Abstract
In semi-arid environments, the effects of irregularly distributed rainfall, flow regulation and water inter-basin transfer enhance the spread of non-native fish to the detriment of native communities. In the River Segura, since the 1980s the number of non-native fish species has progressively increased, also because of the building of water transfer facility connecting the rivers Segura and Tajo. With the aim of highlighting how man-driven changes in the diversity of fish communities affect the diet of top-predators, we compared Eurasian otter Lutra lutra diet in the span of 20 years, i.e. 1997–98 vs. 2016–19. As habitat quality affects the condition of Andalusian barbel Luciobarbus sclateri, the most widespread native fish, we also compared the size of preyed barbels to point out whether human activities may have lowered their profitability to otters. Fish and introduced red swamp crayfish Procambarus clarkii formed the bulk of otter diet in both study periods. In 2016–19 the contribution of non-native species to otter diet increased significantly, both for crayfish and fish, which included ten non-native species. Otter feeding habits faithfully mirrored the variation in the composition of the fish community and confirmed the importance of crayfish as alternative-to-fish prey in the Iberian Peninsula. The average length of preyed barbels was significantly lower in the second study period, consistently with a decline in barbel profitability for otters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Throughout arid lands, water demand is increasing with human population density and climate warming, resulting in the alteration of flow regimes and freshwater communities (Miller et al. 1989; Kingsford 2000; Olden and Poff 2005). Since the second half of the nineteenth century, inter-basin water transfer (IBT) projects have been considered as an effective solution to solve water resource deficits and management problems (Shiklomanov 1999), and, currently, there are over 170 active IBTs globally (Schmidt et al. 2019). Connecting different biogeographic regions, water diversions can act as invasion corridors, facilitating the spread of non-native invasive species (Galil et al. 2007; Gallardo and Aldridge 2018), as demonstrated in all continents (e.g., River Rhine, NW Europe, Leuven et al. 2009; River Huai, China, Qin et al. 2019; Tasmania, rivers Gordon and Pedder, Sanger 2001; rivers Great Fish and Sundays, South Africa, Kadye and Booth 2013; coastal drainages of southern California, Swift et al. 2015). Chronic colonization by non-native fishes, coupled with the buffering of seasonal variability due to damming and flow regulation, can drive the decline of native fish assemblages, especially in water-limited catchments (Propst et al. 2008; Clavero et al. 2015).
Flowing in south-eastern Spain, the River Segura is one of the driest and most regulated (24 dams higher than 10 m and 121 weirs > 2 m; CHS 2007; Grindlay et al. 2011) European watersheds. Agricultural and urban water is mainly supplied from saltwater desalination plants and, since 1978, through the Tajo-Segura IBT, a 286-km-long water transfer facility connecting the Entrepeñas and Buendia dams in the upper River Tajo to the Talave Dam in the catchment of the River Segura (Pittock et al. 2009). Fish dispersion through the Tajo-Segura IBT system has been imputed as the cause of the spread of several non-native fish species in the recipient basin (e.g., Andreu-Soler et al. 2004; Oliva-Paterna et al. 2005).
As in many European countries, in SE Spain, otter populations declined throughout the 1970s and 1980s due to water pollution, man-driven alteration of freshwaters habitats, and hunting (Delibes 1990; Yelo and Calvo 2004). Following improvement in water quality (mainly through water treatment plants) and habitat restoration (Jiménez et al. 2008), in the last three decades, the species has progressively recovered and is currently reported on a ca. 230-km-long stretch of the main river (Dettori et al. 2019). Fish availability is considered a major factor affecting the diet of this semi-aquatic top-predator of freshwater habitats (Kruuk 2006).
Interactions between introduced species and native predators are highly complex, being exacerbated by environmental factors (Mack and D’Antonio 1998). As other native semi-aquatic predators (e.g., kingfisher Alcedo atthis; Nessi et al. 2021), otters usually prey on non-native fish less than expected based on their relative abundance (Balestrieri et al. 2013). Nonetheless, few studies have been carried out in arid and semi-arid freshwater habitats, where anthropogenic impacts add to strong natural environmental stress (Ormerod et al. 2010). In arid rivers of Morocco, otters mainly prey on widespread, native barbels Luciobarbus spp. (Libois et al. 2015; Riesco et al. 2020), despite about 60% of recorded species are non-natives (Clavero et al. 2015, 2017).
With the aim of highlighting if otter feeding behavior in the catchment of the River Segura may be affected by man-driven changes in the diversity and richness of the fish community and the ongoing recovery of this semi-aquatic mustelid hindered by food availability, we compared its diet, as assessed by the analysis of fecal samples, in 1997–1998 and 2016–2019.
Andalusian barbel Luciobarbus sclateri was the most abundant and widespread fish in both study periods (Miñano et al. 2003; Oliva-Paterna et al. 2014). As habitat quality (Vila-Gispert and Moreno-Amich, 2001; Oliva-Paterna et al. 2003) and the spread of introduced species (Castejón Bueno 2010) may affect barbel condition, we expected otters to prey on smaller fishes in the second study period. To test for this hypothesis, the length of preyed barbels was assessed using diagnostic bones and available regression equations.
Study area
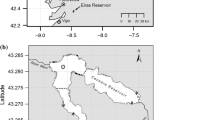
The catchment of the River Segura (352 km) covers 14,432 km2 and hosts a stable population of about 1,850,000 inhabitants (Uche et al. 2015). The climate ranges from sub-humid to semi-arid Mediterranean, with mean annual temperature of 17 °C. Annual rainfall is ca. 400 mm, with large fluctuations in both seasonal and yearly values and pronounced summer droughts (Machado et al. 2011; Belmar et al. 2013). As in arid and semiarid areas of Australia and South Africa, intermittent and ephemeral streams are the predominant watercourse classes in the river catchment (Belmar et al. 2011).
Land use includes shrubland and woodland (45%), crops (52%), urban areas (2%), pastures, and sparsely vegetated areas (Bruno et al. 2014a). Riparian vegetation consists of both European and Ibero-African species (Salix spp., Fraxinus angustifolia, Populus spp., Tamarix spp., Nerium oleander) (Bruno et al. 2014b). From the first half of the 1980s traditional rain-fed crops have been progressively replaced by irrigated ones, and urban areas have largely grown (Alonso-Sarría et al. 2009). River longitudinal connectivity has been recently improved through restoration projects aimed at eliminating or permeabilizing barriers (e.g., LIFE12 ENV/ES/1140 SEGURA RIVERLINK; Sanz-Ronda et al. 2019), and controlling the invasive giant reed Arundo donax using soft engineering techniques (e.g., LIFE13 BIO/ES/001407 RIPISILVANATURA; Bruno et al. 2019).
The catchment of the River Segura currently hosts 18 fish species, of which 13 (72%) are non-natives (Oliva-Paterna et al. 2014). Native Luciobarbus sclateri and introduced Gobio lozanoi, Alburnus alburnus, Lepomis gibbosus, and Pseudochondrostoma polylepis form the bulk of the fish community (Oliva-Paterna et al. 2014).
Methods
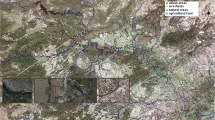
In 1997–1998 and 2016–2019, undisputable spraints were searched for on a 110-km-long stretch of the River Segura (Fig. 1), by surveying typical otter marking sites (e.g., large stones, bridges, pool banks, confluences) on both banks.
The oldest sample consisted of 951 scats collected between February 1997 and March 1998. All fecal samples collected in a same sampling station/date were pooled together.
Between April 2016 and June 2019, 600 fecal samples were collected along 37 sampling stations, consisting of a 0.5 ± 0.05-km-long stretch of the river, stored in test tubes containing ethanol, labeled, and frozen at −20 °C.
For the analysis, each spraint was soaked for 24 h in a solution of hydrogen peroxide 30% w/v (100vol) stabilized pure. Each spraint was then washed by a strong water jet into a sieve with 0.5-mm-wide meshes. Undigested remains were carefully examined using a microscope. Fish remains were identified from their vertebrae, pharyngeal teeth, and scales, using personal collections and the keys of different authors (Prigioni 1997; Oliva-Paterna et al. 2014, 2019). Feathers and chelae and thoracopods were the main diagnostic features for birds and crustaceans, respectively. The diagnostic remains of amphibians and reptiles were identified by the keys of Prigioni (1997) and Smiroldo et al. (2019a).
For both study periods, data were split into two seasons (warm: IV–IX; cold: X–III), and, to allow comparison, results were expressed as percent relative frequency of occurrence [FR% = (number of occurrences of an item/total number of items) × 100]. Raw frequency data were compared by the chi-squared (χ2) test, using Bonferroni’s sequential method as a conservative correction for multiple testing (Rice 1989). Trophic niche breadth was estimated by standardized Levins’ index B = 1/(R Σpi2) (Feinsinger et al. 1981), with pi = RF and R = 20.
The length of preyed barbels was assessed based on key diagnostic bones (maximum length/width of pharyngeal teeth, length of cephalic, thoracic, and caudal vertebrae), using the methods and equations proposed by Ruiz-Olmo (1995). Average total body lengths were compared between the two study periods by Mann–Whitney’s U test.
Results
Fish and introduced red swamp crayfish Procambarus clarkii formed the bulk of otter diet in both study periods (Table 1), with the minor contribution of birds, frogs, and small mammals (< 5% each). In 1997–1998, fish (%RF = 76.8), particularly native Andalusian barbel (59.9), was the main otter prey, while in the second study period crayfish dominated (47.9). In the late 1990s, preyed fish included only four species, of which two (Eastern mosquitofish Gambusia holbrooki and Iberian nase Pseudochondrostoma polylepis) were non-natives. In 2016–2019, otter diet was more diverse than in 1997–1998 (B = 0.2 vs. 0.1), including twelve fish species, of which ten were non-natives (Table 1). The contribution of introduced species to otter diet increased significantly, both for crayfish (47.9 vs. 17.5, χ2 = 255.6, P < 0.001) and fish, particularly Iberian nase (3.4 vs. 0.1, χ2 = 93.3, P < 0.001) and mosquitofish (2.3 vs. 0.3, χ2 = 65.6, P < 0.001), while the relative frequency of Andalusian barbel declined (15.5 vs. 59.9, χ2 = 234.4, P < 0.001). The frequency of non-fish prey, frogs (2.9 vs. 0.3, χ2 = 72.6, P < 0.001), and small mammals (4.5 vs. 0.9, χ2 = 76.4, P < 0.001) also increased.
In both study periods, crayfish were preyed on more frequently in the warm season (1997–1998: 20.9 vs. 15.5, χ2 = 7.2, P < 0.05; 2016–2019: 64.8 vs. 40.2, χ2 = 18.7, P < 0.01), while barbels prevailed in autumn–winter (1997–1998: 63.0 vs. 54.8, χ2 = 9.7, P < 0.05; 2016–2019: 20.3 vs. 11.5, χ2 = 8.9, P < 0.05) (Fig. 2). In 1997–1998, insects were used only in the warm season, while birds were preyed on in the cold season of 2016–2019.
The average (± SD) length of preyed barbels was significantly lower in the second study period (18.9 ± 5.6 cm vs. 16.1 ± 5.1 cm; U = 2936, P < 0.001, N = 200 and 46, respectively; Fig. 3).
Discussion
Since the 1980s, the number of non-native fish species in the River Segura has progressively increased, with some species – bleak Alburnus alburnus, Iberian gudgeon Gobio lozanoi, pumpkinseed Lepomis gibbosus and Iberian nase – currently occurring as frequently as native barbels (Oliva-Paterna et al. 2014). The initial spread of non-native species coincided with the completion of the Tajo-Segura IBT, which probably promoted the invasion by Iberian gudgeon (Mas 1986), golden carp Carassius auratus (García de Jalon et al. 1992), Iberian nase (Torralva and Oliva-Paterna 1997), and zander Sander lucioperca (Miñano et al. 2002), but also with a phase of exponential increase in the cumulative number of alien fish acclimatized in Spain, suggesting that the “improvement” of fish resources for sport fishing and aquaculture also played a major role in fish introductions (Elvira and Almodóvar 2001).
These deep changes in the composition of the river’s fish community were mirrored by the feeding habits of the otter, which shifted from a native barbel-based diet in the late 1990s to a non-native prey-based diet 20 years later. Although the frequency of occurrence of non-native fish in otter diet in 2016–2019 was still rather low, consistently with its disinclination to prey on introduced fish (Balestrieri et al. 2013), their contribution was by far higher than in the late 1990s, both in terms of relative frequency and species diversity. Moreover, most preyed non-native fishes were also the most widespread, except for bleak, which, as recorded for southern Italy (Remonti et al. 2010), was rarely preyed despite being as spread as Andalusian barbel (Oliva-Paterna et al. 2014).
While non-native fish usually represent a minor component of otter diet (Balestrieri et al. 2013), throughout the Iberian Peninsula red swamp crayfish are often preyed by otters (Delibes and Adrián 1987; Beja 1996). North American crayfish were introduced in the marshes of the River Guadalquivir, southwestern Spain, in 1973, and by the end of the decade spread throughout eastern Spain (Gutiérrez-Yurrita et al. 1999; Oficialdegui et al. 2019). Being highly resistant to adverse conditions, red swamp crayfish are capable to spread through dry land and temporary habitats, coping with large seasonal fluctuations in water levels (Barbaresi and Gherardi 2000), as those occurring in our study area.
In a study carried out between October 2004 and February 2005 by the analysis of 943 spraints (Pastor González 2011), the relative frequencies of occurrence of fish and crayfish in otter diet were intermediate (48% and 38.2%, respectively) to those recorded in 1997–1998 and 2016–2019, suggesting that throughout the study period the importance of crayfish as prey has progressively increased. Although otters can rely on a relatively wide range of aquatic food resources, fish are their preferred prey (Ruiz-Olmo and Palazón 1997; Clavero et al. 2003; Remonti et al. 2008; Smiroldo et al. 2019a), and use by otters of alternative-to-fish prey mainly depends on fish shortage (Remonti et al. 2010; Krawczyk et al. 2016; Smiroldo et al. 2019b).
Thus, the recorded trend may indicate the progressive decline of native fish, which may have been worsened by either competition with non-native fish or human-altered flow regimes (Lytle and Poff 2004). Particularly, growth, age structure, body condition, and population abundance of Andalusian barbels have been demonstrated to be affected by waterflow regulation in the River Segura catchment (Torralva et al. 1997; Oliva-Paterna et al. 2003, 2019). The smaller size of preyed barbels recorded in 2016–2019 with respect to the late 1990s, together with their lower contribution to otter diet, is consistent with a decline in barbel profitability for otters, which may have forced the mustelid to switch to alien crayfish, particularly in the warm season, when large crayfish are most abundant and native fish availability is the lowest (Beja 1996; Kruuk 2006).
Our results stress the dichotomous role played by red swamp crayfish in introduction areas, as they both alter freshwater food webs and prey on fingerlings (Geiger et al. 2005; Loureiro et al. 2015; Souty-Grosset et al. 2016), affecting fish communities and food availability to otters and, at the same time, provide a major alternative food resource for several predators in man-altered freshwater ecosystems (Correia 2001; Tablado et al. 2010; Musseau et al. 2015), possibly contributing to otter ongoing recolonization of Iberian catchments (see Beja 1996), particularly in peri-urban and agricultural land, where native fish are often replaced by non-native species (Dettori et al. 2021). On the other hand, crayfish can store large amounts of xenobiotic substances, such as heavy metals (Alcorlo and Baltanás 2013), with serious risks for otter health and recovery (Rodríguez-Estival et al. 2020).
As climate warming is expected to increase water deficit, with direct and indirect consequences for cyprinid populations (Beja 1995; Ilheu et al. 2007), information on the ability of semi-aquatic species to face the expansion of arid areas is pivotal for implementing conservation actions (Harms et al. 2008). The spread of invasive aquatic species, interacting with climate change (Burgiel and Muir 2010; Capdevila-Arguelles et al. 2011), will pose additional threats on the whole trophic web of semiarid rivers. In the Iberian Peninsula, strategies aimed at otter conservation should focus on the restoration of freshwater habitats, which have been demonstrated to improve both habitat quality and otter abundance (Bruno et al. 2019; Dettori et al. 2019, 2021). Specific actions should be directed at improving native fish species through site-specific measures (Santos et al. 2018), whereas invasive crayfish are difficult to eradicate. In addition, the design of flows that mimic natural flow regime patterns may provide more suitable environmental conditions for native fish species (Sánchez-Perez et al. 2020). The Eurasian otter should be regarded as a useful indicator of the effectiveness of such actions, as already demonstrated with respect to both the recovery of fish in depleted rivers (Narváez et al. 2020) and contaminant accumulation (Rodríguez-Estival et al. 2020).
Availability of data and material
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
Alcorlo P, Baltanás A (2013) The trophic ecology of the red swamp crayfish (Procambarus clarkii) in Mediterranean aquatic ecosystems: a stable isotope study. Limnetica 32:121–138
Alonso Sarría F, Gomariz Castillo F, Cánovas García F, Moreno Brotóns J (2009) Análisis espacio-temporal de los cambios de usos del suelo en la Cuenca del Río Segura. In Advances in studies on desertification: contributions to the International Conference on Desertification in memory of Professor John B. Thornes. Universidad de Murcia, Spain
Andreu-Soler A, Oliva-Paterna FJ, Verdiell D, Torralva M (2004) Primeras citas de Alburnus alburnus (Linnaeus, 1758) y Tinca tinca en la cuenca del río Segura (Murcia, sudeste de la Península Ibérica). Anales De Biología 26:222–224
Barbaresi S, Gherardi F (2000) The invasion of the alien crayfish Procambarus clarkii in Europe, with particular reference to Italy. Biol Invasions 2:259–264
Balestrieri A, Remonti L, Vezza P, Prigioni G, Copp GH (2013) Do non-native fish as prey favour the conservation of the threatened indigenous Eurasian otter?. Freshw Biol 58:995–1007
Belmar O, Bruno D, Martínez-Capel F, Barquín J, Velasco J (2013) Effects of flow regime alteration on fluvial habitats and riparian quality in a semiarid Mediterranean basin. Ecol Ind 30:52–64
Belmar O, Velasco J, Martinez-Capel F (2011) Hydrological classification of natural flow regimes to support environmental flow assessments in intensively regulated Mediterranean rivers, Segura River Basin (Spain). Environ Manage 47:992–1004
Beja PR (1995) Patterns of availability and use of resources by otters (Lutra lutra L.) in Southwest Portugal. PhD thesis, University of Aberdeen
Beja R (1996) An analysis of otter Lutra lutra predation on introduced American crayfish Procambarus clarkii in Iberian streams. J Appl Ecol 33:1156–1170
Bruno D, Belmar O, Sánchez-Fernández D, Guareschi S, Millán A, Velasco J (2014a) Responses of Mediterranean aquatic and riparian communities to human pressures at different spatial scales. Ecol Ind 45:456–464
Bruno D, Belmar O, Sánchez-Fernández D, Velasco J (2014b) Environmental determinants of woody and herbaceous riparian vegetation patterns in a semi-arid Mediterranean basin. Hydrobiologia 730:45–57
Bruno D, Zapata V, Guareschi S, Picazo F, Dettori E, Carbonell JA, Millan A, Velasco J, Robledano F (2019) Short-term responses of aquatic and terrestrial biodiversity to riparian restoration measures designed to control the invasive Arundo donax L. Water 11(12):2551
Burgiel SW, Muir AA (2010) Invasive species, climate change and ecosystem-based adaptation: addressing multiple drivers of global change. Global Invasive Species Programme (GISP), Washington DC and Nairobi
Capdevilla Argüelles L, Zilleti B, Suárez Álvarez VA (2011) Cambio climático y especies exóticas invasoras en España. Diagnóstico preliminar y bases de conocimiento sobre impacto y vulnerabilidad. Oficina Española de Cambio Climático - Ministerio de Medio Ambiente y Medio Rural y Marino, Madrid
Castejón Bueno D (2010) Estado de condición de Barbus sclateri Günther, 1868 (Cypriniformes: Cyprinidae) en gradientes longitudinales de la cuenca del Segura (SE Península Ibérica). Master thesis, Departamento de Zoología y Antropología Física, Universidad de Murcia
CHS (2007) Estudio general sobre la Demarcación Hidrográfica del Segura. Confederación Hidrográfica del Segura (Ministry for the Environment). https://chsegura.es/export/descargas/planificacionydma/planificacion/docsdescarga/Estudio_general_de_la_Demarcacion_V4.pdf
Clavero M, Esquivias J, Qninba A, Riesco M, Calzada J, Ribeiro F, Fernández N, Delibes M (2015) Fish invading deserts: non-native species in arid Moroccan rivers. Aquatic Conservation: Marine and Freshwaters Ecosystems 25:49–60
Clavero M, Prenda J, Delibes M (2003) Trophic diversity of the otter (Lutra lutra L.) in temperate and Mediterranean freshwater habitats. J Biogeogr 30:761–769
Clavero M, Qninba A, Riesco M, Esquivias J, Calzada J (2017) Delibes M (2017) Fish in Moroccan desert rivers: the arid extreme of Mediterranean streams. Fishes in Mediterranean Environments 003:21p
Correia AM (2001) Seasonal and interspecific evaluation of predation by mammals and birds on the introduced red swamp crayfish Procambarus clarkii (Crustacea, Cambaridae) in a freshwater marsh (Portugal). J Zool Lond 255:533–541
Delibes M (1990) La Nutria (Lutra lutra) en Espana. ICONA, Serie Técnica, Madrid
Delibes M, Adrián I (1987) Effects of crayfish introduction on otter Lutra lutra food in the Doñana National Park, SW Spain. Biol Cons 42:153–159
Dettori EE, Zapata-Perez VM, Bruno D, Soto-Otón IC, Rubio-Saura N, Millán A, Velasco J, Balestrieri A, Robledano-Aymerich F (2019) Improving knowledge of Eurasian otter ecology in the basin of the River Segura (SE Iberian Peninsula). 1st Iberian Ecological Society & XIV AEET Meeting, 4–7 February 2019, Barcelona, Spain
Dettori, EE, Balestrieri A, Zapata-Perez VM, Bruno D, Rubio-Saura N, Robledano-Aymerich F (2021) Distribution and diet of recovering Eurasian otter (Lutra lutra) along the natural-to-urban habitat gradient (River Segura, SE Spain). Urban Ecosystems. https://doi.org/10.1007/s11252-021-01109-3
Elvira B, Almodóvar A (2001) Freshwater fish introductions in Spain: facts and figures at the beginning of the 21st century. J Fish Biol 59 (Supplement A):323–331
Feinsinger P, Spers EE, Poole RW (1981) A simple measure of niche breadth. Ecology 62:27–32
Galil BS, Nehring S, Panov VE (2007) Waterways as invasion highways–impact of climate change and globalization. In: Nentwig W (ed) Biological invasions. Ecological studies 93:59–74
Gallardo B, Aldridge DC (2018) Inter-basin water transfers and the expansion of aquatic invasive species. Water Res 143:282–291
García de Jalon D, González Del Tánago M, Casado C (1992) Ecology of regulated streams in Spain: an overvicw. Litnnetica 8:161–166
Geiger W, Alcorlo P, Baltanás A, Montes C (2005) Impact of an introduced Crustacean on the trophic webs of Mediterranean wetlands. Biol Invasions 7:49–73
Grindlay AL, Zamorano M, Rodriguez MI, Molero E, Urrea MA (2011) Implementation of the European Water Framework Directive: integration of hydrological and regional planning at the Segura River Basin, southeast Spain. Land Use Policy 28:242–256
Gutiérrez-Yurrita PJ, Martínez JM, Bravo-Utrera MA, Montes C, Ilhéu M, Bernardo JM (1999) The status of crayfish populations in Spain and Portugal. In Crayfish in Europe as alien species: How to make the best of a bad situation?. (edited by Gherardi F, Holdich DM). AA Balkema/Rotterdam/Brookfield
Harms TK, Sponseller RA, Grimm NB (2008) Desert streams. In Encyclopedia of Ecology (edited by Jørgensen SE, Fath BD). Elsevier, Oxford
Ilhéu M, Bernardo JM, Fernandes S (2007) Predation of invasive crayfish on aquatic vertebrates: the effect of Procambarus clarkii on fish assemblages in Mediterranean temporary streams. In Biological invaders in inland waters: profiles, distribution, and threats. (edited by Gherardi F). Invading Nature - Springer Series in Invasion Ecology, vol 2. Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-6029-8_29
Jiménez J, López-Martín JM, Ruiz-Olmo J, Delibes M (2008) ¿ Por qué se está recuperando la nutria en España. In La nutria en España, veinte años de seguimiento de un mamífero amenazado (edited by López-Martín JM & Jiménez J). Sociedad Española para la Conservación y Estudio de los Mamíferos, Málaga
Kadye W, Booth A (2013) An invader within an altered landscape: one catfish, two rivers and an inter-basin water transfer scheme. River Res Appl 29:1131–1146
Kingsford RT (2000) Ecological impacts of dams, water diversions and river management on floodplain wetlands in Australia. Austral Ecol 25:109–127
Krawczyk A, Bogdziewicz M, Majkowska K, Głazaczow A (2016) Diet composition of the Eurasian otter Lutra lutra in different freshwater habitats of temperate Europe: a review and meta-analysis. Mammal Rev 46(2):106–113
Kruuk H (2006) Otters: ecology, behaviour and conservation. Oxford University Press, Oxford
Leuven RSEW, van der Velde G, Baijens I, Snijders J, van der Zwart C, Lenders HJR, de Vaate A (2009) The river Rhine: a global highway for dispersal of aquatic invasive species. Biol Invasions 11:1989–2008
Libois R, Fareh M, Brahimi A, Rosoux R (2015) Régime alimentaire et stratégie trophique saisonnière de la loutre d’Europe, Lutra lutra, dans le Moyen Atlas (Maroc). Revue D’ecologie 70:314–327
Loureiro TG, Anastácio PMSG, Araujo PB, Souty-Grosset C, Almerão MP (2015) Red swamp crayfish: biology, ecology and invasion - an overview. Nauplius 23:1–19
Lytle DA, Poff NL (2004) Adaptation to natural flow regimes. Trends Ecol Evol 19:94–100
Machado MJ, Benito G, Barriendos M, Rodrigo FS (2011) 500 years of rainfall variability and extreme hydrological events in southeastern Spain drylands. J Arid Environ 75:1244–1253
Mack MC, D’Antonio CM (1998) Impacts of biological invasions on disturbance regimes. Trends Ecol Evol 13:195–198
Mas J (1986) La ictiofauna continental de la cuenca del río Segura, evolución histórica y estado actual. Annales De Biología 8:3–17
Miller RR, Williams JD, Williams JE (1989) Extinctions of North American fisheries during the past century. Fisheries 14:22–38
Miñano PA, Oliva-Paterna FJ, Andreu A, García-Mellado A, García-Rodríguez J, Garcia de Jalon D, Torralva M (2003) Recursos piscícolas en los embalses de la Región de Murcia (SE de España). Boletín De La Real Sociedad Española De Historia Natural 98:103–113
Miñano PA, Oliva-Paterna FJ, Torralva M (2002) Primera cita de Sander lucioperca (L.) (Actinopterygii, Percidae) en la cuenca del río Segura. SE De España Anales De Biología 24:77–79
Musseau C, Boulenger C, Crivelli AJ, Lebel I, Pascal M, Boulêtreau S, Santoul F (2015) Native European eels as a potential biological control for invasive crayfish. Freshw Biol 60:636–645
Narváez M, Cabezas S, Blanco-Garrido F, Baos R, Clavero M, Delibes M (2020) Eurasian otter (Lutra lutra) diet as an early indicator of recovery in defaunated river communities. Ecol Ind 117:106547
Nessi A, Winkler A, Balestrieri A, Casoni AG, Tremolada P (2021) Kingfisher (Alcedo atthis) diet and prey selection as assessed by the analysis of pellets collected under resting sites (River Ticino, North Italy). Aquatic Ecology, https://doi.org/10.1007/s10452-020-09817-2
Oficialdegui FJ, Clavero M, Sánchez MI, Green AJ, Boyero L, Michot TC, Klose K, Kawai T, Lejeusne C (2019) Unravelling the global invasion routes of a worldwide invader, the red swamp crayfish (Procambarus clarkii). Freshw Biol 64:1382–1400
Olden JD, Poff NL (2005) Long-term trends of native and non-native fish faunas in the American Southwest. Anim Biodivers Conserv 28:75–89
Oliva-Paterna FJ, Andreu A, Verdiell D, Torralva M (2005) First occurrence of Lepomis gibbosus (L.,1758) in the Segura river basin (SE, Spain). Limnetica 24(3–4):199–202
Oliva-Paterna FJ, Miñano PA, Torralva MM (2003) Condition of Barbus sclateri as an indicator of habitat quality for fish in Mediterranean semi-arid streams. Environ Biol Fishes 67:13–22
Oliva-Paterna FJ, Verdiell-Cubedo D, Ruiz-Navarro A, Torralva M (2014) La ictiofauna continental de la Cuenca del río Segura (S.E. Península Ibérica): décadas después de Mas (1986) Anales de Biología 36:37–45
Oliva Paterna FJ, Zamora Marín JM, Franco Galera JM, Zamora López A, Sánchez Pérez A, Amat Trigo F, Guillén Beltrán A, Guerrero Gómez A, Torralva Forero M (2019) Peces dulceacuícolas de la cuenca del río Segura. ANSE, Asociación de Naturalistas del Sureste, Spain.
Ormerod SJ, Dobson M, Hildrew AG, Townsend CR (2010) Multiple stressors in freshwater ecosystems. Freshw Biol 55:1–4
Pastor González A (2011) La nutria (Lutra lutra, Linnaeus 1758) en la Cuenca del Segura: Situación actual y factores que determinan su distribución. Master thesis, University of Murcia.
Pittock J, Meng J, Geiger M, Chapagain AK (2009) Interbasin water transfers and water scarcity in a changing world - a solution or a pipedream?. WWF Germany, Frankfurt am Main, p 61
Prigioni C (1997) La Lontra. Una vita silenziosa negli ambienti acquatici. Edagricole, Bologna
Propst DL, Gido KB, Stefferud JA (2008) Natural flow regimes, non-native fishes, and native fish persistence in arid-land river systems. Ecol Appl 18:1236–1252
Qin J, Cheng F, Zhang L, Schmidt B, Liu J, Xie S (2019) Invasions of two estuarine gobiid species interactively induced from water diversion and saltwater intrusion. Management of Biological Invasions 10:139–150
Remonti L, Prigioni C, Balestrieri A, Sgrosso S, Priore G (2008) Trophic flexibility of the otter (Lutra lutra) in southern Italy. Mamm Biol 73:293–302
Remonti L, Prigioni C, Balestrieri A, Sgrosso S, Priore G (2010) Eurasian otter (Lutra lutra) prey selection in response to a variation of fish abundance. Ital J Zool 77:331–338
Rice WR (1989) Analysing tables of statistical tests. Evolution 43:223–225
Riesco M, Delibes M, Calzada J, Esquivias J, Qninba A, Clavero M (2020) Desert otters: distribution, habitat use and feeding ecology in arid rivers of Morocco. J Arid Environ 178:104165
Rodríguez-Estival J, Ortiz-Santaliestra ME, Mateo R (2020) Assessment of ecotoxicological risks to river otters from ingestion of invasive red swamp crayfish in metal contaminated areas: Use of feces to estimate dietary exposure. Environ Res 181:108907
Ruiz-Olmo J (1995) Estudio bionómico de la nutria (Lutra lutra L 1758) en aguas continentales de la Península Ibérica. PhD thesis, Universidad de Barcelona, 321 pp
Ruiz-Olmo J, Palazón S (1997) The diet of the European otter (Lutra lutra L., 1758) in Mediterranean freshwater habitats. J Wildlife Res 2:171–181
Sánchez-Pérez A, Oliva-Paterna FJ, Colin N, Torralva M, Górski K (2020) Functional response of fish assemblage to multiple stressors in a highly regulated Mediterranean river system. Science of The Total Environment 138989
Sanger AC (2001) Prospects and problems for the restoration of the Pedder galaxias. Lake Pedder - values and restoration, Occasional Paper No. 27. C. Sharpels. Hobart, Centre for Environmental Studies, University of Tasmania:125–130
Santos JM, Rivaes R, Boavida I, Branco P (2018) Structural microhabitat use by endemic cyprinids in a Mediterranean-type river: Implications for restoration practices. Aquat Conserv Mar Freshwat Ecosyst 28:26–36
Sanz-Ronda FJ, Bravo-Córdoba FJ, Sánchez-Pérez A, García-Vega A, Valbuena-Castro J, Fernandes-Celestino L, Torralva M, Oliva-Paterna FJ (2019) Passage performance of technical pool-type fishways for potamodromous cyprinids: novel experiences in semiarid environments. Water 11:2362
Schmidt BV, Wang Z, Ren P, Guo C, Qin J, Cheng F, Xie S (2019) A review of potential factors promoting fish movement in inter-basin water transfers, with emergent patterns from a trait-based risk analysis for a large-scale project in China. Ecology of Freshwater Fish, Early View. https://doi.org/10.1111/eff.12530
Shiklomanov IA (1999) Water transfer as one of the most important ways to eliminate water resources deficits and to solve water management problems. Interbasin water transfer. Proceedings of the International Workshop (UNESCO, Paris, 25–27 April 1999). IHP-V Technical documents in hydrology I No. 28. Int Hydrologic Program Paris, UNESCO:203–210
Smiroldo G, Villa A, Tremolada P, Gariano P, Balestrieri A, Delfino M (2019a) Amphibians in Eurasian otter Lutra lutra diet: osteological identification unveils hidden prey richness and male-biased predation on anurans. Mammal Rev 49:240–255
Smiroldo G, Gariano P, Balestrieri A, Manenti R, Pini E, Tremolada P (2019b) Predation on amphibians may enhance Eurasian otter recovery in southern Italy. Zoolog Sci 36:273–283
Souty-Grosset C, Anastacio PM, Aquiloni L, Banha F, Choquer J, Chucholl C, Tricarico E (2016) The red swamp crayfish Procambarus clarkii in Europe: impacts on aquatic ecosystems and human well-being. Limnologica 58:78–93
Swift C, Howard S, Mulder J, Pondella D, Keegan T (2015) Expansion of the non-native Mississippi Silverside, Menidia audens (Pisces, Atherinopsidae), into fresh and marine waters of coastal southern California. Bull South Calif Acad Sci 113:153–164
Tablado Z, Tella JL, Sanchez-Zapata JA, Hiraldo F (2010) The paradox of the long-term positive effects of a North American crayfish on a European community of predators. Conserv Biol 24:1230–1238
Torralva M, Oliva-Paterna FJ (1997) Primera cita de Chondrostoma polylepis Steindachner, 1865 (Ostariophysi, Cyprinidae) en la cuenca del río Segura, S.E. de España. Limnetica 13:1–3
Torralva M, Puig MA, Fernández-Delgado C (1997) Effect of river regulation on the life-history patterns of Barbus sclateri in the Segura river basin (south-east Spain). J Fish Biol 51:300–311
Uche J, Martínez-Gracia A, Círez F, Carmona U (2015) Environmental impact of water supply and water use in a Mediterranean water stressed region. J Clean Prod 88:196–204
Vila-Gispert A, Moreno-Amich R (2001) Mass–length relationship of Mediterranean barbel as an indicator of environmental status in South-west European stream ecosystems. J Fish Biol 59:824–832
Yelo ND, Calvo JF (2004) Aproximación a la distribución y estatus de los mamíferos carnívoros en la región de Murcia. Galemys 16(2):21–37
Acknowledgements
We are grateful to Jose Manuel Zamora-Marin and Antonio Zamora-López (Department of Zoology and Physical Anthropology, University of Murcia) for their cooperation.
Funding
Open access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement. The research was supported by the LIFE13 BIO/ES/001407 RIPISILVANATURA. Daniel Bruno was funded by “PTI ECOBIODIV” through the Vicepresidencia Adjunta de Áreas Científico-Técnicas (VAACT-CSIC).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by EED, AB, and SP. The first draft of the manuscript was written by EED and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Research involving human and animal participants
The study did not involve humans or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dettori, E.E., Balestrieri, A., Zapata-Pérez, V.M. et al. Eurasian otter Lutra lutra diet mirrors the decline of native fish assemblages in a semi-arid catchment (River Segura, SE Spain). Eur J Wildl Res 68, 38 (2022). https://doi.org/10.1007/s10344-022-01588-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10344-022-01588-5