.048; p < 0.05) with an annual decrease of 12.1%. The highest abundance index was reached in 2010, which was followed by a continuous decline from 2013 onwards that reached its minimum historical value in 2019 (Table 1, Fig. 2).
Discussion
We cannot share a common conclusion for all the studied species, so we will discuss every species separately to better understand each case.
The red fox (Vulpes vulpes) is the most widespread and generalist carnivore species in both the world (Macdonald and Reynolds 2004) and the Iberian Peninsula (Purroy and Varela 2003). It thus has an advantage over other carnivore species with narrower niches that are more susceptible to disturbance (Barrul et al. 2014; Curveira-Santos et al. 2019). The densities found in DNP are similar to other reported for Iberian ecosystems (1.4–1.7 indiv./km2) (Gortázar 1999; Barrul and Mate 2015). In this sense, it is not surprising that the red fox is the only species with a positive population trend in DNP over the past 15 years. This contradicts previous long-term studies of carnivore communities in Iberia that report stable trends for red fox populations (Sobrino et al. 2009), but agrees with the popular idea that red fox numbers are increasing in the region. However, it should be noted that there are no other recent studies of fox population trends in the Iberian Peninsula, although research in northern Europe has highlighted a positive trend in red fox populations using the same census method (Jahren et al. 2020). In terms of population dynamics over the years, periods of abundance alternate with marked negative events—for example, low population density events occurred in 2010–2011, and more importantly, in 2013—that could be related to rabbit population dynamics (see Carro et al. 2019). These two events seem to coincide with the above-mentioned declines in the fox population and probably reflect foxes’ preference for rabbits when this prey is abundant and available (Delibes-Mateos et al. 2008). However, foxes have very diverse and opportunistic diets that include insects, vegetables, mammals, birds, and carcasses (Diaz-Ruiz et al. 2013).
Fox culling has been reported to be an ineffective management tool for controlling fox populations not only in the DNP (see above) but also in other areas (Curveira-Santos et al. 2019; Kämmerle et al. 2019) and may even suppose a serious threat for nontarget species when specific and illegal methods are used (Duarte et al. 2012). Although population dynamics show variations in relative abundance between years, the global trend in the DNP fox population is positive, suggesting that isolated culling campaigns are not successful.
From a more local perspective, red fox population increases have been reported before from DNP and have even been suggested to be responsible for the decrease in the Iberian lynx population (Rau et al. 1985). Nevertheless, the most likely explanation is that, although red foxes are widely distributed throughout DNP and are the most abundant carnivore in the area (e.g. Soto-Navarro 2013), they are less common in Coto del Rey, the only zone where lynxes are present continuously in the National Park and a high-density rabbit population exists. Moreover, the intolerance of the presence of the Iberian lynx has been established as one of the main reasons why mesocarnivores avoid areas occupied by lynx, which leads to spatial segregation (Palomares et al. 1996; Palomares and Caro 1999; Alonso and de Ayala 2019). The red fox exhibits fine-scale habitat and temporal avoidance of lynxes, which allows them to co-exist to some extent (Soto-Navarro 2013; Soto and Palomares 2015), even though lynx predate on foxes as well as on other mesocarnivore species (Palomares and Caro 1999).
Both badgers and Egyptian mongooses show stable trends in the study area. The Egyptian mongoose is a native mesocarnivore species in Iberia that, despite its expanding population, is understudied in certain aspects of its biology, including population trends (Palomares 2017, 2020). Although its diet, expansion factors, and effects on prey populations have been the focus of recent research (Descalzo 2022), our data enable us to undertake the first population trend analysis of an Egyptian mongoose population in Europe. This is especially important in the context of the negative social perception towards this expansive species, wrongly considered exotic and invasive by some people (Descalzo 2022). In our study area, although no significant population increases or decreases have occurred over the past 15 years, high-abundance peaks have been detected periodically, especially in 2010, 2012, and 2017, which alternate with years and periods of low abundance (2009, 2011, and 2013–2014). This dynamic could be explained by variations in resource availability, which may determine population growth or detection. It is worth noting that in 2012 rabbit density experimented with a conspicuous peak followed by a rapid decline due to the arrival of a new RHD variant. According to our data, the DNP mongoose population also declined markedly after 2012, and these two events could be related, although no significant relationship between these two species was tested for in this study.
More significantly, the fact that the Egyptian mongoose DNP population trend is stable—the only long-term trend currently available—is especially relevant given the current popular belief that this species has increased its numbers in some parts of its Iberian territories after having expanded its distribution range (Descalzo 2022). This idea also derives from the report of conflicts with human activities that have led certain collectives (Recio and Virgós 2010) to occasionally request mongoose population control. The legal situation of H. ichneumon in Spain is different from that of Portugal, where this species can legally be controlled. However, the control of mongoose populations only seems to benefit more generalist species like the red fox (Curveira-Santos et al. 2019) and only serves to aggravate the overall situation. Moreover, in DNP, in the absence of any mesopredator control activities targeting Herpestes ichneumon, this species has maintained a stable population trend over the past 15 years. Nevertheless, it is true that H. ichneumon has expanded into new territories in recent decades in Iberia (Balmori and Carbonell 2012; Barros et al. 2016; Palomares and Román 2020; Descalzo et al. 2021; Descalzo 2022), which has led to problems with certain collectives. The origin of this problem could be related to the fact that most carnivore communities in Mediterranean Iberia are highly modified and mesopredator populations have increased in light of the absence of the main top predators, the European wolf (Canis lupus) and Iberian lynx (Lynx pardinus) (Monterroso et al. 2015, 2016; Jimenez et al. 2019). In conclusion, given the lack of other information, our data provide a reference for how Egyptian mongoose populations may change over time.
The case of the badgers was expectable for a Mediterranean habitat like DNP. Badgers are expected to live in stable but low abundances given that limitations on resources prevent them from establishing large social groups and increasing in number, as other populations do in areas where resources are more abundant (Rodríguez et al. 1996; Revilla 1998; Revilla and Palomares 2002). Therefore, a stable trend classification is coherent with this assumption. However, due to its traditional dependence on rabbits in DNP (Martín et al. 1995; Revilla and Palomares 2002), a decline in the badger population would have been expected. However, badgers display great diet plasticity (Roper 2010), and based on the changes noted in badgers’ diets after the RHD outbreak (Fedriani et al. 1998; Zapata et al. 2007), it seems that badgers are now exploiting different trophic resources. These resources also seem to be more abundant in Coto del Rey than in the rest of the DNP, which may possibly explain why badgers are more frequently detected in Coto del Rey. Moreover, badgers are not affected by Iberian lynx intragremial predation behavior (Palomares and Caro 1999) and can occupy without problems areas where lynxes are present (Fedriani et al. 1999; Donadio and Buskirk 2006).
Genets and Iberian lynxes are the only species showing uncertainly decreasing or clearly decreasing trends. Our data (see also Sobrino et al. 2009) indicate that in DNP common genet population dynamics are cyclical, with periods of slightly greater abundance alternating with population decrease events. In the DNP, genets have been reported to range between 0.03 indiv/km2 in areas where lynxes are present to 0.33–0.67 indiv/km2 outside lynx presence area, being similar to other areas in Iberia (see Camps 2015). We have classified the trend in the genet population as “uncertain,” as these annual variations in counts do not reveal any precise trend, although a slight decrease can be discerned from the graph in Fig. 2 and, based on the OSMM value, a slight annual decrease of 5.3% seems to have occurred. Both this suspected population decrease and the count variability over the years could reflect small mammal population dynamics and changes in community composition (Santoro et al. 2017), although further investigation is still needed to prove this hypothesis.
As well, common genets and other mesocarnivores shun certain areas to avoid being preyed upon by Iberian lynx. This confines them to more peripheral areas (Monterroso et al. 2020), as has been previously documented (Palomares et al. 1996; Jiménez et al. 2019), and could be the main reason for this suspected decline.
The Iberian lynx (Lynx pardinus) is still one of the most endangered vertebrates in the world. However, its numbers have recovered dramatically since conservation actions have been undertaken, and today its remaining populations are increasing, and it is now occupying new areas such as Montes de Toledo or Matachel Valley due to reintroductions and an ex situ breeding program (life + Iberlince). Therefore, it is somewhat surprising that the DNP lynx population trend has undergone a moderate decrease over the past 15 years, which contrasts with the situation outside the National Park where the lynx population has increased during the past decade (Simón et al. 2009; Simón 2018; Doñana Memoria de actividades y resultados 2018). Thus, the conclusions from our data cannot be extrapolated for the Iberian lynx to other areas due to certain problems present in DNP that do not exist elsewhere.
As previously mentioned and reported in all papers on the subject, all current L. pardinus territories in DNP are located in the Coto del Rey area, in the northern sector of the park, where almost 70% of all lynx tracks were detected. This could be related to the dependence of this feline on rabbits (Ferrer and Negro 2004), which they need to be able to establish their territories (Delibes 1980; Rodríguez 2017). In this way, the decline of rabbits over the past century (Moreno et al. 2008), together with their even more recent decline inside DNP due to the arrival of a new variant of hemorrhagic viral disease (Delibes et al. 2014), could be directly related to the decrease in lynx relative abundance (Delibes-Mateos et al. 2014; Monterroso et al. 2016). Nonetheless, a recent study suggests that lynx can successfully occupy areas with medium- or low-density rabbit populations thanks to their efficiency in capturing prey and even goes as far as to report a lack of association between lynx and high-density rabbit areas (Monterroso et al. 2020). Thus, other factors could be playing an important role in the population dynamics of this threatened feline.
The other confirmed cause of the Iberian lynx decline in DNP was the Feline Leukemia Virus outbreak that occurred during the 2007 breeding season and affected the Coto del Rey subpopulation (López et al. 2009), which seems to be reflected in our data (i.e., the low number of tracks detected that year). Moreover, disease has been demonstrated to be one of the main threats affecting the Iberian lynx (Millán et al. 2009). Feral cats are frequently detected in some track transects, especially those located near urban areas such as Matalascañas and El Rocío, which underlines the fact that the situation could worsen if similar incidents occur in the future. As previously mentioned, it is difficult to establish a clear difference between feral and wildcat tracks. However, previous studies related to feline leukemia carried out in the study area (Meli et al. 2010), as well as naturalist studies from the 1990s (Palomares and Delibes 1993), show that feral cats are often found in and around DNP. Although it cannot be established as a firm conclusion, it is clear from our data that the trend in the Iberian lynx population in DNP is negative and that feline leukemia has caused mortality events in the past.
Conclusion
The long-term population trends of the main species of carnivores in DNP have varied over the past 15 years and suggest that more generalist predators have tended to increase their population (red fox); however, if the species is more specialist, the trend is more negative. Thus, badgers and Egyptian mongooses show stable population trends. This is especially important for Egyptian mongoose, given the absence of similar data from any other region in Iberia and Europe. Finally, the population trend for the common genet, the most specialist of the generalist mesocarnivores present, is unclear, and a moderate decline seems to have occurred, while the Iberian lynx has undergone a marked decrease. Thus, over the past 15 years, the particular population dynamics of these mesocarnivore species have generated a variety of different population trends.
References
Alonso GG, de Ayala RP (2019) Spatial segregation between Iberian lynx and other carnivores. Anim Biodivers Conserv 42(2):347–354. https://doi.org/10.32800/abc.2019.42.0347
Arroyo B, Delibes-Mateos M, Díaz-Fernández S, Viñuela J (2012) Hunting management in relation to profitability aims: red-legged partridge hunting in central Spain. Eur J Wildl Res 58(5):847–855. https://doi.org/10.1007/s10344-012-0632-4
Balme GA, Hunter LT, Slotow ROB (2009) Evaluating methods for counting cryptic carnivores. J Wildl Manag 73(3):433–441. https://doi.org/10.2193/2007-368
Balmori A, Carbonell R (2012) Expansion and distribution of the Egyptian mongoose (Herpestes ichneumon) in the Iberian Peninsula. Galemys 24:83–85
Barros T, Gaubert P, Rocha RG, Bandeira V, Souto L, Mira A, Fonseca C (2016) Mitochondrial demographic history of the Egyptian mongoose (Herpestes ichneumon), an expanding carnivore in the Iberian Peninsula. Mamm Biol 81(2):176–184. https://doi.org/10.1016/j.mambio.2015.09.003
Barroso P, Barasona JA, Acevedo P, Palencia P, Carro F, Negro JJ, ... Vicente J (2020) Long-term determinants of tuberculosis in the ungulate host community of Doñana National Park. Pathogens 9(6):445. https://doi.org/10.3390/pathogens9060445
Barrul J, Mate I (2015) El Zorro. Monografías zoológicas, Serie Ibérica, Vol.3. Tundra Ediciones, Castellón
Barrull J, Mate I, Ruiz-Olmo J, Casanovas JG, Gosàlbez J, Salicrú M (2014) Factors and mechanisms that explain coexistence in a Mediterranean carnivore assemblage: an integrated study based on camera trapping and diet. Mamm Biol 79:123–131. https://doi.org/10.1016/j.mambio.2013.11.004
Blaum N, Engeman RM, Wasiolka B, Rossmanith E (2008) Indexing small mammalian carnivores in the southern Kalahari. S Afric Wildl Res 35(1):72–79. https://doi.org/10.1071/WR07023
Camps D (2015) La Gineta, Monografías Zoológicas, Serie Ibérica, Vol 2. Tundra Ediciones. Castellón
Carro F, Ortega M, Soriguer RC (2019) Is restocking a useful tool for increasing rabbit densities?. Global Ecol Conserv 17. https://doi.org/10.1016/j.gecco.2019.e00560
Carro F, Soriguer RC (2017) Long‐term patterns in Iberian hare populationdynamics in a protected area (Doñana National Park) in the southwestern Iberian Peninsula: Effects of weather conditions and plant cover. Integrative Zoology 12(1):49–60. https://doi.org/10.1111/1749-4877.12212
Cazacu C, Adamescu MC, Ionescu O, Ionescu G, Jurj R, Popa M, ... Cotovelea A (2014) Mapping trends of large and medium size carnivores of conservation interest in Romania. Ann Forest Res 57(1):97–107
Cimatti M, Ranc N, Benítez‐López A, Maiorano L, Boitani L, Cagnacci F, ... Santini L (2021) Large carnivore expansion in Europe is associated with human population density and land cover changes. Divers Distrib. https://doi.org/10.1111/ddi.13219
Conroy MJ (1996) Abundance Indices. In: Wilson DE, Cole FR, Nichols JD, Rudran R, Foster MS (eds) Measuring and monitoring biological diversity. Smithsonian Institution Press, Washington and London, Standard Methods for Mammals, pp 179–193
Crawford J (1991) The calculation of index numbers from wildlife monitoring data. In: Goldsmith B (ed) Monitoring for Conservation and Ecology. Chapman and Hall, London, pp 225–248
Curveira-Santos G, Pedroso NM, Barros AL, Santos-Reis M (2019) Mesocarnivore community structure under predator control: unintended patterns in a conservation context. PloS one 14(1). https://doi.org/10.1371/journal.pone.0210661
Custodio E, Manzano M, Montes C (2010) Las aguas subterráneas en Doñana. Aspectos ecol_ogicos y sociales. Agencia Andaluza del Agua. Consejería de Medio Ambiente de Andalucía, Sevilla
Delibes M (1980) Feeding ecology of the Spanish lynx in the Coto Doñana (Huelva, Spain). ActaTheriol 25:309–324
Delibes-Mateos M, De Simon JF, Villafuerte R, Ferreras P (2008) Feeding responses of the red fox (Vulpes vulpes) to different wild rabbit (Oryctolagus cuniculus) densities: a regional approach. Eur J Wildl Res 54(1):71–78. https://doi.org/10.1007/s10344-007-0111-5.
Delibes-Mateos M, Díaz-Fernández S, Ferreras P, Viñuela J, Arroyo B (2013) The role of economic and social factors driving predator control in small-game estates in central Spain. Ecol Soc 18(2)
Delibes-Mateos M, Ferreira C, Carro F, Escudero MA, Gortázar C (2014) Ecosystem effects of variant rabbit hemorrhagic disease virus. Iberian Peninsula Emerging Infectious Diseases 20(12):21–66. https://doi.org/10.3201/eid2012.140517
Descalzo E (2022) Situation of Egyptian mongoose (Herpestes ichneumon) in Castilla-La Mancha, effects on its prey and social perception. Thesis dissertation. 433 pp. University of Castilla-La Mancha.
Descalzo E, Díaz-Ruiz F, Delibes-Mateos M, Salgado I, Martínez-Jauregui M, Soliño M, ... Ferreras P (2021) Update of the Egyptian mongoose (Herpestes ichneumon) distribution in Spain/Actualización de la distribución del meloncillo (Herpestes ichneumon) en España. Galemys 33:29–38. https://doi.org/10.7325/Galemys. 2021.A4
Donadio E, Buskirk SW (2006) Diet, morphology, and interspecific killing in Carnivora. Am Nat 167(4):524–536. https://doi.org/10.1086/501033
Díaz‐Ruiz F, Delibes‐Mateos M, García‐Moreno JL, María López‐Martín J, Ferreira C, Ferreras P (2013) Biogeographical patterns in the diet of an opportunistic predator: the red fox Vulpes vulpes in the Iberian Peninsula. Mamm Rev 43(1):59–70. https://doi.org/10.1111/j.1365-2907.2011.00206.x
Doñana, memoria anual de actividades y resultados (2018) Conserjería de Agricultura, Ganadería, Pesca y Desarrollo Sostenible. Junta de Andalucía
Duarte J, Farfán MA, Fa JE, Vargas JM (2012) How effective and selective is traditional Red Fox snaring? Galemys 24:1–11
Durant SM, Bashir S, Maddox T, Laurenson MK (2007) Relating long-term studies to conservation practice: the case of the Serengeti Cheetah Project. Conserv Biol 21(3):602–611. https://doi.org/10.1111/j.1523-1739.2007.00702.x
Durant SM, Craft ME, Hilborn R, Bashir S, Hando J, Thomas L (2011) Long-term trends in carnivore abundance using distance sampling in Serengeti National Park, Tanzania. J Appl Ecol 48(6):1490–1500. https://doi.org/10.1111/j.1365-2664.2011.02042.x
Engeman RM (2005) Indexing principles and a widely applicable paradigm for indexing animal populations. Wildl Res 32:203–210. https://doi.org/10.1071/WR03120
Fabiano EC, Sutherland C, Fuller AK, Nghikembua M, Eizirik E, Marker L (2020) Trends in cheetah Acinonyx jubatus density in north-central Namibia. Popul Ecol 62(2):233–243. https://doi.org/10.1002/1438-390X.12045
Fedriani JM, Ferreras P, Delibes M (1998) Dietary response of the Eurasian badger, Meles meles, to a decline of its main prey in the Doñana National Park. J Zool 245(2):214–218
Fedriani JM, Palomares F, Delibes M (1999) Niche relations among three sympatric Mediterranean carnivores. Oecologia 121(1):138–148.
Ferrer M, Negro JJ (2004) The near extinction of two large European predators: super specialists pay a price. Conserv Biol 18(2):344–349
Funston PJ, Frank L, Stephens T, Davidson Z, Loveridge A, Macdonald DM, Ferreira SM (2010) Substrate and species constraints on the use of track incidences to estimate African large carnivore abundance. J Zool 281(1):56–65. https://doi.org/10.1111/j.1469-7998.2009.00682.x
García Barrón L, Aguilar M, Sousa A (2011) Evolution of annual rainfall irregularity in the southwest of the Iberian Peninsula. Theor Appl Climatol 103:13–26
Garrote G, De Ayala RP, Pereira P, Robles F, Guzman N, García FJ, ... Barroso JL (2011) Estimation of the Iberian lynx (Lynx pardinus) population in the Doñana area, SW Spain, using capture–recapture analysis of camera-trapping data. Eur J Wildl Res 57(2):355–362. https://doi.org/10.1007/s10344-010-0440-7
Gortázar C (1999) Ecología y Patología del zorro (Vulpes vulpes L.) en el valle medio del Ebro. Consejo de Protección de la Naturaleza de Aragón, Zaragoza
Jahren T, Odden M, Linnell JD, Panzacchi M (2020) The impact of human land use and landscape productivity on population dynamics of red fox in southeastern Norway. Mammal Research 65:503–516. https://doi.org/10.1007/s13364-020-00494-y
Jiménez J, Nuñez-Arjona JC, Mougeot F, Ferreras P, González LM, García-Domínguez F, ... Villaespesa F (2019) Restoring apex predators can reduce mesopredator abundances. Biol Conserv 238:108234. https://doi.org/10.1016/j.biocon.2019.108234
Jiménez J, Nuñez-Arjona JC, Rueda C, González LM, García-Domínguez F, Muñoz-Igualada J, López-Bao JV (2017) Estimating carnivore community structures. Sci Rep 7(1): 1–10. https://doi.org/10.1038/srep41036
Kämmerle JL, Niekrenz S, Storch I (2019) No evidence for spatial variation in predation risk following restricted-area fox culling. BMC Ecol 19(1):17. https://doi.org/10.1186/s12898-019-0235-y
Karanth KU, Nichols JD, Kumar NS, Link WA, Hines JE (2004) Tigers and their prey: predicting carnivore densities from prey abundance. Proc Natl Acad Sci 101(14):4854–4858. https://doi.org/10.1073/pnas.0306210101
Kuebbing SE, Reimer AP, Rosenthal SA, Feinberg G, Leiserowitz A, Lau JA, Bradford MA (2018) Long‐term research in ecology and evolution: a survey of challenges and opportunities. Ecol Monographs 88(2):245–258. https://doi.org/10.1002/ecm.1289
Lindenmayer DB, Likens GE, Andersen A, Bowman D, Bull CM, Burns E, ... Lowe AJ (2012) Value of long‐term ecological studies. Austral Ecol 37(7):745–757. https://doi.org/10.1111/j.1442-9993.2011.02351.x
Long RA, MacKay P, Ray J, Zielinski W (Eds) (2012) Noninvasive survey methods for carnivores. Island Press
López G, López‐Parra M, Fernández L, Martínez‐Granados C, Martínez F, Meli ML, ... Lutz H (2009) Management measures to control a feline leukemia virus outbreak in the endangered Iberian lynx. Anim Conserv 12(3):173–182. https://doi.org/10.1111/j.1469-1795.2009.00241.x
Lozano J, Urra F (2014) El gato doméstico, Felis catus (Linnaeus, 1758) En: Calzada J., Clavero M. & Fernández A. (eds). Guía virtual de los indicios de los mamíferos de la Península Ibérica, Islas Baleares y Canarias. Sociedad Española para la Conservación y Estudio de los Mamíferos (SECEM). http://www.secem.es/guiadeindiciosmamiferos/ Downloaded on “dd/mm/aaaa”
Macdonald DW, Reynolds JC (2004) Red fox (Vulpes vulpes Linnaeus, 1758). Pp. 129–136. En: Sillero-Zubiri, C., Hoffmann, M. y Macdonald, D.W. (Eds.). Canids: Foxes, Wolves, Jackals and Dogs. Status Survey and Conservation Action Plan. IUCN/SSC Canid Specialist Group, Gland, Switzerland and Cambridge, UK
Magurran AE, Baillie SR, Buckland ST, Dick JM, Elston DA, Scott EM, ... Watt AD (2010) Long-term datasets in biodiversity research and monitoring: assessing change in ecological communities through time. Trends Ecol Evol 25(10):574–582. https://doi.org/10.1016/j.tree.2010.06.016
Martín R, Rodríguez A, Delibes M (1995) Local feeding specialization by badgers (Meles meles) in a Mediterranean environment. Oecologia 101(1):45–50
Meli M, Cattori V, Martínez F, López G, Vargas A, Palomares F, López-Bao JV, Hofmann-Lehmann R, Lutz H (2010) Feline leukemia virus infection: a threat for the survival of the critically endangered Iberian lynx (Lynx pardinus). Vet Immunol Immunopathol 134(1–2):61–67. https://doi.org/10.1016/j.vetimm.2009.10.010
Millán J, Candela MG, Palomares F, Cubero MJ, Rodríguez A, Barral M, ... León-Vizcaíno L (2009) Disease threats to the endangered Iberian lynx (Lynx pardinus). Vet J 182(1):114–124. https://doi.org/10.1016/j.tvjl.2008.04.005
Monterroso P, Alves PC, Ferreras P (2015) Ecological interactions and species coexistence in Iberian mesocarnivore communities-extended summary and main results. Galemys 27:47–57
Monterroso P, Díaz‐Ruíz F, Lukacs PM, Alves PC, Ferreras P (2020) Ecological traits and the spatial structure of competitive coexistence among carnivores. Ecology e03059. https://doi.org/10.1002/ecy.3059
Monterroso P, Garrote G, Serronha A, Santos E, Delibes-Mateos M, Abrantes J, ... Lopes AM (2016) Disease-mediated bottom-up regulation: an emergent virus affects a keystone prey, and alters the dynamics of trophic webs. Sci Rep 6:36072. https://doi.org/10.1038/srep36072
Moreno S, Beltrán JF, Cotilla I, Kuffner B, Laffite R, Jordán G, ... Cabezas S (2008) Long-term decline of the European wild rabbit (Oryctolagus cuniculus) in south-western Spain. Wildl Res 34(8):652–658
Morrison TA, Estes AB, Mduma SA, Maliti HT, Frederick H, Kija H, ... Kohi EM (2018) Informing aerial total counts with demographic models: population growth of Serengeti elephants not explained purely by demography. Conserv Lett 11(3):e12413. https://doi.org/10.1111/conl.12413
Mueller SA, Reiners TE, Middelhoff TL, Anders O, Kasperkiewicz A, Nowak C (2020) The rise of a large carnivore population in Central Europe: genetic evaluation of lynx reintroduction in the Harz Mountains. Conserv Genet 21(3):577–587. https://doi.org/10.1007/s10592-020-01270-w
O’Donoghue M, Slough BG, Poole K, Boutin S, Hofer EJ, Mowat G, Murray D, Krebs CJ Snow (2022) track counts for density estimation of mammalian predators in the boreal forest. Wildl Res. https://doi.org/10.1071/WR21159.
Palomares F (2017) Meloncillo -Herpestes ichneumon. En: Enciclopedia Virtual de los Vertebrados Españoles. Salvador A, Barja I (Eds.). Museo Nacional de Ciencias Naturales, Madrid. http://www.vertebradosibericos.org
Palomares F (2020) El Meloncillo, Monografías Zoológicas, Serie Ibérica, Vol 8. Tundra Ediciones
Palomares F, Caro TM (1999) Interspecific killing among mammalian carnivores. Am Nat 153(5):492–508. https://doi.org/10.1086/303189
Palomares F, Delibes M (1993) A note on the movements of a free-ranging male domestic cat in sothwestern Spain. Hystrix 5:119–123
Palomares F, Ferreras P, Fedriani JM, Delibes M (1996) Spatial relationships between Iberian lynx and other carnivores in an area of south-western Spain. J Appl Ecol 5–13
Palomares F, Román J (2020) Nuevos datos sobre la distribución y hábitat usados por el meloncillo en la península ibérica: ¿ Es más común y generalista de hábitat de lo que se conocía?/New data on the distribution and habitat used by the Egyptian mongoose in the Iberian Peninsula: Is it more common and generalist of habitat than was known? Galemys 32:21–30
Palomares F, Soto C, López-Bao JV, Rodríguez A, Godoy JA, Roldán E, Gomendio M, Göritz F, Jewgenow K (2011) Estudio de las poblaciones de carnívoros del Parque Nacional de Doñana usando métodos no invasivos. In: Ramírez L, Asensio B (eds) Proyectos De Investigación En Parques Nacionales 2007–2010. Organismo Autónomo de Parques Nacionales, Madrid, pp 253–276
Pannekoek J, Van Strien AJ (2005) TRIM 3 manual trends and indices for monitoring data. CBS Statistics Netherlands Voorburg Neetherlands, Published by Prentice Hall. Available from URL: http://www.cbs.nl/en-GB/menu/themas/natuur-milieu/methoden/trim/default.htm.
Prugh LR, Stoner CJ, Epps CW, Bean WT, Ripple WJ, Laliberte AS, Brashares JS (2009) The rise of the mesopredator. Bioscience 59(9):779–791. https://doi.org/10.1525/bio.2009.59.9.9
Purroy Iraizoz FJ, Varela JM (2003) Guía de los mamíferos de España. Lynx Edicions
Rau JR, Beltran JF, Delibes M (1985) Can the increase of fox density explain the decrease in lynx numbers at Doñana? Rev Ecol Terre Vie 40:145–150
Recio MR, Virgós E (2010) Predictive niche modelling to identify potential areas of conflicts between human activities and expanding predator populations: a case study of game management and the grey mongoose, Herpestes ichneumon. Spain Wildl Res 37(4):343–354. https://doi.org/10.1071/WR09096
Reinke BA, Miller DA, Janzen FJ (2019) What have long-term field studies taught us about population dynamics? Annu Rev Ecol Evol Syst 50:261–278
Revilla E (1998) Organización social del tejon en Doñana (Doctoral dissertation, Universidad de León), pp 222
Revilla E, Palomares F (2002) Spatial organization, group living and ecological correlates in low-density populations of Eurasian badgers, Meles meles. J Anim Ecol 71(3):497–512. https://doi.org/10.1046/j.1365-2656.2002.00617.x
Rodríguez A (2017) Lince ibérico –Lynx pardinus. En: Enciclopedia Virtual de los Vertebrados Españoles. Salvador A, Barja I (Eds.). Museo Nacional de Ciencias Naturales, Madrid. http://www.vertebradosibericos.org/
Rodríguez A, Martin R, Delibes M (1996) Space use and activity in a Mediterranean population of badgers Meles meles. Acta Theriol 41:59–72
Rogers PM (1974) Land classification and patterns of animal distributions in the management of national parks. Coto Doñana, Spain. Msc. thesis. University of Guelph. Microfiche, National Library of Canada, Ottawa
Roper TJ (2010) Badger. Collins New Naturalist Library, Book 114. (Vol. 114). HarperCollins UK
Santoro S, Sanchez-Suarez C, Rouco C, Palomo LJ, Fernández MC, Kufner MB, Moreno S (2017) Long-term data from a small mammal community reveal loss of diversity and potential effects of local climate change. Curr Zool 63(5):515–523. https://doi.org/10.1093/cz/zow109
Schradin C, Hayes LD (2017) A synopsis of long-term field studies of mammals: achievements, future directions, and some advice. J Mammal 98(3):670–677. https://doi.org/10.1093/jmammal/gyx031
Simón MA (2018) Censo de las poblaciones Andaluzas de Lince Ibérico año 2017. Life+ Iberlince, Junta de Andalucía, Sevilla, pp 11
Simón MA, Cadenas R, Gil-Sánchez JM, López-Parra M, García J, Ruiz G, López G (2009) Conservation of free-ranging Iberian lynx (Lynx pardinus) populations in Andalusia. Vargas, A., Breitenmoser, c., Breitenmoser, U.(Eds.), Iberian Lynx Ex situ conservation: An Interdisciplinary Approach. Fundación Biodiversidad, Madrid, Spain
Sinclair ARE, Mduma SA, Hopcraft JGC, Fryxell JM, Hilborn RAY, Thirgood S (2007) Long-term ecosystem dynamics in the Serengeti: lessons for conservation. Conserv Biol 21(3):580–590. https://doi.org/10.1111/j.1523-1739.2007.00699.x
Smallwood KS, Fitzhugh EL (1995) A track count for estimating mountain lion Felis concolor californica population trend. Biol Cons 71(3):251–259
Smith JE, Lehmann KD, Montgomery TM, Strauss ED, Holekamp KE (2017) Insights from long-term field studies of mammalian carnivores. J Mammal 98(3):631–641. https://doi.org/10.1093/jmammal/gyw194
Sobrino R, Acevedo P, Escudero MA, Marco J, Gortázar C (2009) Carnivore population trends in Spanish agrosystems after the reduction in food availability due to rabbit decline by rabbit haemorrhagic disease and improved waste management. Eur J Wildl Res 55(2):161–165. https://doi.org/10.1007/s10344-008-0230-7
Soto Navarro C (2013) Patrones de distribución, abundancia e interacciones entre carnivoros simpátridos en un área mediterránea protegida (Doctoral dissertation, Universidad de Sevilla), pp 212
Soto C, Palomares F (2015) Coexistence of sympatric carnivores in relatively homogeneous Mediterranean landscapes: functional importance of habitat segregation at the fine-scale level. Oecologia 179(1):223–235. https://doi.org/10.1007/s00442-015-3311-9
Stansbury CR, Ausband DE, Zager P, Mack CM, Miller CR, Pennell MW, Waits LP (2014) A long-term population monitoring approach for a wide-ranging carnivore: noninvasive genetic sampling of gray wolf rendezvous sites in Idaho, USA. J Wildl Manag 78(6):1040–1049. https://doi.org/10.1002/jwmg.736
Torres RT, Fonseca C (2016) Perspectives on the Iberian wolf in Portugal: population trends and conservation threats. Biodivers Conserv 25(3):411–425. https://doi.org/10.1007/s10531-016-1061-6
Treves A, Karanth KU (2003) Human-carnivore conflict and perspectives on carnivore management worldwide. Conserv Biol 17(6):1491–1499. https://doi.org/10.1111/j.1523-1739.2003.00059.x
Treves A, Naughton-Treves LISA, Harper EK, Mladenoff DJ, Rose RA, Sickley TA, Wydeven AP (2004) Predicting human-carnivore conflict: a spatial model derived from 25 years of data on wolf predation on livestock. Conserv Biol 18(1):114–125. https://doi.org/10.1111/j.1523-1739.2004.00189.x
Valverde JA (1967) Estructura de una comunidad de vertebrados terrestres. Monografías de la Estación Biológica de Doñana. CSIC, Madrid, España
Virgós E, Travaini A (2005) Relationship between small-game hunting and carnivore diversity in central Spain. Biodivers Conserv 14(14):3475. https://doi.org/10.1007/s10531-004-0823-8
Winterbach CW, Ferreira SM, Funston PJ, Somers MJ (2016) Simplified large African carnivore density estimators from track indices. PeerJ 4:e2662. https://doi.org/10.7717/peerj.2662
Zapata SC, Travaini A, Ferreras P, Delibes M (2007) Analysis of trophic structure of two carnivore assemblages by means of guild identification. Eur J Wildl Res 53(4):276–286. https://doi.org/10.1007/s10344-007-0095-1
Acknowledgements
Data provided by ICTS-RBD Biodiversity Monitoring Program (Doñana Biological Station, CSIC). We are also indebted to the Doñana Biological Monitoring Team (ESPN-EBD-ICTS-CSIC), especially Diego Lopez, Olga Ceballos, David Paz, and Isidro Román. Logistical support was provided by the laboratory of GIS and remote sensing (LAST, EBD-CSIC), especially David Aragones. Xosé Pardavila also helped with the figures and GIS support. In memoriam Rafael Lafitte, technician of Doñana Biological station.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. Singular Scientific and Technical Infrastructures from the Spanish Science and Innovation Ministry (ICTS-MICINN); Ministry of Agriculture, Livestock, Fisheries, and Sustainable Development from the Regional Government of Andalusia (CAGPDES-JA); Doñana Biological Station from the Spanish National Research Council (EBD-CSIC); Ministry of Environmetal sustainability and blue economy from the Regional Goverment of Andalusia and Ministry of Science and Innovation.
Author information
Authors and Affiliations
Contributions
F.C. and R.C.S. contributed to the conception, design, data collection, data analysis, drafting, and writing of the manuscript. J.S-C contributed to data analysis, drafting, and writing of the manuscript. The authors agree with the contents of the manuscript and its submission to the journal.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sereno-Cadierno, J., Soriguer, R.C. & Carro, F. Shedding light on long-term trends in Mediterranean carnivore populations: five species, one scenario, different responses. Eur J Wildl Res 69, 55 (2023). https://doi.org/10.1007/s10344-023-01683-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-023-01683-1